Translate this page into:
Synthesis, characterization and effect of bis-1,3,4-oxadiazole rings containing glycine moiety on the activity of some transferase enzymes
*Corresponding author. Tel.: +964 7901965123 ivanhrtomy@yahoo.com (Ivan H.R. Tomi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 10 June 2010
Abstract
We described herein the synthesis of bis-1,3,4-oxadiazole containing glycine moiety. N-{5-[5-(4-Methoxyphenyl)-1,3,4-oxadiazole-2-yl-sulfanyl]-1,3,4-oxadiazole-2-yl-methyl}-4-methoxybenzamide was fully characterized by elemental analysis, FT-IR and 1H NMR spectroscopy. Also this study was designed to show the effects of bis-oxadiazole compound on the activities of some transferase enzymes such as: GOT, GPT and γ-GT in sera. This compound demonstrated activation on GOT and GPT activities, inhibitory effects on the γ-GT activity. These effects increased with increasing the concentration of the compound. The causes of the increases and decreases in the enzymes activities are discussed.
Keywords
Bisoxadiazole
Transferase enzymes
Glycine
Activity of enzymes
GOT
GPT
GGT
1 Introduction
Derivatives of 1,3,4-oxadiazole constitute an important family of heterocyclic compounds (Hill, 1984). Substituted 1,3,4-oxadiazole are of considerable pharmaceutical and material interest, which is documented by a steadily increasing number of publications and patents (Kartitzky et al., 1995–2007). Reported among these activities were: nervous system depressing (Maillard et al., 1962), muscle relaxants (Vousooghi et al., 2007), analgesic (Mishra et al., 1995), herbicidal (Kennedy and Summers, 1981), hypoglycemic (Girges and Arzneim-Forsch, 1994), antifungal (Xia et al., 2002), anti-inflammatory (Suman and Bahel, 1979) and antibacterial activities (Tashfeen et al., 2005). In addition to that, derivatives of 1,3,4-oxadiazole are used in agriculture (Hodogaya, 1980), photosensitizer (Jai et al., 1999) and liquid crystals (Parra et al., 2006).
Also, the derivatives of heterocyclic compounds were studied to show their effects on activities of some transferase enzymes. Dere and Polat (2001) were studied the biochemical analysis of (PQ) (1,1-dimethyl-4,4′-bipiridillium) on some transferase enzymes and they found some changes (increases or decreases) in these enzymes activities.
In view of the importance of transferase enzymes reactions like GOT, GPT and GGT which form links between the metabolism of amino acids, carbohydrates and fats.
Glutamate oxaloacetate transaminase (GOT) Enzyme EC 2.6.1.1: it is called also aspartate amino transfer (AST), it is one of the most important of transferase enzymes, catalyzes the transfer of the amino group of aspartate to α-ketoglutarate. GOT is widely distributed in human tissues; heart, liver, skeletal muscle and kidney. The optimum conditions of maximum enzyme activity are pH 7.4 and temperature = 37 °C. The enzyme stability 3 days in 25 °C, 1 week in 5 °C and 1 month in −25 °C. The clinical usefulness of the enzyme is largely restricted to the diagnosis of heart and liver diseases. Large amount of GOT may be released in to the blood. Very high levels are observed in acute liver disease while lesser elevation is seen in chronic liver disease.
Glutamate pyruvate transaminase (GPT) Enzyme EC 2.6.1.2: it is also called alanine amino transferase (ALT) which is prevalent in mammalian tissue catalyzes the transfer of the amino group of alanine to α-ketoglutarate. GPT found in a highest concentration in livers in spite of its active occurrence in skeletal muscles, heart and kidneys. The GPT activity in tissues is generally less than GOT. GPT level found to increase in the following diseases; infection hepatitis, liver cirrhosis and biliary cirrhosis, obstructive jaundice and liver cancer.
Gamma glutamate transferase (γ-GT) Enzyme EC 2.3.2.2: it is also called gamma glutamate transpeptides (γ-GT) or (GGT), it is found in kidneys and liver and catalyzes the transfer of gamma-glutamyl group from glutathione to an amino acid. GGT levels are increased in most forms of liver disease, especially cholestasis. GGT, a plasma membrane-bound enzyme, provides the only activity capable to effect the hydrolysis of extra cellular glutathione (GSH), thus favoring the cellular utilization of its constituent amino acids.
The common synthetic approaches to oxadiazoles involve cyclization of diacylhydrazines (Georg, 2006). A variety of reaction conditions influence the cyclization reaction. Typically, the reaction is promoted by heat and anhydrous reagents including thionyl chloride (Al-Talib et al., 1990), phosphorous oxychloride (Theocharis and Alexandrou, 1990), phosphorous pentoxide (Carlsen and Jorgensen, 1994), triphenylphosphine (Brown et al., 1997) and triflic anhydride (Liras et al., 2000). Also Feray et al. (2002) are synthesized the oxadiazole derivatives using the synthetic procedure based on the ring closure reactions of appropriate acid hydrazides with carbon disulfide in alkali media.
In the literature, we did not found any studies of the effect of oxadiazole derivatives on the activity of transferase enzymes. So, we studied here the effective of bis-1,3,4-oxadiazole compound containing sulfur atom between the two oxadiazole rings derived from N-protected glycine attached at C-5 of the oxadiazole ring, on the activities of some transferase enzymes such as; aspartate amino transferase (AST), alanine amino transferase (ALT) and gamma glutamate transferase (γ-GT) in sera. This product might be potential compound for biological activity tests on transferase enzymes. A literature search revealed that no such oxadiazoles derived from amino acids have yet been prepared except Sebastiao et al. (1998) synthesized some 3-aryl-1,2,4-oxadiazoles carrying protected l-alanine side chain.
2 Experimental
2.1 Materials and physical measurements
All starting materials and solvents were purchased from Aldrich and Fluka and used without further purification. Melting points were determined on electrothermal capillary apparatus and are uncorrected, elemental analysis (C, H, N) were carried out using a Perkin-Elmer model 2400 instrument, FT-IR measurements were recorded on Shimadzu model FTIR-8400S. 1H NMR spectra were obtained with Bruker spectrophotometer model ultra shield at 300 MHz in DMSO-d6 solution with the TMS as internal standard. Note: in some 1H NMR spectra, the peaks at δ 2.5 and 3.35 are for the solvent (DMSO-d6) and dissolved water in (DMSO-d6) respectively.
2.2 Materials and methods of biological activity section
2.2.1 Effect of compound (7) on GOT and GPT activities
Colorimetric determination of GOT or GPT activity according to the following reactions:
The pyruvate or oxaloacetate formed was measured in its derived from 2,4-dinitrophenylhydrazine, which was absorbed at wave length 505 nm (Reitman and Frankel, 1957).
2.2.2 Effect of compound (7) on γ-GT activity:
Kinetic colorimetric method for the determination of γ-GT activity was assayed by Persijn and Van Der Slik (1976). The principle of the method was measurement of the 5-amino-2-nitro benzoate form from reaction, which was absorbed at wave length 405 nm
2.2.3 A stock solution (0.01 M) of compound (7)
A stock solution (0.01 M) of compound (7) was prepared by dissolving it in ethanol/DMSO (10/1), and the following concentrations (10−2, 10−3, 10−4, 10−5 M) were prepared by diluting with absolute ethanol. The enzymes GOT, GPT and GGT activities were measured in human serum by using the same methods of these enzymes with replace 100 μl of buffer with 100 μl of compound (7). The inhibition percentage was calculated by comparing the activity with and without the compound (7) and under the same conditions, according to the equation:
The activation percentage was calculated by comparing the activity with and without the activator and under the same conditions, according to the equation:
2.2.4 A constant concentration of compound (7) (10−2 M)
A constant concentration of compound (7) (10−2 M) was used with different substrate concentrations of (40, 80, 120, 160, 200) mmol/L for GOT and GPT, and (0.4, 0.8, 1.2, 1.62) mmol/L for γ-GT, to study the type of inhibition or activation. Buffers were used to prepared different substrates concentrations of these enzymes, GOT, GPT (phosphate buffer pH = 7.40, 100 mmol/L) and γ-GT (TRIS buffer pH = 8.25, 100 mmol/L). The enzymes activities were determined with and without compound (7), by using the Linweaver and Burke (1934) equation and plotting 1/v against 1/[s] were evaluated values; Ki, apparent Vmax (Vmapp), apparent Km (Kmapp), type of inhibition or activation.
2.3 Preparation methods and physical data of synthesized compounds (1–7)
2.3.1 4-Methoxymethyl benzoate (1)
This compound was prepared following the procedure described by Brian et al. (1989). Yield (95%); mp: 49–51 °C.
2.3.2 4-Methoxybenzoyl hydrazine (2)
This compound was prepared following the procedure described by Smith (1946). Yield (91%); mp: 135–137 °C.
2.3.3 5-(4-Methoxyphenyl)-1,3,4-oxadiazole-2-thione (3)
To a solution of hydrazide 2 (1.66 g, 0.01 mol) in ethanol (20 mL), potassium hydroxide (0.56 g, 0.01 mol) in water (5 mL) and carbon disulfide (2 mL, 0.03 mol) were added. The reaction mixture was heated under reflux till the evolution of hydrogen sulfide ceased (approximately 5 h), therefore, it was cooled, diluted with cold water (30 mL) and acidified with 10% hydrochloric acid. The solid that separated was collected by filtration, washed with water and recrystallized from ethanol. Yield (84%); mp: 199–201 °C; FT-IR (KBr disk, cm−1) 3217 (N—H), 1616 (C⚌N), 1253, 1080 (C—O—C); 1H NMR (DMSO-d6, 300 MHz, δ) 14.38–14.78 (s, br. 1H, NH), 7.79–7.85 (dd, 2H, Ar.H), 7.11–7.16 (dd, 2H, Ar.H), 3.86 (s, 3H, OCH3). Anal. Calc. for C9H8N2O2S (208 g/mol): C, 51.92; H, 3.85; N, 13.46. Found: C, 51.88; H, 3.80; N, 13.48%.
2.3.4 5-(4-Methoxyphenyl)-1,3,4-oxadiazole-2-ethoxycarbonylsulfanyl (4)
Compound 3 (2.08 g, 0.01 mol) in an excess of ethyl chloroformate (3 mL) was heated under reflux for 5 h. After cooling the residue of ethyl chloroformate was removed under reduced pressure and the solid residue obtained was recrystallized from ethanol. Yield (79%); mp: 124–125 °C; FT-IR (KBr disk, cm−1) 2980, 2937, 2841 (C—H aliph.), 1773 (C⚌O), 1626 (C⚌N), 1259, 1078 (C—O—C); 1H NMR (DMSO-d6, 300 MHz, δ) 7.88–7.85 (dd, 2H, Ar.H), 7.17–7.14 (dd, 2H, Ar.H), 4.48–4.41 (q, 2H, ), 3.86 (s, 3H, OCH3), 1.38–1.33 (t, 3H, CH3). Anal. Calc. for C12H12N2O4S (280 g/mol): C, 51.43; H, 4.29; N, 10.00. Found: C, 51.44; H, 4.32; N, 10.03%.
2.3.5 5-(4-Methoxyphenyl)-1,3,4-oxadiazole-2-hydrazinecarbonylsulfanyl (5)
This compound was prepared by the same method described for compound 2. Yield (87%); mp: 222–224 °C; FT-IR (KBr disk, cm−1) 3316, 3146 (N—H), 2935, 2920, 2851 (C—H aliph.) 1640 (C⚌O), 1610 (C⚌N), 1255, 1074 (C—O—C); 1H NMR (DMSO-d6, 300 MHz, δ) 13.85–13.75 (s, br. 1H, NH), 7.95–7.92 (dd, 2H, Ar.H), 7.05–7.02 (dd, 2H, Ar.H), 5.72 (s, 2H, NH2), 3.78 (s, 3H, OCH3). Anal. Calc. for C10H10N4O3S (266 g/mol): C, 45.11; H, 3.76; N, 21.05. Found: C, 45.14; H, 3.77; N, 21.08%.
2.3.6 4-(4-Methoxybenzenesulfonyl)-hippuric acid (6)
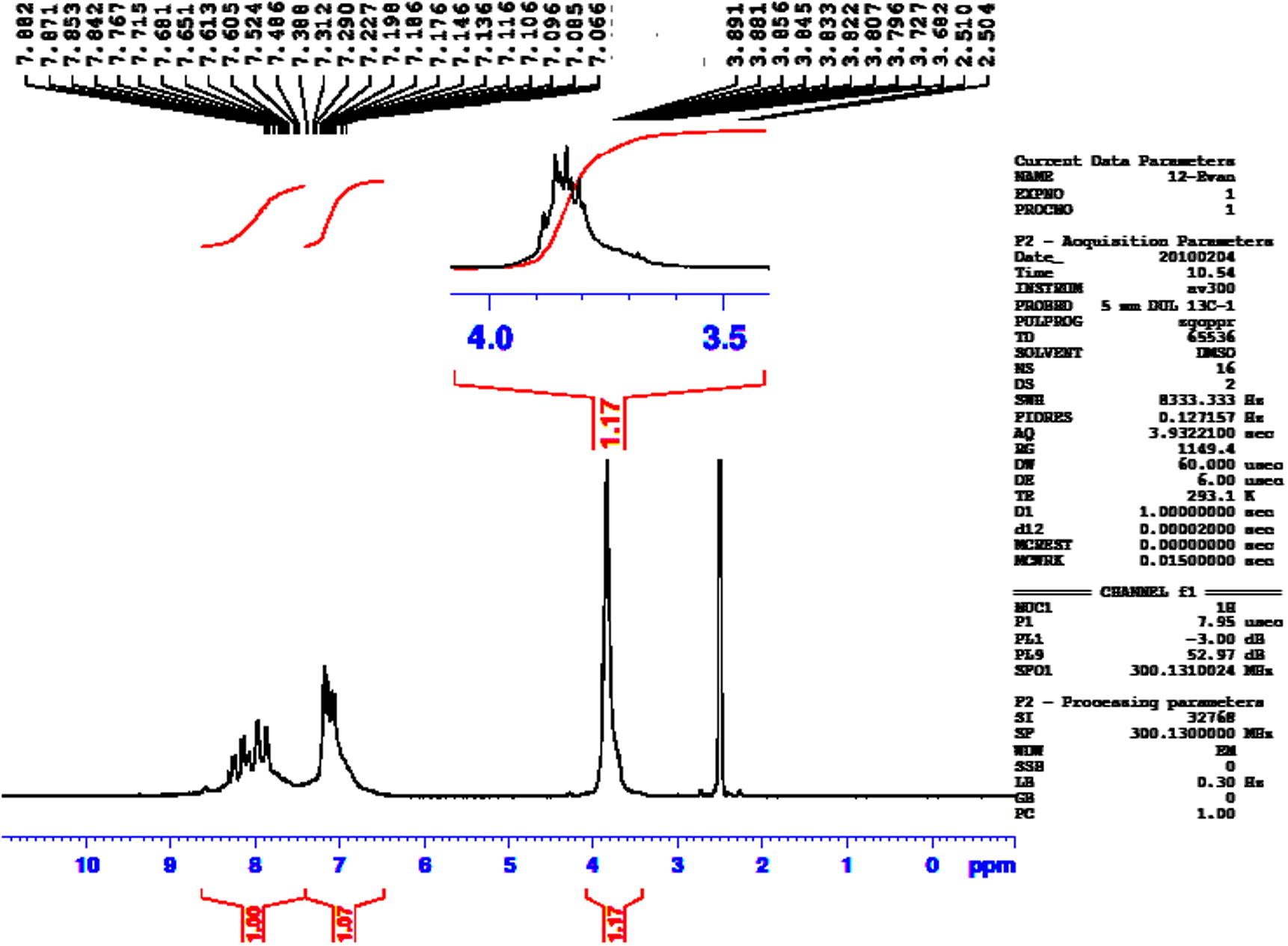
Glysine (1.5 g, 0.02 mol) in 1 N sodium hydroxide solution (20 mL) was cooled at 0–5 °C and the cold solution was added dropwise to a solution of 4-methoxybenzoyl chloride (3.41 g, 0.02 mol) in chloroform (30 mL). The reaction mixture was continued under stirring for an additional 1 h. The aqueous layer was separated and acidified with 2 N hydrochloric acid. The product 6 was collected by filtration and recrystallized from ethanol as colorless needles. Yield (83%); mp: 178–180 °C; FT-IR (KBr disk, cm−1) 3366 (N—H), 3190–2534 (O—H, carboxylic), 2933, 2847 (C—H aliph.) 1743 (C⚌O, acid), 1625 (C⚌O, amide); 1H NMR (DMSO-d6, 300 MHz, δ) 8.65–8.63 (t, 1H, NH), 7.86–7.79 (dd, 2H, Ar.H), 6.98–6.95 (dd, 2H, Ar.H), 3.86–3.84 (d, 2H, CH2), 3.77 (s, 3H, OCH3). Anal. Calc. for C10H11N1O4 (209 g/mol): C, 57.41; H, 5.26; N, 6.69. Found: C, 57.44; H, 5.23; N, 6.72%.
2.3.7 N-{5-[5-(4-Methoxyphenyl)-1,3,4-oxadiazole-2-yl-sulfanyl]-1,3,4-oxadiazole-2-yl-methyl}-4-methoxybenzamide (7)
Compound 5 (2.66 g, 0.01 mol) and Compound 6 (2.09 g, 0.01 mol) were refluxed with phosphorous oxychloride (5 mL) for 24 h. and the reaction mixture was then treated with ice water carefully and made basic by adding concentrated sodium bicarbonate solution. The resulting solid was filtered, dried and recrystallized by ethanol–DMSO (10/1). Yield (69%); mp: 193–195 °C; FT-IR (KBr disk, cm−1) 3362 (N—H), 2920, 2839 (C—H aliph.), 1620 (C⚌O, amide), 1610 (C⚌N), 1255, 1026 (C—O—C); 1H NMR (DMSO-d6, 300 MHz, δ) 8.29–8.22 (t, 1H, NH), 8.17–8.11 (dd, 2H, Ar.H), 7.88–7.81 (dd, 2H, Ar.H), 7.23–7.18 (dd, 2H, Ar.H), 7.09–7.02 (dd, 2H, Ar.H) 3.87 (s, 3H, OCH3 attached to phenyl-oxadiazole), 3.81 (s, 3H, OCH3 attached to benzamide), 3.79–3.82 (d, 2H, CH2). Anal. Calc. for C20H17N5O5S (439 g/mol): C, 54.67; H, 3.87; N, 15.94. Found: C, 54.71; H, 3.89; N, 15.98%.
3 Result and discussion
3.1 Synthesis
The characterization data of all compounds 1–7 are given in the experimental section. All the newly synthesized compounds gave satisfactory analysis for the proposed structures, which were confirmed on the basis of their elemental analysis, FT-IR and 1H NMR data. Our synthetic strategy is show in Scheme 1.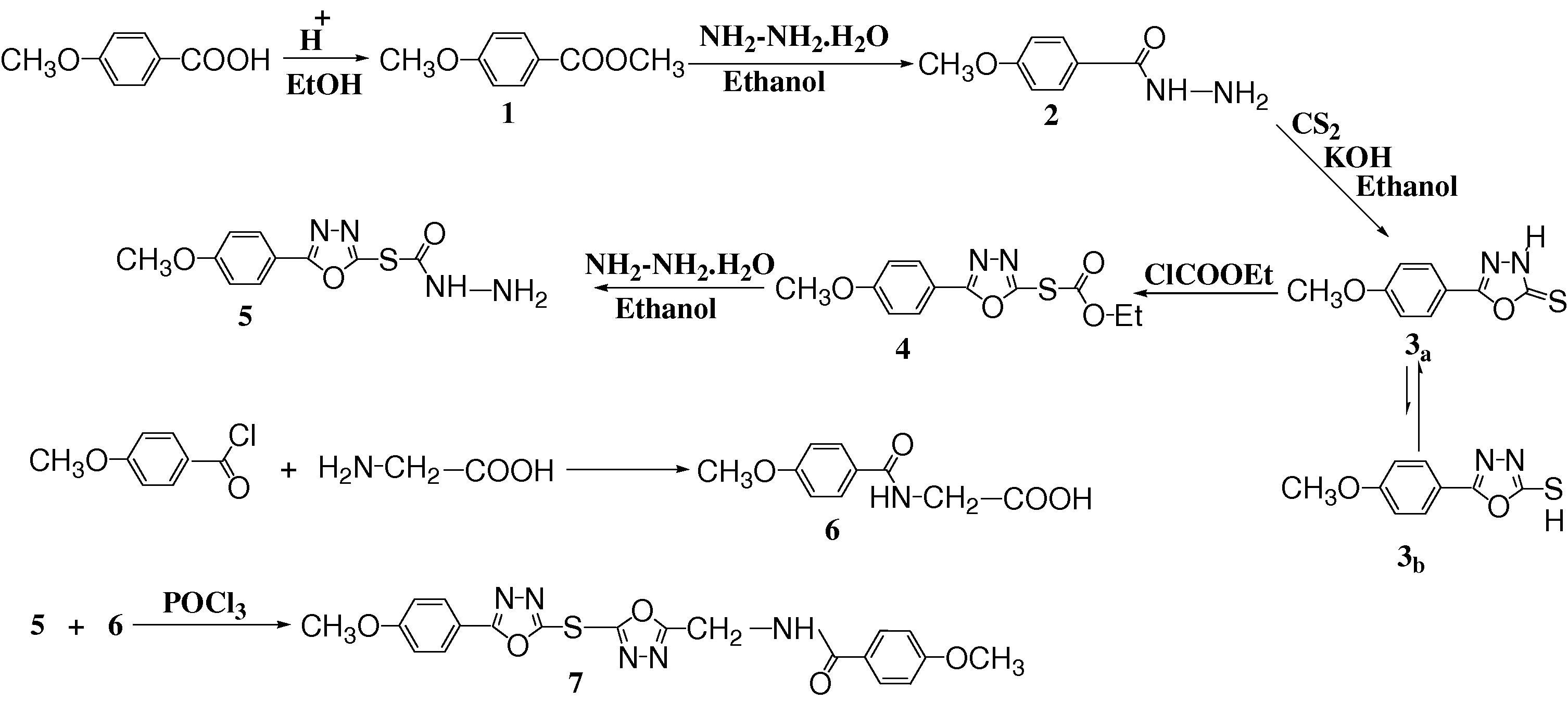
Synthesis of compounds (1–7).
The first step was begun to convert 4-methoxybenzoic acid to 4-methoxymethyl benzoate 1 by the standard esterification method (Brian et al., 1989). 4-Methoxybenzoyl hydrazine 2 was synthesized in good yield from the reaction of ester 1 with excess of hydrazine hydrate in ethanol. Compound 3 was synthesized by the ring closure reaction of acid hydrazide 2 with carbon disulfide in ethanolic KOH.
The tautomeric equilibrium in compound 3 (3a and 3b) was investigated both for isolated and for solvated species considering the solution medium. Tsoleridis et al., 1997 studied the same equilibrium and he found that the thiol form (3b) is more stable in the gas phase. However, this is contrary to the known experimental finding. On the basis of FT-IR and 1H NMR data, it is know that 5-(4-methoxyphenyl)-1,3,4-oxadiazole-2-thione (3a) exist in solution in the thione rather than the thiol form (Horning and Muchowski, 1972). Parallel to experimental data, the thione tautomer is more stable than thiol in the solution. The equilibrium is even more favored to wards the thione because the thione is better solvated than thiol. In the 1H NMR spectrum of compound 3 (Fig. 1), the broad peak at δ 14.38–14.78 of the NH proton was recorded, although it was very weak. It has been reported that the crystal structure of compound 3 correspond to the thione form (Ozturk et al., 2004), but the reaction conditions for the synthesis of compound 4 prove that compound 3 can be in the thiol form too. Finally, the crystal structure of compound 3 corresponded to the thione form, but they showed thiol–thione tautomerism in solution.
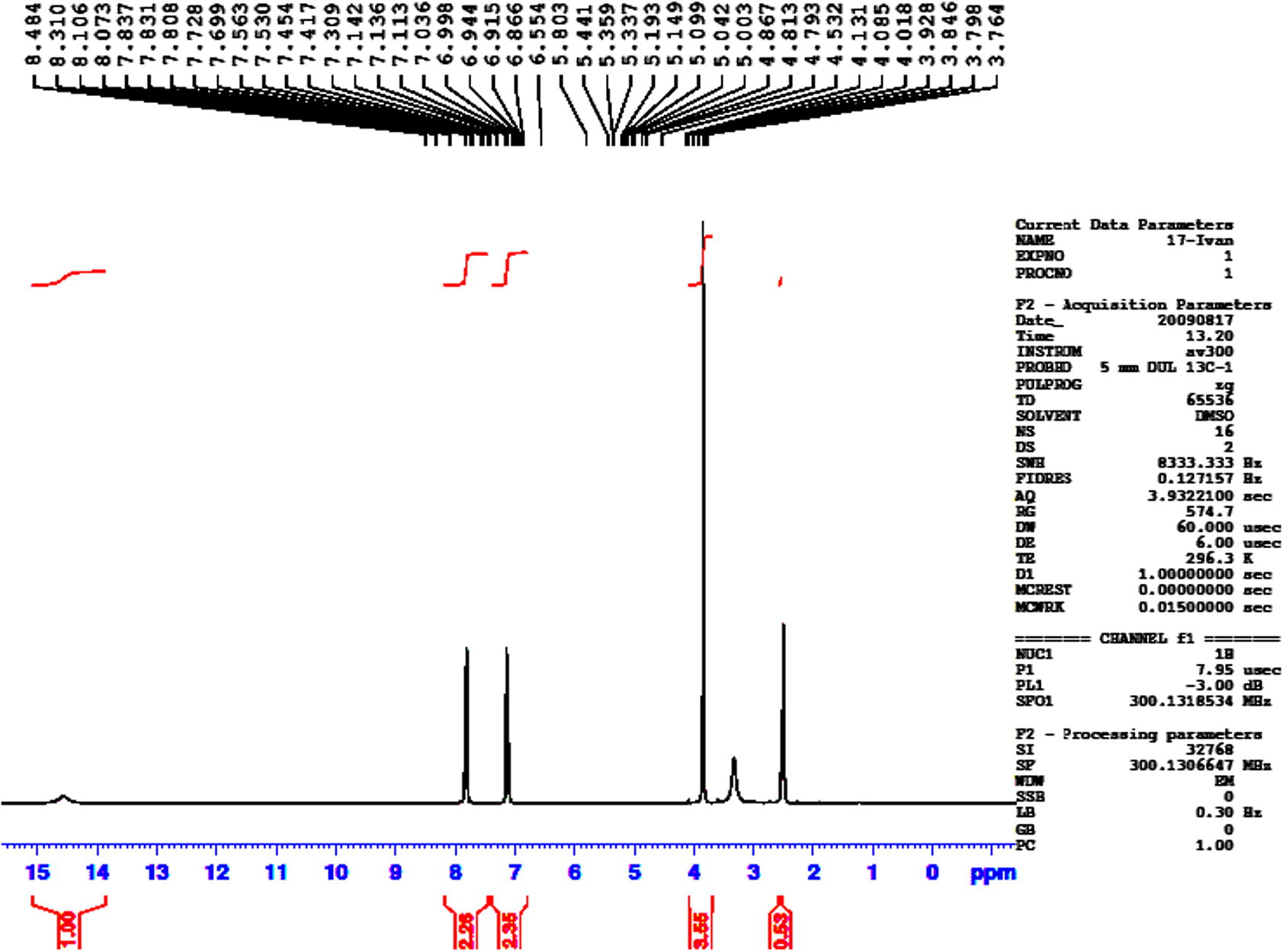
1H NMR spectrum of compound 3.
An attempt to prepare the thio ester of oxadiazole ring 4 by treatment of compound 3 with an excess of ethyl chloroformate was successful and the product obtained was identified by elemental analysis, FT-IR and 1H NMR, Fig. 2 shows the 1H NMR spectrum of compound 4. The hydrazide of oxadiazole ring 5 was prepared in good yield by the reaction of compound 4 with excess of 80% hydrazine hydrate in ethanol. The peaks at δ 5.75 and 13.75 in 1H NMR spectrum (Fig. 3) are the good evidence to formation oxadiazoles hydrazide 5.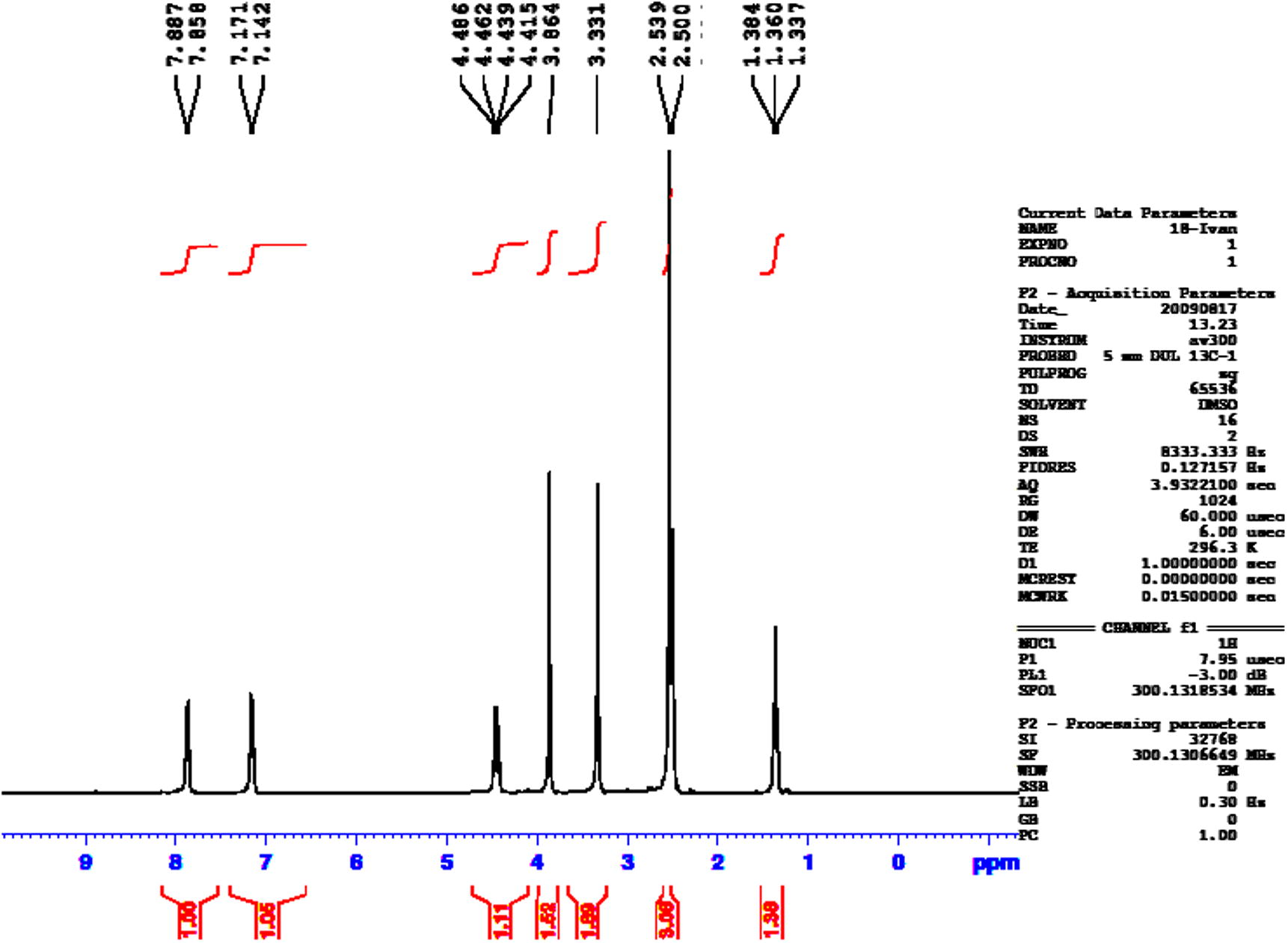
1H NMR spectrum of compound 4.
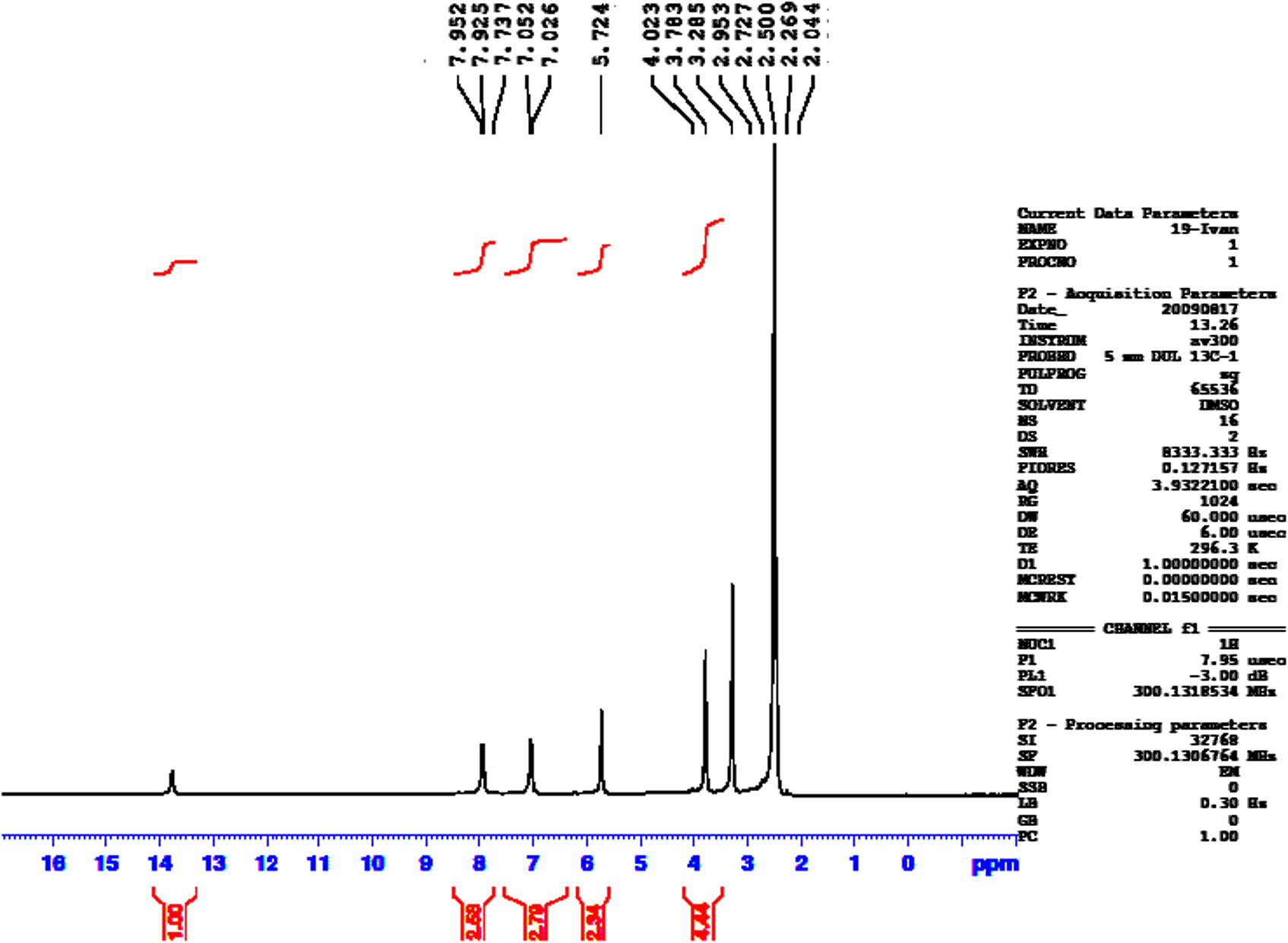
1H NMR spectrum of compound 5.
The compound 6 was synthesized by the reaction of 4-methoxybenzoyl chloride with glycine according to Steiger’s (1944) procedure to afford the corresponding hippuric acid 6. The 1H NMR spectrum of compound 6 was shown in Fig. 4. The hydrazide of oxadiazole ring 5 and compound 6 could be smoothly cyclicdehydrated by boiling in phosphorus oxychloride affording the bis oxadiazole 7 in good yield. The 1H NMR spectra of this compound was shown in Fig. 5. The mechanism of dehydration in presence of POCl3 is depicted in the following steps: (Scheme 2).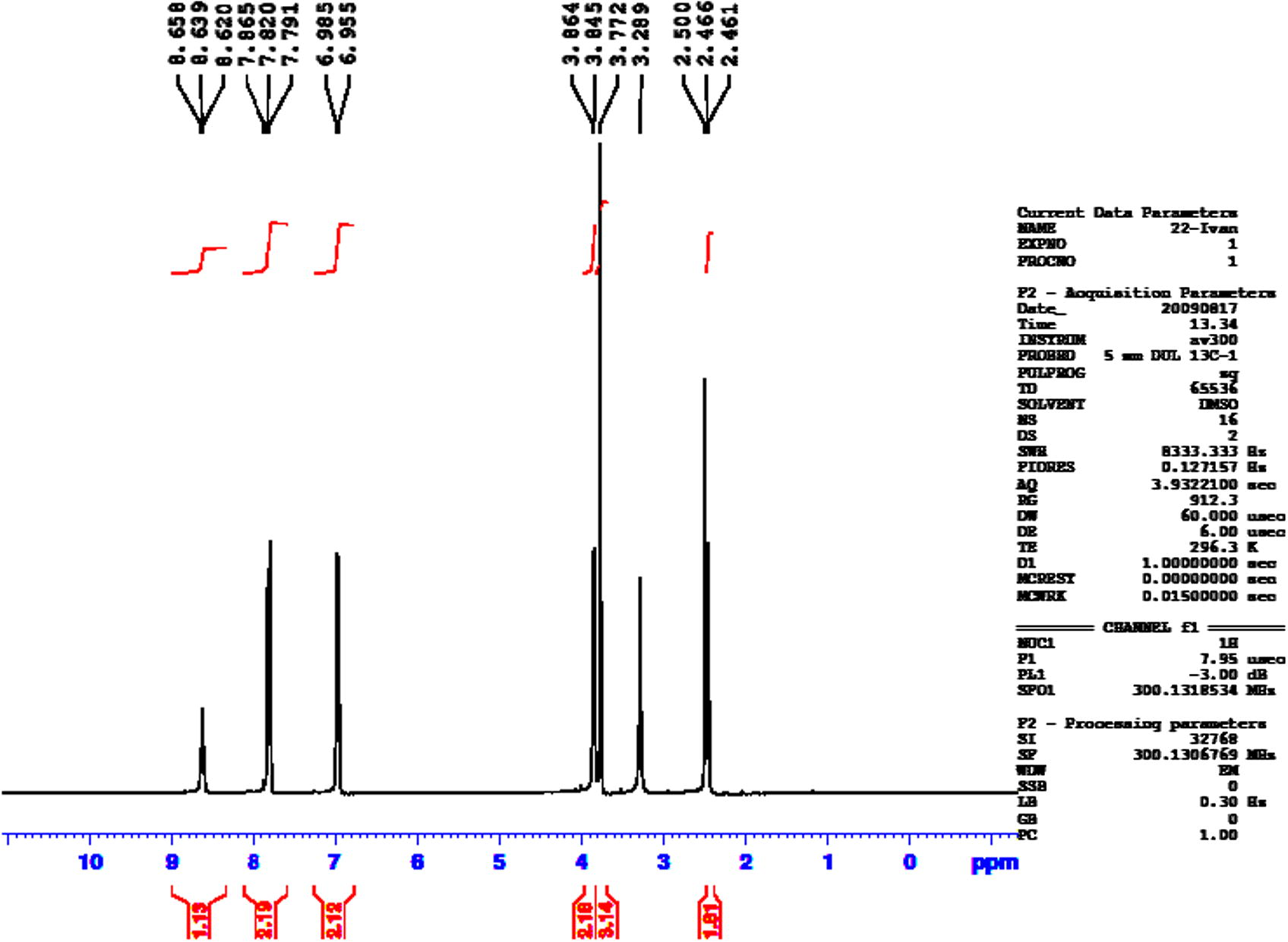
1H NMR spectrum of compound 6.
1H NMR spectrum of compound 7.
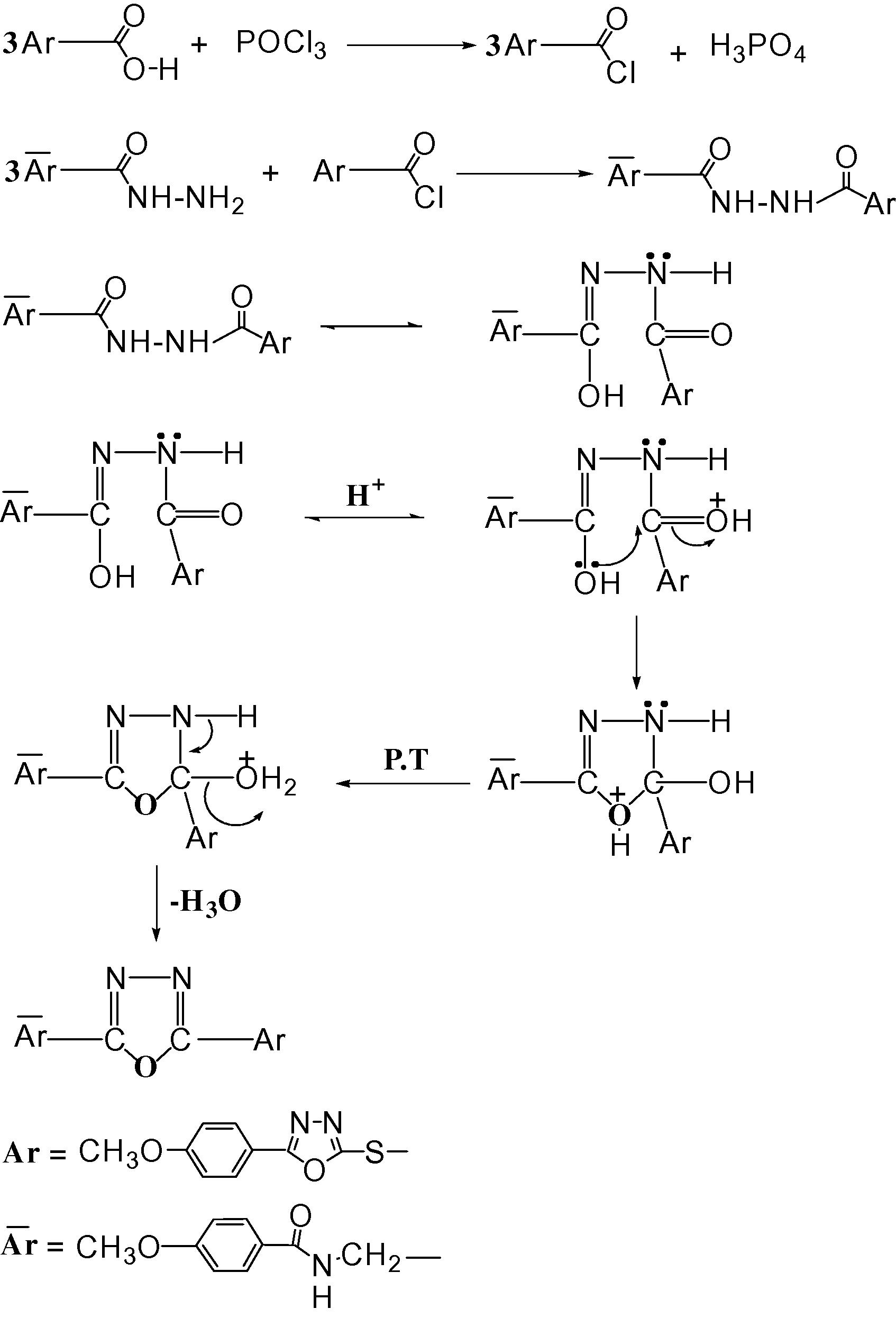
The mechanism steps of formation of compound 7.
3.2 Biological activity of transferase enzymes (GOT, GPT and GGT)
Molecular structure of compound (7) has: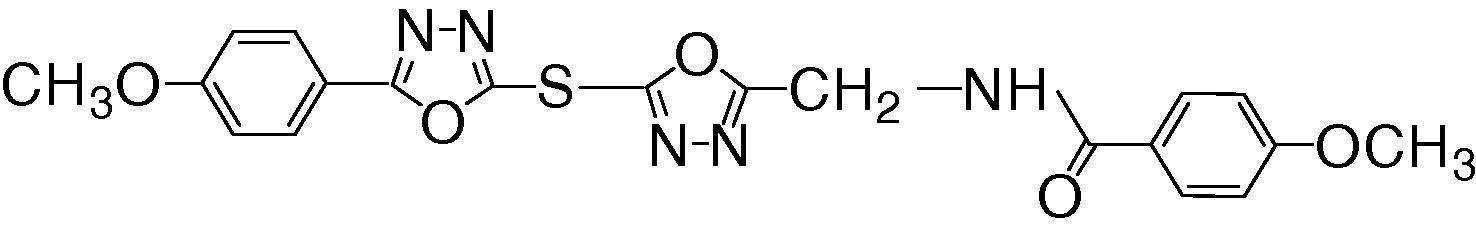
This research addresses investigation of the effects of compound (7) of GOT, GPT and γ-GT enzymes. The biochemical tests revealed that this compound caused inhibitory effects on γ-GT enzyme activity and activatory effects on GOT and GPT enzymes activities. Table 1 shows the effect of different concentrations of compound (7) on the activity of GOT, GPT and γ-GT enzymes in human serum.
No.
Concentration (M)
GOT activity (U/L)
Activation (%)
GPT activity (U/L)
Activation (%)
γ-GT activity (U/L)
Inhibition (%)
1
0
17
0.000
16
0.000
6.545
0.000
2
10−2
79
364.705
64
300.000
1.303
0.000
3
10−3
30
76.470
20
25.000
2.618
20.000
4
10−4
29
70.580
18
12.500
5.236
60.000
5
10−5
24
41.176
17
6.250
6.545
80.000
The normal value of the GOT and GPT enzyme activities were (17 and 16 U/L) respectively. The relationship between compound (7) concentrations versus and the activity of enzymes were shown in Fig. 6. These results observed that any increase in compound concentrations caused increase in percentage of activation of enzymes. The greater activation of compound (7) was demonstrated at concentration 10−2 M (364.705%) for GOT and 10−2 M (300%) for GPT as shown in Fig. 7.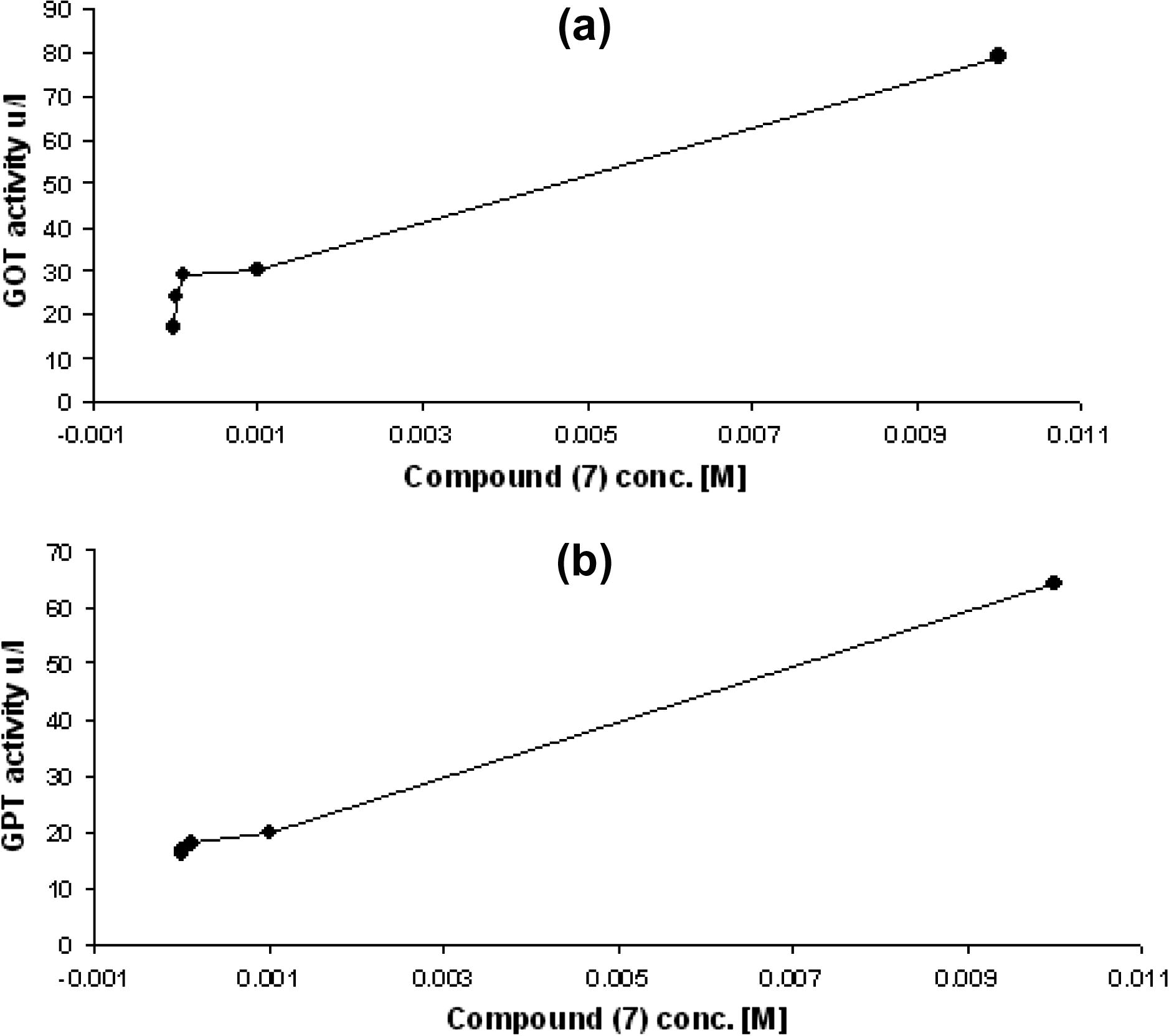
(a) The relationship between concentration of compound 7 and GOT enzyme activity. (b) The relationship between concentration of compound 7 and GPT enzyme activity.
(a) The percentage of activation GOT enzyme and compound 7 concentration. (b) The percentage of activation GPT enzyme and compound 7 concentration.
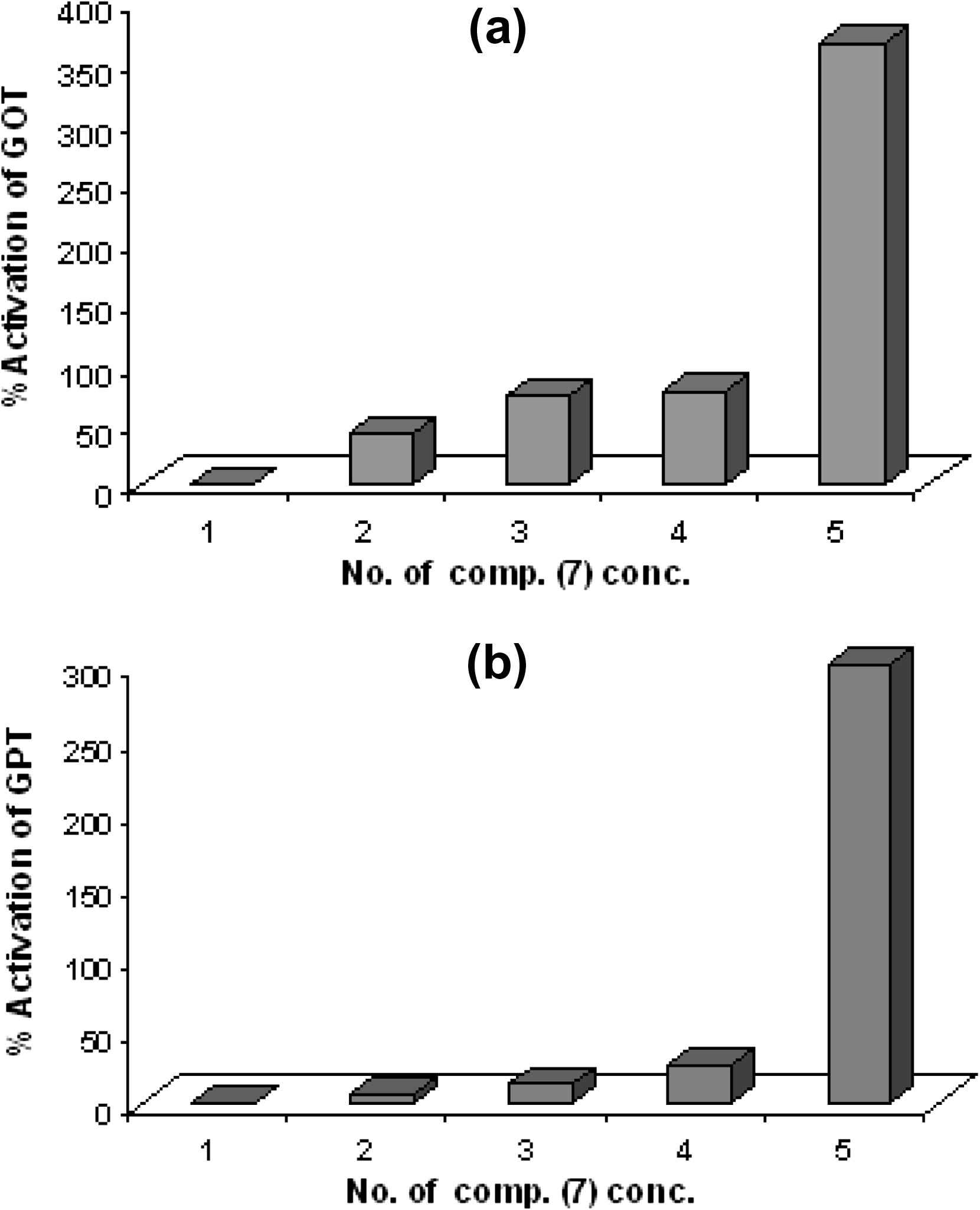
The normal value of the γ-GT enzyme activity was (6.545 U/L). The relationship between compound (7) concentration versus and the activity of enzyme was shown in Fig. 8(a). These results observed that any increase in compound concentrations caused increase in percentage of inhibition of enzyme. The greater inhibition of compound (7) was demonstrated at concentration 10−5 M (55%) as shown in Fig. 8(b).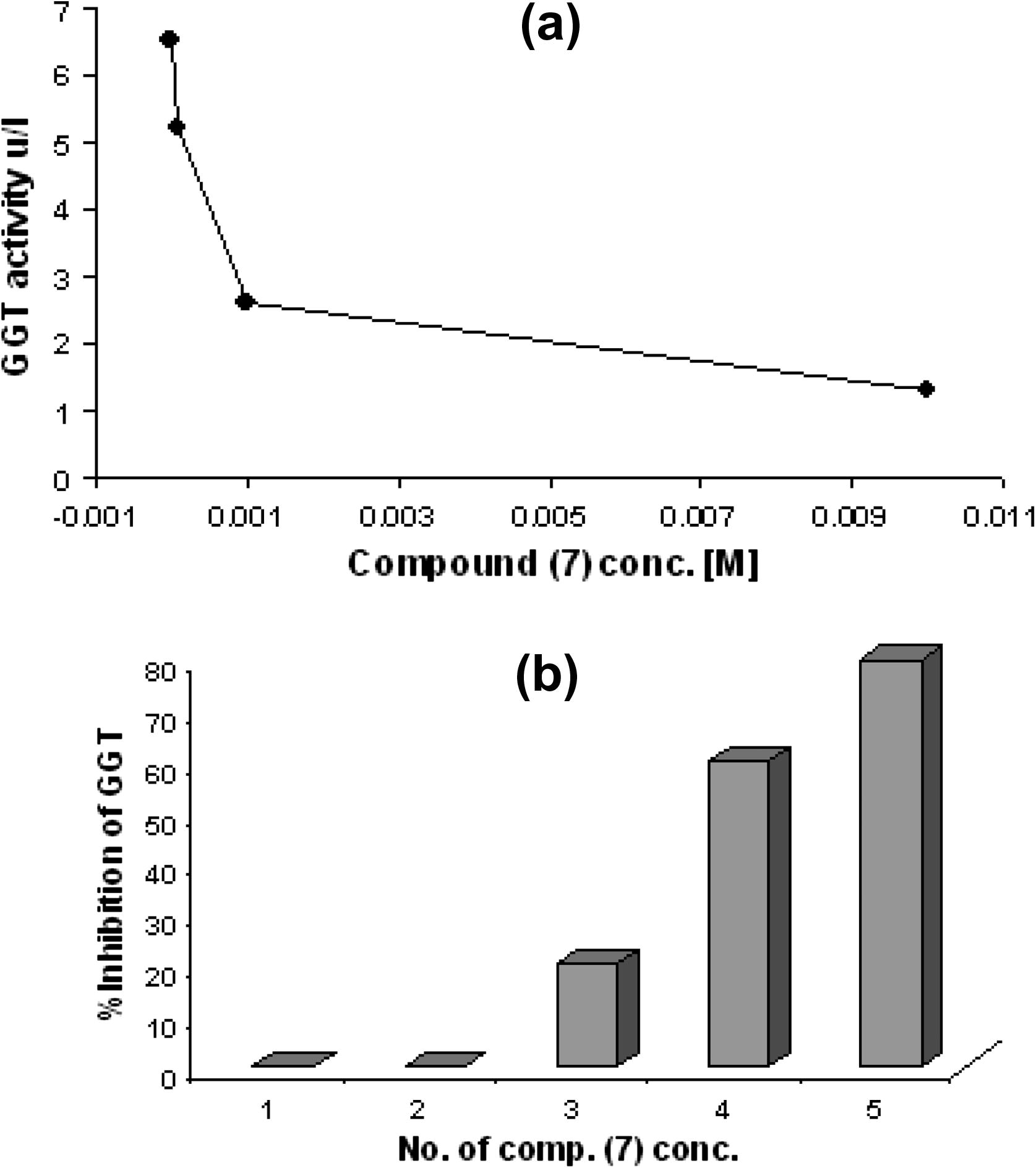
(a) The relationship between concentration of compound 7 and GGT enzyme activity. (b) The percentage of activation GGT enzyme and compound 7 concentration.
Competitive, noncompetitive and uncompetitive inhibition can be easily distinguished with the use of double reciprocal plot of the Lineweaver–Burk plot. Two sets of rate determination in which enzyme concentration was held constant, were carried out. In the first experiment the velocity of uninhibited enzyme was established, in the second experimental constant amount of inhibitor is included in each enzyme assay. Varieties of substances have the ability to reduce or eliminate the catalytic activity of specific enzyme (Satyanarayna, 2003).
Table 2 and Fig. 9(a) showed that the type of enzyme activation using Lineweaver–Burk plot for compound (7) on serum GOT activity. The Vmax and Km values determined with 10−2 M of compound (7) and without it. Vmax and Km values without compound (7) were 67 U/L, 200 M respectively. A liquate 10−2 M of compound (7) was noncompetitive activation for enzyme activity. Noncompetitive activation changed the Vmax of the enzyme but not the Km. When concentration of compound was 10−2 M, the Vmax was 50 M. By using Lineweaver–Burk equation, the Ki values of enzyme for compound which was studied in different concentrations. The Ki of compound (7) in 10−2 M was 0.0129 M.
Enzymes
Kmapp (M)
Vmapp (U/L)
Ki (M)
Type of effect
GOT
100
50
0.0129
Noncompetitive
GPT
400
50
0.02
Noncompetitive
γ-GT
1
0.256
0.000263
Noncompetitive
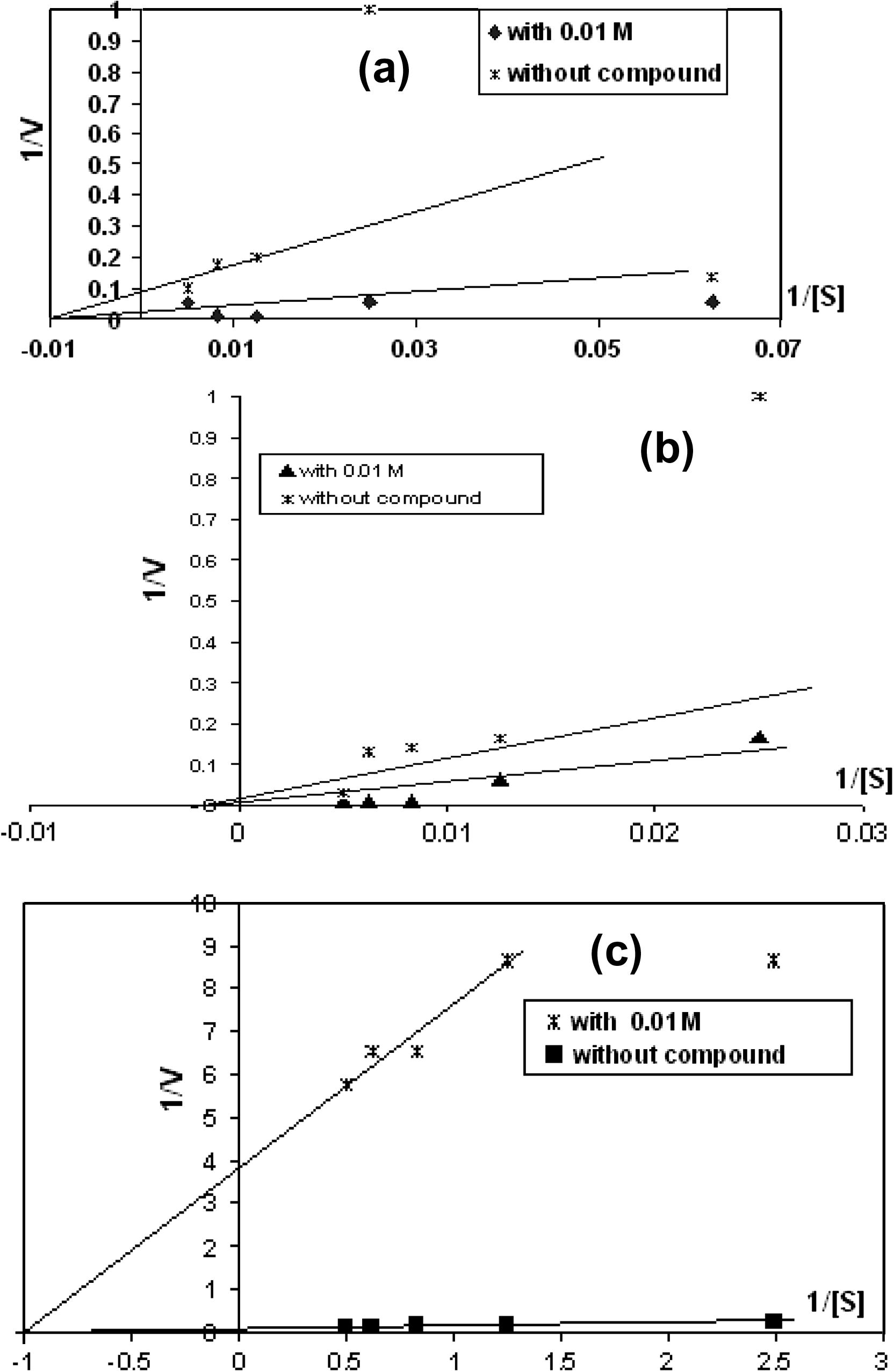
Lineweaver–Burk plots for compound 7 effects on (a) GOT, (b) GPT and (c) GGT.
Table 2 and Fig. 9(b) show that the type of enzyme activation using Linweaver and Burke (1934) plot for compound (7) on serum GPT activity. The Vmax and Km values determined with 10−2 M of compound (7) and without it. Vmax and Km values without compound (7) were 100 U/L, 400 M respectively. A liquate 10−2 M of compound (7) was noncompetitive activation for enzyme activity. Noncompetitive activation changed the Vmax of the enzyme but not the Km. When concentration of compound was 10−2 M, the Vmax was 50 M. By using Lineweaver–Burk equation, the Ki values of enzyme for compound which was studied in different concentrations. The Ki of compound (7) in 10−2 M was 0.02 M.
The enzymes play important role in amino acid metabolism and in urea and tricarboxylic acid cycles. We suggested that compound (7) molecule has (N– and O⚌) groups by which, it activities the active sides of amino acids of GOT and GPT enzymes by increasing affinity of active sides of enzymes to react with the substrates.
Table 2 and Fig. 9(c) show that the type of enzyme inhibitor using Lineweaver–Burk plot for compound (7) on serum γ-GT activity. The Vmax and Km values determined with 10−2 M of compound (7) and without it. Vmax and Km values without compound (7) were 37.037 U/L, 0.4 M respectively. A liquate 10−2 M of compound (7) was noncompetitive inhibition for enzyme activity. Competitive inhibition changed the Km of the enzyme but not the Vmax. When concentration of compound was 10−2 M, the Km was 1 M. By using Lineweaver–Burk equation, the Ki values of enzyme for compound which was studied in different concentrations. The Ki of compound (7) in 10−2 M was 0.000263 M. Molecule of compound (7) interaction between the groups (N– and O⚌) with active sides of amino acids of γ-GT enzyme.
4 Conclusion
Bis-1,3,4-oxadiazole compound containing glycine moiety was prepared and structurally characterized using spectroscopic techniques. The synthetic route started from esterification of anisic acid followed by reaction the ester with hydrazine hydrate. The acid hydrazide was converted to thione–thiol oxadiazole tautomer (3) by ring closure mechanism. The thio ester of oxadiazole (4) was prepared by the reaction of tautomer (3) with ethyl chloroformate. The acid hydrazide of oxadiazole (5) was synthesized by the same conditions for prepared compound (2). The compound (7) was prepared by dehydration mechanism in presence of POCl3. The biochemical studies revealed that the bis-oxadiazole caused activatory effects on GOT and GPT enzymes activities, and inhibitory effects on γ-GT enzyme activity.
Acknowledgment
We thank Mr. Mohanad H.M. Masad (Al Al-Bayt University, Jordan) for helpful about doing the elemental analysis (CHN) and 1H NMR spectra, and Mrs. Zainab K. Mohammed Jawad (Al-Mustansiriya University, Chem. Dept.) about doing the FT-IR spectra. Also we are very grateful to Dr. Selma A. Abbass (Al-Mustansiriya University, Chem. Dept.) for performing the biological experiments.
References
- A convenient synthesis of alkyl and aryl substituted bis-1,3,4-oxadiazoles. Synth. Commun.. 1990;20:1811.
- [Google Scholar]
- Vogel’s: Practical Organic Chemistry (fifth ed.). New York: Longman Scientific Technical; 1989. p. 1077
- The chemistry of pseudomonic acid. 18. Heterocyclic replacement of the α,β-unsaturated ester: synthesis, molecular modelling, and antibacterial activity. J. Med. Chem.. 1997;40:2563.
- [Google Scholar]
- Synthesis of unsymmetrically substituted 4H-1,2,4-triazoles. J. Heterocyclic Chem.. 1994;31:805.
- [Google Scholar]
- The effect of paraquat on the activity of some enzymes in different tissues of mice (mus musculus–swiss albino) Turk. J. Biol.. 2001;25:323.
- [Google Scholar]
- Synthesis and electronic structure of new aryl- and alkyl-substituted 1,3,4-oxadiazole-2-thione derivatives. Turk. J. Chem.. 2002;26:159.
- [Google Scholar]
- Georg Thieme Verlag, Stuttgart, 2006. Science of Synthesis, vol. 13, p. 219.
- Synthesis and pharmacological evaluation of novel series of sulfonate-ester containing 1,3,4-oxadiazole derivatives with anticipated hypoglycimic activity. Drug Res.. 1994;44:490.
- [Google Scholar]
- Hill, J., 1984. In: Katritzky, A.R., Ress, C.W. (Eds.), 1,3,4-Oxadiazoles in Comprehensive Heterocyclic Chemistry, vol. 6. Pergamon press, Oxford, New York, Toronto, Sydney, Paris and Frankfurt, p. 427.
- Hodogaya, 1980. Chemical Co., Ltd., Japan. Tokkyo Koho, 8027.042, Chem. Abstr. 93, 232718g.
- Five-membered heterocyclic thiones. Part I. 1,3,4-Oxadiazole-2-thione. Can. J. Chem.. 1972;50:3079.
- [Google Scholar]
- Jai, F.W., Ghassan, E.J., Eugene, A.M., Jeff, A., Yadong, Z., Paul, A.L., Neal, R.A., Nasser, P., Bernard, K., 1999. Oxadiazole metal complex for organic light-emitting diodes. Adv. Mater. 11, 15, 1266.
- Katritzky, A.R., Charles, W.R., Scriven, E.F.V., 1995–2007. Comprehensive Heterocyclic Chemistry, vol. 5. CHEC III, p. 397; Kartitzky, A.R., Taylor, R.J.K., Ramsden, C.A., Scriven, E.F.V., 1996. Comprehensive Heterocyclic Chemistry, vol. 4. CHEC II, p. 267.
- Reduction potential and herbicidal activity of 4,4-(1,3,4-thiadiazole-2,5-diyl)-and4,4-(1,3,4-oxadiazole2,5diyl)bis(1-methylpyridinium)diiodides. J. Heterocyclic Chem.. 1981;XIV:409.
- [Google Scholar]
- The determination of enzyme dissociation constants. J. Am. Chem. Soc.. 1934;56:658.
- [Google Scholar]
- A mild method for the preparation of 1,3,4-oxadiazoles: triflic anhydride promoted cyclization of diacylhydrazines. Synth. Commun.. 2000;30:437.
- [Google Scholar]
- Maillard, J., Vincent, M., Morin, R., Benard, M., 1962. Hypnotic and sedative drug, 2-(o-hydroxyphenyl)-1,3,4-oxadizole. French Patent M 379, Chem. Abstr. 57, 15251.
- Antitumor and antimicrobial activities of Fe(II)/Fe(III) complexes derived from some heterocyclic compounds. Bioorg. Med. Chem. 1995;3(9):1241.
- [Google Scholar]
- New 1,2,4- and 1,3,4-oxadiazole materials: synthesis, mesomorphic and luminescence properties. Liq. Cryst.. 2006;33(8):875.
- [Google Scholar]
- A new method for the determination of gamma-glutamyl transferase. J. Chem. Clin. Biochem.. 1976;4:421.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Path.. 1957;28:56.
- [Google Scholar]
- Satyanarayna, U., 2003. Biochemistry, second ed. Books and Allied (P) Ltd., India, p. 91.
- Sebastiao, J.M., Antonio, D.S., Heron, L.L., Srivastava, R.M., 1998. Synthesis of some 3-aryl-1,2,4-oxadiazoles carrying a protected l-alanine side chain. J. Braz. Chem. Soc. 19, 5, 465.
- Organic Reactions. 1946;3:366.
- 5-Substituted-1,3,4-oxadiazoles and related compounds as possible fungicides. J. Indian Chem. Soc.. 1979;56:712.
- [Google Scholar]
- In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta. Pharm.. 2005;58:135.
- [Google Scholar]
- Synthesis and spectral data of 4,5-bis[5-aryl-1,3,4-oxadiazole-2-yl]-1-benzyl-1,2,3-triazole. J. Heterocyclic Chem.. 1990;27:1685.
- [Google Scholar]
- UV and MO study on the deprotonation of some 2-aryl-Δ2-1,3,4-oxadiazole-5-thiones. J. Heterocyclic Chem.. 1997;34:1715.
- [Google Scholar]
- Synthesis, anticonvulsant and muscle relaxant activities of substituted 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole. Acta Chim. Slov.. 2007;54:317.
- [Google Scholar]
- Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole. J. Agric. Food Chem.. 2002;50:3757.
- [Google Scholar]







