Translate this page into:
Synthesis, characterization, biological evaluation, and kinetic study of indole base sulfonamide derivatives as acetylcholinesterase inhibitors in search of potent anti-Alzheimer agent
⁎Corresponding author. mtaha@iau.edu.sa (Muhammad Taha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Alzheimer is a prolonged neurodegenerative disease which degenerate the brain cells and particularly affects the person ability to function independently. Despite of dynamic research, there is no proper treatment but can limit their persistent effect in early stages. In search of more potent drug for Alzheimer treatment, we have synthesized indole-based sulfonamide derivatives (1–17). All analogs were screened to find out lead candidate against acetylcholinesterase enzyme under positive control of donepezil as standard drug. Herein this study, analogs 1–4, 6–9, and 13–15 showed potent inhibition while kinetic studies further confirmed their mode of inhibition. All the synthesized analogs were characterized through HR-EI-MS, 1H NMR and 13C NMR spectroscopic techniques.
Keywords
Alzheimer
Sulfonamide derivatives
Acetylcholinesterase enzyme
Kinetic study
1 Introduction
Alzheimer is a chronic disease and showed to effect 10% of world population and its prevalence increases with age (Seshadri et al., 1997). The foremost cause of dementia is Alzheimer especially in the elderly people (Blennow, 2010). It is irremediable disease predominantly degenerates the nervous system and particularly affect memory, thought and language skill respectively (Marlatt et al., 2005; Singh et al., 2013). By all considerations, till date - the main cause and mechanism of this disease is entirely unexplored but as per general consensus there are certain factors like hyperchlorination of neuron protein of center nervous system, low concentration of acetylcholine (Ach), beta amyloid peptide aggregations and oxidative stress play vital roles to prompt further this disease (Huang et al., 2004; Savelieff et al., 2013; Tumiatti et al., 2010). Although cholinergic hypothesis concerned to the low concentration of acetylcholine (ACh) which has direct links with learning and memory function, immediately hydrolyze by butyrylcholinesterase and acetylcholinesterase enzyme (AChE) into choline and acetic acid which causes the termination of neurotransmission signal. It has been observed that acetylcholinesterase play major role in this disintegration as compare to butyrylcholinesterase enzyme (Francis et al., 1999; Massoulié et al., 1993). An advanced method to cure Alzheimer’s disease (AD) includes the strategy to design AChE and BuChE inhibitors. Cholinesterases (ChEs) are the eye-catching objects for the medicinal community, hence several FDA-approved drugs [e.g., donepezil (Aricept), rivastigmine (Exelon), and galantamine (Reminyl)] have been marketed to cure AD. By this consideration to treat AD, acetylcholinesterase is the active target to be inhibit and to improve the cholinergic neurotransmission (Herrmann et al., 2011; Mohamed & Rao, 2017).
Indole based sulfonamide analogs clearly proved its versatility like anti-tumor (Deng et al., 2018), antidiabetic (Kawde et al., 2020), anti-fibrotic (Boubia et al., 2018), anti-viral (Zhao et al., 2008) respectively. Indole has been broadly explored for numerous biological activities due to its fortunate structure that can bind several receptors with high affinity (Gomtsyan, 2012). A large number of marketed medications covers the indole based moiety, for example, indomethacin, (Sukul et al., 2016) apaziquone (indolequinone), (Witjes & Kolli, 2008) and delavirdine (Xu & Lv, 2009). Li et al. reported the design, synthesis, and evaluation of a series of 2-(2-indolyl)-4(3H)-quinazolines derivatives and compounds 1a and 1b (Fig. 1) as inhibitors of AChE (Li et al., 2013). Filali et al. discussed the inhibitory activity of harmine and its isoxazoline derivatives (Fig. 1) against AChE. Harmine and its derivatives (isoxazoline) showed AChE inhibitory effect, and harmine showed the potent inhibition with an IC50 value of 10.4 µm (Filali et al., 2015). A series of PHY derivatives was synthesized and evaluated for their anticholinesterase activity (Brufani et al., 1986). Among the synthesized analogs, heptylphysostigmine (Eptastigmine, Fig. 1) showed a competitive inhibition of AChE. The sulfonamide derivatives also reported for anti-Alzheimer potential in recent past (Bag et al., 2015; Swetha et al., 2019)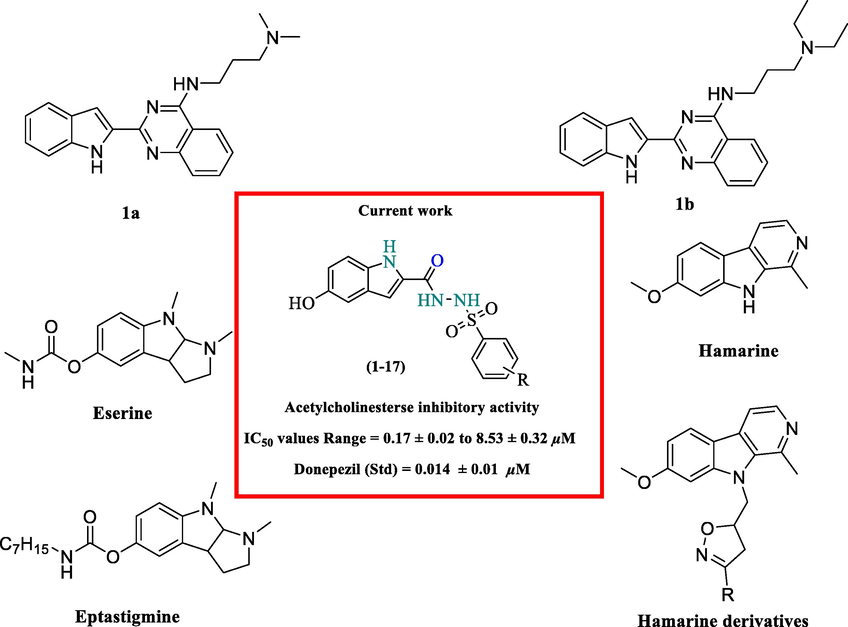
Indole based analogues reported in recent past as anti-Alzheimer agent.
In the continuation of our work and pronounced pharmacological potential of indole derivatives we have synthesized indole-based sulfonamide analogs (1–17). All analogs were screened against acetylcholinesterase enzyme in order to explore their potential in search of potent anti-Alzheimer agent (Fig. 1).
2 Result and discussion
2.1 Chemistry
Methyl 5-hydroxy-1H-indole-2-carboxylate and hydrazine hydrate were mixed together methanol and reflux the reaction mixture for six hours to afford the desire intermediate 5-hydroxy-1H-indole-2-carbohydrazide. Finally, 5-hydroxy-1H-indole-2-carbohydrazide and various aromatic substituted sulfonyl-chlorides were reacted in pyridine. When the reaction was complete, the reaction product was poured in cold distil water in order to precipitate the target analogs. Subsequently filter the precipitate to afford pure analogs (1–17) (Scheme 1) and washed with n-hexane to afford pure compounds (1–17) (Table 1).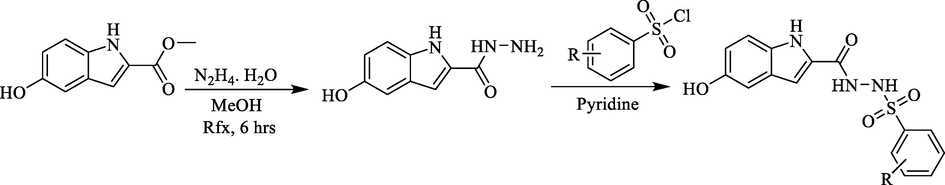
Synthesis of indole base sulfonamide analogs (1–17).
Comp.
R
IC50 ± SEM (µM)
Comp.
R
IC50 ± SEM(µM)
1
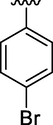
0.19 ± 0.08
10
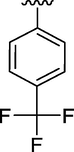
1.12 ± 0.12
2
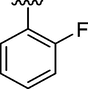
0.20 ± 0.12
11
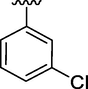
5.71 ± 0.32
3
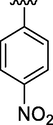
0.37 ± 0.15
12
8.53 ± 0.32
4
0.41 ± 0.17
13
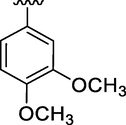
7.37 ± 0.08
5
6.32 ± 0.03
14
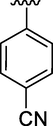
0.20 ± 0.06
6
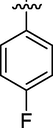
0.17 ± 0.02
15
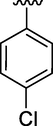
0.18 ± 0.03
7
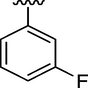
0.51 ± 0.12
16
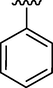
5.12 ± 0.12
8
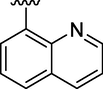
5.63 ± 0.02
17
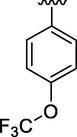
7.52 ± 0.02
9
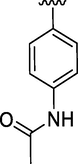
0.41 ± 0.17
–
–
–
Donepezil (Standard)
0.014 ± 0.01 µM
SEM = Standard Error Mean
2.2 In vitro acetylcholinesterase inhibitory activity
To date with endless efforts, our group has been reported various heterocyclic moieties in search of potent inhibitors (Chigurupati et al., 2016; Imran et al., 2015; Taha et al., 2019a, 2015a, 2015b, 2015c, 2015d, 2019b). With hope, particularly for search of potent anti-Alzheimer agent we have synthesized indole base sulfonamide analogs and therefore all analogs were screened for acetylcholinesterase inhibitory activity in search of potent inhibitors under positive control of donepezil (IC50 = 0.014 ± 0.01 µM) as reference drug. The whole series of analogs were active and exhibited inhibitory activity ranging in between 0.17 ± 0.02 µM to 8.53 ± 0.32 µM as shown in table 1. Almost all analogs exhibited acetylcholinesterase inhibitory activity, but analogs 1, 2, 6, 14 and 15 displayed very significant acetylcholinesterase inhibitory potential (IC50 = 0.19 ± 0.08, 0.2 ± 0.12, 0.17 ± 0.02, 0.20 ± 0.06 and 0.18 ± 0.03 µM respectively) as compared to standard donepezil (IC50 = 0.014 ± 0.32 µM), while the remaining analogs exhibited good inhibitory activity. The effects of substituents on phenyl ring were illustrated through structure activity relationship (SAR) study.
Here in this study, it was found that the substituents on phenyl ring which have electron withdrawing affect deactivated the phenyl ring, but the phenyl ring with such substitution at ortho and para position interacted well with fit coordination with active site of enzyme. Among the analogs, analog 6 (IC50 = 0.17 ± 0.02 µM) and analog 2 (IC50 = 0.20 ± 0.12 µM) having fluoro group at para position and ortho position respectively on phenyl ring emerged the most active one as compared with analog 7 (0.51 ± 0.12 µM) which have the same fluoro-substitution at meta position on phenyl ring. Also, analog 1 (IC50 = 0.19 ± 0.08 µM) and analog 15 (IC50 = 0.18 ± 0.03 µM) showed good activity with bromo and choro substitutions at para position.
It was clearly observed from activity profile when the electron donating substituents are present, the inhibitory activity of analog decreased. Among analog 12 and 13 (IC50 = 8.5 ± 0.32 and 7.37 ± 0.08 µM) respectively. In case of analog 13 there is only one methoxy substituents at 4 position exhibited better inhibitory potential than analog 12 which have methoxy substituents at meta and para positions on phenyl ring and might hindered the binding interactions of analog 12 with active site of enzyme due to which the inhibitory potential decreased.
Moreover, the position of substituent some time more favor the binding interaction of analogs with active sites and this was illustrated by comparison the inhibitory activity of analog 11 and 15 with each other. Analog 15 which have chloro substituent at para position exhibited greater inhibitory potential than analog 11 which have the same chloro substituent at meta position on phenyl ring. The analog 14 also showed good activity having 4-cyno group.
2.3 Kinetic studies of enzyme inhibition
Herein this work kinetic study of enzyme inhibition was carried out to know about the mechanism of inhibition and types of inhibition of most active analogs (1, 2, 6, 14, and 15). The kinetic parameters Vmax, Km, Ki, AICc and R2 values were calculated and shown in Table 2. The type of enzyme inhibitions was confirmed by Km and Vmax values obtained by Michaelis-Menten and Lineweaver-Burk double reciprocal plots and all the analogs showed reduced Km and Vmax values. Whereas the Ki value obtained by Dixon plot, showed that the Ki values of all the analogs showed one-half that of the IC50 numerical values. Moreover, the type enzyme inhibitor mechanism was supported by regression coefficient (R2) of curve fitting and low AICc value. Based on the above evidence confirmed that all the selected compounds displayed Uncompetitive type enzyme inhibitor mechanism (Figs. 2–4). The calculated Vmax and Km values for pure enzyme (without inhibitor) were 980.5 ± 22 µM/min and 422.1 ± 015 µM respectively.
Comp.
Vmax (µM/min)
Km (µM)
Ki (µM)
AICc
R2
Type of inhibition
1
668.2 ± 32.0
310 ± 0.015
0.095
234.1
0.912
Uncompetitive type
2
690.6 ± 26.0
300 ± 0.011
0.100
266.4
0.904
Uncompetitive type
6
702.7 ± 12.0
380 ± 0.020
0.110
199.4
0.912
Uncompetitive type
14
692.1 ± 54.0
350 ± 0.012
0.100
258.2
0.910
Uncompetitive type
15
686.5 ± 70.0
350 ± 0.015
0.090
230.5
0.902
Uncompetitive type
Donepezil
107.8 ± 25.0
124 ± 0.018
0.010
85.2
0.921
Mixed Type
![Lineweaver-Burk plot of analog 6 1/[S] Vs 1/Rate in the different concentrations of Uncompetitive inhibitor. Where the reciprocal of the y-axis intercept gives information about Vmax while reciprocal of the x-axis gives information about Km.](/content/185/2021/33/3/img/10.1016_j.jksus.2021.101401-fig4.png)
Lineweaver-Burk plot of analog 6 1/[S] Vs 1/Rate in the different concentrations of Uncompetitive inhibitor. Where the reciprocal of the y-axis intercept gives information about Vmax while reciprocal of the x-axis gives information about Km.
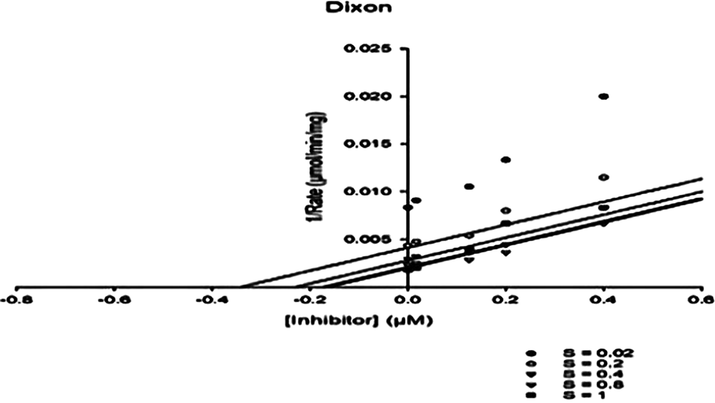
Dixon plot of analog 6 at different concentration of Uncompetitive inhibitor Vs 1/Rate. The regression line for each concentration of the substrate was obtained, and Ki was calculated from the intersection of the five lines.
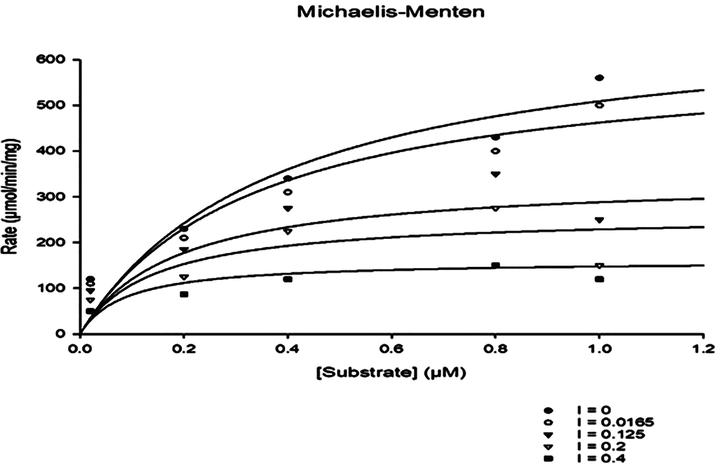
Michaelis-Menten plot of analog 6 by substrate Vs Rate in the different concentration of Uncompetitive inhibitor.
Note: Values expressed as mean ± SD (n = 3).
Moreover, various kinetics plots were used such as Dixon, Lineweaver-Burk, and Michaelis-Menten models to interpret the reaction rate which proved Uncompetitive inhibition (Figs. 2–4).
2.4 Molecular docking
Indole base sulfonamide derivatives showed potent acetylcholinesterase (AChEs) inhibition based on their IC50 values (Table 1). AChEs inhibition of synthesized compounds may be influenced by the number, position and type of the varied functional groups in the aromatic ring of their parent skeleton of indole base sulfonamide (Table 1). To realize the observed enzymatic inhibition by the synthesized compounds, molecular docking studies has been carried out to find the binding interactions between the synthesized compounds 1–17 from one side and the active residues of the acetylcholinesterase from either side. The selected compounds varied by their number and position of the substituted functional groups in the aromatic ring (Table 1). For example, compounds 2, 6 and 7 are substituted by a fluorine group in ortho, para and meta positions, respectively (Table 1). Compounds 12 and 13 differ by the number and positions of substituted methoxy groups (Table 1).
The binding energies of the stable complex's ligand-acetylcholinesterase, between synthesized analogs and AChEs active site have been summarized in Table 3 to show the closest residues among the docked compounds and their IC50 values.
Comps.
Free binding energy (kcal/mol)
H-Bonds (HBs)
Number of closest residues to the docked ligand in the active site
IC50 ± SEM (µM)
1
−10.55
5
9
0.19 ± 0.08
2
−10.00
4
6
0.20 ± 0.12
3
−10.15
8
8
0.37 ± 0.15
4
−10.28
3
9
0.41 ± 0.17
5
−10.07
5
7
6.32 ± 0.03
6
−9.78
4
6
0.17 ± 0.02
7
−10.10
4
9
0.51 ± 0.12
8
−12.23
2
10
5.63 ± 0.02
9
−10.98
5
5
0.41 ± 0.17
10
−9.57
6
10
1.12 ± 0.12
11
−10.35
4
7
5.71. ± 0.32
12
−10.34
6
7
8.53 ± 0.32
13
−9.90
5
6
7.37 ± 0.08
14
−10.28
5
6
0.20 ± 0.06
15
−10.36
3
5
0.18 ± 0.03
16
−10.20
4
5
5.12 ± 0.12
17
−9.67
4
5
7.52 ± 0.02
Donepezil (Std)
−11.11
1
8
0.014 ± 0.01
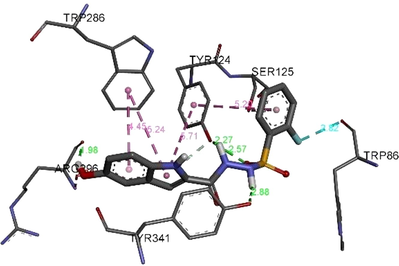
All the complexes formed between the indole base sulfonamide derivatives and the active residues of acetylcholinesterase displayed negative bending energies, which reveals that AChEs inhibition by indole base sulfonamide derivatives is a thermodynamic favorable process (Table 3). Except 8, binding energies of the stable complexes vary slightly with variation less than 1.5 kcal mol−1. Such variation is considerably not enough to show strong descriptor in justifying the observed acetylcholinesterase inhibition. However, the number of hydrogen bonding, its distances, and the intermolecular interactions among the substitute groups of the selected derivatives and the active residues may strongly help in identifying the observed acetylcholinesterase inhibition. The compounds 2, 7 and 6 vary by the position of the substituted fluorine atom at the aromatic. Experimentally, 2and 6 show higher activity than 7. Their corresponding complexes formed with acetylcholinesterase exhibit similar binding energies with variation less than 0.32 kcalmol−1. As can be seen in Fig. 5 and Table 2, these compounds forms four hydrogen bonding with amino acids ARG B:296, TYR B:296 and TRY B:341 of acetylcholinesterase. The greater acetylcholinesterase inhibition of 2 and 6 compared with 7 may refer to the π-π T-shaped interaction established between TYR B:124 and the substitute aromatic ring of the formers compared with the latter (Fig. 5).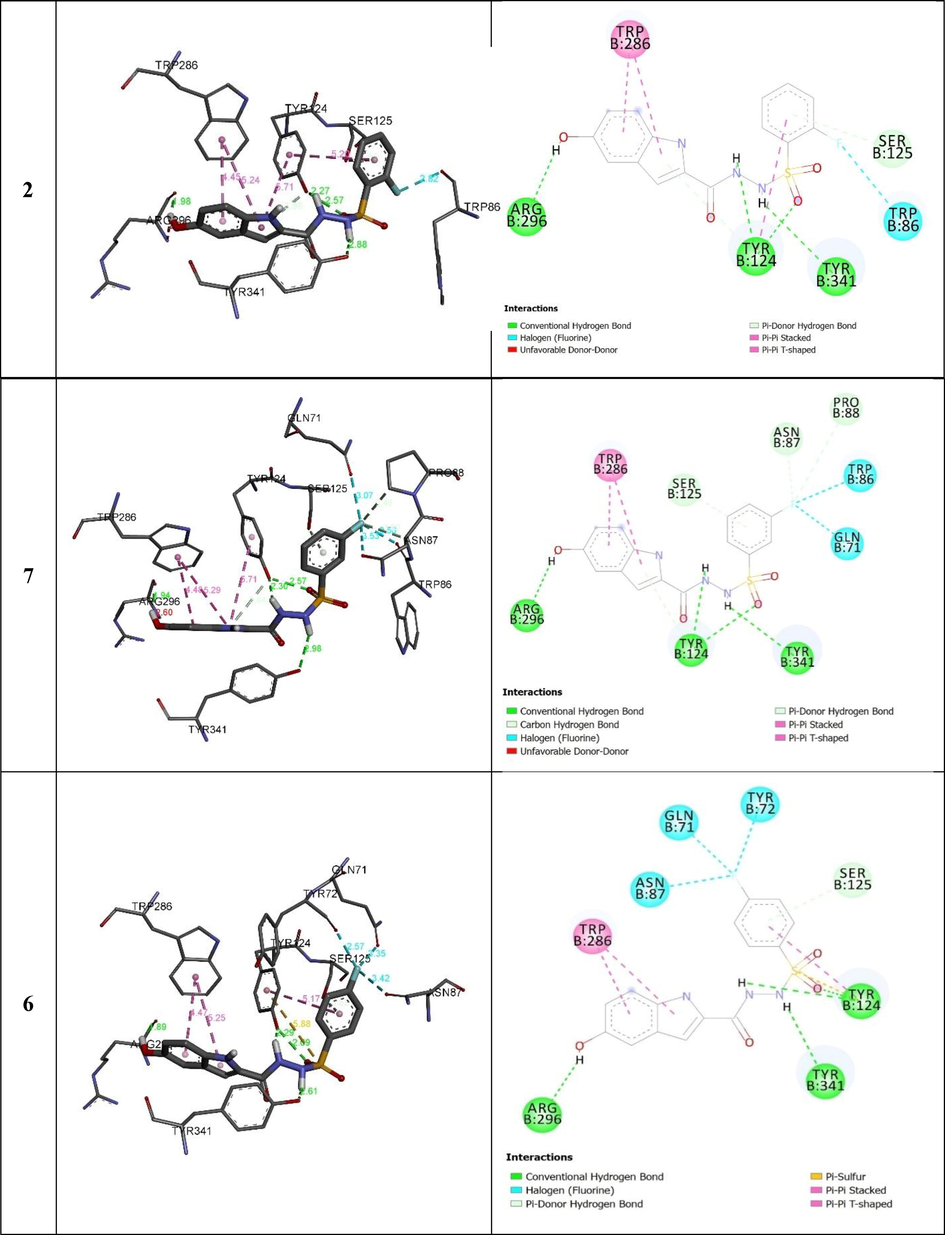
3D (right) and 2D (left) closest interactions between active site residues of urease and selected compounds 2, 7, and 6.
3 Conclusion
Herein the present work all analogs (1–17) of indole base sulfonamide were screened for their acetylcholinesterase inhibitory activity and the results obtained about their potentials revealed that it acts as suitable class of acetylcholinesterase inhibition. Almost all analogs of the series displayed inhibitory potentials comparable with standard drug donepezil but analogs 1–4, 6, 7, 9, 14 and 15 were found most potent the series. Moreover, kinetic studies were carried which clearly showed that analog 1, 2, 6, 14 and 15 involved in this inhibition through uncompetitive manner. Further molecular docking studies revealed that compounds having considerably good inerteraction with enzyme at active site. These findings is the first step towards the development of potent analog against Alzheimer disease. The most active compounds can further be optimized to get lead compounds.
Acknowledgments
Authors acknowledge IRMC support given for the research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. Lett.. 2015;25(3):626-630.
- [Google Scholar]
- Biomarkers in Alzheimer's disease drug development. Nat. Med.. 2010;16(11):1218-1222.
- [Google Scholar]
- Design, synthesis, and evaluation of a novel series of indole sulfonamide peroxisome proliferator activated receptor (PPAR) α/γ/δ triple activators: discovery of lanifibranor, a new antifibrotic clinical candidate. J. Med. Chem.. 2018;61(6):2246-2265.
- [Google Scholar]
- Anticholinesterase activity of a new carbamate, heptylphysostigmine, in view of its use in patients with Alzheimer-type dementia. Eur. J. Biochem.. 1986;157(1):115-120.
- [Google Scholar]
- Identification of novel acetylcholinesterase inhibitors: indolopyrazoline derivatives and molecular docking studies. Bioorg. Chem.. 2016;67:9-17.
- [Google Scholar]
- Design, Synthesis, and Evaluation of Dihydrobenzo [cd] indole-6-sulfonamide as TNF-α Inhibitors. Front. Chem.. 2018;6:98.
- [Google Scholar]
- Synthesis of new isoxazoline derivatives from harmine and evaluation of their anti-Alzheimer, anti-cancer and anti-inflammatory activities. J. Enzyme Inhib. Med. Chem.. 2015;30(3):371-376.
- [Google Scholar]
- The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):137-147.
- [Google Scholar]
- Gomtsyan, A., 2012. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. (N. Y., NY, U. S.), 48(1), 7-10.
- Current and emerging drug treatment options for Alzheimer’s disease. Drugs. 2011;71(15):2031-2065.
- [Google Scholar]
- Redox-active metals, oxidative stress, and Alzheimer's disease pathology. Ann. N. Y. Acad. Sci.. 2004;1012(1):153-163.
- [Google Scholar]
- Synthesis of novel flavone hydrazones: In-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. Eur. J. Med. Chem.. 2015;105:156-170.
- [Google Scholar]
- Kawde, A.-N., Taha, M., Alansari, R. S., Almandil, N. B., Uddin, N., Rahim, F., Chigurupati, S., Nawaz, M., Hayat, S., & Ibrahim, M. 2020. Exploring efficacy of indole-based dual inhibitors for α-glucosidase and α-amylase enzymes: In silico, biochemical and kinetic studies. Int. J. Biol. Macromol.
- 2-(2-indolyl-)-4 (3 H)-quinazolines derivates as new inhibitors of AChE: design, synthesis, biological evaluation and molecular modelling. J. Enzyme Inhib. Med. Chem.. 2013;28(3):583-592.
- [Google Scholar]
- Therapeutic opportunities in Alzheimer disease: one for all or all for one? Curr. Med. Chem.. 2005;12(10):1137-1147.
- [Google Scholar]
- Molecular and cellular biology of cholinesterases. Prog. Neurobiol.. 1993;41(1):31-91.
- [Google Scholar]
- 2, 4-Disubstituted quinazolines as amyloid-β aggregation inhibitors with dual cholinesterase inhibition and antioxidant properties: Development and structure-activity relationship (SAR) studies. Eur. J. Med. Chem.. 2017;126:823-843.
- [Google Scholar]
- Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem. Biol.. 2013;8(5):856-865.
- [Google Scholar]
- Lifetime risk of dementia and Alzheimer's disease: The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498-1504.
- [Google Scholar]
- Acetylcholinesterase inhibitors as Alzheimer therapy: from nerve toxins to neuroprotection. Eur. J. Med. Chem.. 2013;70:165-188.
- [Google Scholar]
- Sukul, A., Kumar Poddar, S., Haque, S., Kumar Saha, S., Chandra Das, S., Al Mahmud, Z., & Abdur Rahman, S. 2016. Synthesis, Characterization and Comparison of Local Analgesic, Anti-Inflammatory, Anti-Ulcerogenic Activity of Copper and Zinc Complexes of Indomethacin. Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents), 15(3), 221-233.
- Multifunctional hybrid sulfonamides as novel therapeutic agents for Alzheimer’s disease. Future Med. Chem.. 2019;11(24):3161-3178.
- [Google Scholar]
- Antiglycation and antioxidant potential of novel imidazo [4, 5-b] pyridine benzohydrazones. Arabian J. Chem.. 2019;12(8):3118-3128.
- [Google Scholar]
- Synthesis of new oxadiazole derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem.. 2015;23(15):4155-4162.
- [Google Scholar]
- Synthesis of novel benzohydrazone–oxadiazole hybrids as β-glucuronidase inhibitors and molecular modeling studies. Bioorg. Med. Chem.. 2015;23(23):7394-7404.
- [Google Scholar]
- Synthesis of novel derivatives of oxindole, their urease inhibition and molecular docking studies. Bioorg. Med. Chem. Lett.. 2015;25(16):3285-3289.
- [Google Scholar]
- Synthesis of novel inhibitors of α-glucosidase based on the benzothiazole skeleton containing benzohydrazide moiety and their molecular docking studies. Eur. J. Med. Chem.. 2015;92:387-400.
- [Google Scholar]
- Synthesis of 3, 4, 5-trihydroxybenzohydrazone and evaluation of their urease inhibition potential. Arabian J. Chem.. 2019;12(8):2973-2982.
- [Google Scholar]
- Tacrine derivatives and Alzheimer's disease. Curr. Med. Chem.. 2010;17(17):1825-1838.
- [Google Scholar]
- Apaziquone for non-muscle invasive bladder cancer: a critical review. Expert Opin. Invest. Drugs. 2008;17(7):1085-1096.
- [Google Scholar]
- Developments of indoles as anti-HIV-1 inhibitors. Curr. Pharm. Des.. 2009;15(18):2120-2148.
- [Google Scholar]
- Novel indole-3-sulfonamides as potent HIV non-nucleoside reverse transcriptase inhibitors (NNRTIs) Bioorg. Med. Chem. Lett.. 2008;18(2):554-559.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101401.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







