Translate this page into:
Synthesis and characterization of eco-friendly TiO2 nanoparticle from combine extract of onion and garlic peel
⁎Corresponding author at: Department of Chemistry, Maulana Azad National Institute of Technology, Bhopal, India. humali.manit@yahoo.com (Huma Ali)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plant extracts are increasingly being used to make green nanoparticles since they are harmless and environmentally friendly. The process provides a cheap technique to create nanoparticles. TiO2 nanoparticles were created in this work using titanium isopropoxide and a combination of onion and garlic peel extracts. X-ray diffraction (XRD), scanning electron microscope-energy dispersive X-ray spectroscopy (SEM-EDS), ultraviolet–visible spectroscopy (UV–Vis), and Fourier transform infrared (FTIR) spectroscopy were used to analyze the green-generated TiO2 nanoparticles containing the extract. The sharp and potent peaks at 2 values of 27.45°, 37.97°, 44.07°, 54.40°, and 69.85° have been used to confirm the configuration of the crystalline nanoparticles (NPs) of TiO2. With the exception of the inescapable adsorption of moisture on the surface in the open air, calcined TiO2 NPs exhibit FT-IR absorption bands that show the purity of the produced nanoparticles. The bactericidal activity of green TiO2 NPs and unprocessed extracts of onion and garlic peels were examined using the disc diffusion method. In comparison to a crude mixed extract, NP is more efficient against Gram-positive and Gram-negative bacteria because to its smaller average crystalline size and consistent shape, which are visible in SEM pictures.
Keywords
Synthesis
Nanoparticles
Antimicrobial activity
Onion and Garlic peel
1 Introduction
Nanotechnology is described by Professor Norio Taniguchi of Tokyo Science University as “the splitting and bending of matter by one atom or molecule.” He claims it is a scientific idea that focuses on changing things at the atomic or molecular level. The study of nanotechnology is a brand-new area that has emerged in the twenty-first century. The creation, processing, and application of materials with a size of less than 100 nm are the main topics of this interdisciplinary study. It now focuses on cell-level matter control and has spread into other domains (Marimuthu et al., 2013). Surface-enhanced Raman scattering (SERS), quantum dots, applied microbiology, and nanobiotechnology are some examples of cutting-edge components and applications in nanotechnology. Examples of nanotechnology include the use of nanoscale structures in numerous technologies, the electrical sector, and photoelectrochemical applications (Singh et al., 2016) Because of their extremely small size (in nm) and high surface-to-volume ratio, which significantly affect physical and chemical changes in their properties, nanoparticles are of immense significance. Even though there are many useful uses for nanoparticle materials, many of them have shown toxicity at the nanoscale level. Green chemistry and nanotechnology are used to make environmentally beneficial nanoparticles from plants, microbes, and other natural resources (Lateef et al., 2016). Through “green chemistry” methods including bacteria, fungus, and plants, numerous synthetic ways have been created to produce nanoparticles, and they have demonstrated to have considerable advantages for the environment and nature (Duan et al., 2015). Numerous research have used Bacillus subtilis and other microbes to produce metal nanoparticles. These microorganisms include Penicillium sp. and Fusarium oxysporum. The most common and environmentally benign method is that of using plant extracts to create various nanoparticles, therefore this is the main topic of discussion. Scientists and researchers are interested in this subject because plants are accessible and plentiful. The fact that it is safe to use and can be a source of a number of metabolites are additional factors that increase its worth (Sundaram et al., 2012, Du et al., 2011).
The creation of green nanoparticles has attracted a lot of attention in nanotechnology studies. This innovative approach aims to regulate, control, clean up, and remediate these substantial particles to make them more environmentally friendly. By reducing the harmful byproducts produced during the production of conventional nanoparticles, less dangerous and unsustainable commodities will be produced. A greener and more sustainable economy will be built using natural resources, including biological systems, but acceptance of these alternative methods depends on corporate support.
For antibacterial and antimalarial activities, respectively, TiO2 nanoparticles (NPs) were produced utilising the leaf extracts of Artemisia vulgaris and aerial portions of Callistemon citrinus (Rasheed et al., 2017, Larayetan et al., 2019). TiO2 nanoparticles were more useful in the study of chemistry and nanomedicine because of their distinctive chemical characteristics and antibacterial activities. TiO2 NPs are utilised in cosmetic products, and lotions and ointments containing these nanoparticles are applied to the skin to delay skin ageing and avoid sunburns (Zhang et al., 2022). TiO2 NPs and other metal nanoparticles have been produced using both chemical and physical techniques. Business entities use the first most frequently. However, these technologies have disadvantages due to their high cost, hazard potential, and environmental effect (Amanulla et al., 2019, Ahmad et al., 2013).
The synthesis of novel antibacterial inorganic and organic compounds has grown quickly in recent decades due to the growth of infectious diseases that are resistant to antibiotics, which have become serious challenges in modern medicine (Chauhan et al., 2013). It is innovative to use an extract of onion and garlic peels in combination to inhibit both Gram-positive and Gram-negative human pathogenic bacterial strains.
2 Materials and methods
2.1 Preparation of aqueous combined extract of onion and garlic outer peel
The Allium sativum (garlic) and Allium cepa L. (onion) bulbs came from Bhopal, Madhya Pradesh, India's Big Bazaar. Both of their dry exterior peels were collected, thoroughly cleaned, and then given two washings before being cut into little pieces. The soft powder of each peel was mixed with 500 cc of distilled water to make the extract that would be used as a stabilising agent. Using magnetic stirring, the solution was heated to 70 °C for two hours. After that, it is kept in the refrigerator for further use.
2.2 Phytochemical characterization of combined extract of onion and garlic
The phytochemical content of the combined onion and garlic peel extract in methanol, acetone, and water was spectrophotometrically analysed. The total amounts of polyphenols present in the aforementioned generated extract were calculated in mg galic acid equivalent using the Folin-Ciocalteau reagent and the equation for the gallic acid calibration curve (Wafula et al., 2023). The total flavonoid concentration was measured using a Shimadzu UV-spectrophotometer at a wavelength of 415 nm. The flavonoid content was converted to quercetin (quercetin equivalent) using the equation for the quercetin calibration curve in mg QE/L sample.
2.3 Synthesis of TiO2 onion and garlic nano particles (OGNPs)
After being filtered via Whatman filter paper, the extract was heated before being employed as a stabilising element in the creation of nanoparticles. The green approach was used to combine 70 mL of 5 mM Titanium isopropoxide (TTIP) solution and 70 mL of extract in a 1:1 (volume/volume) ratio to produce TiO2 NPs. After that, the mixture was mixed for 8 h at room temperature. The process behind their formation was the hydrolysis of TTIP, which is the main pathway for the production of TiO2 NPs (Muniandy et al., 2017). In the mixture of solutions, the extract served as a stabilising agent, preventing agglomeration and allowing the TiO2 NPs to adopt the necessary shape and size. The mixture was agitated and centrifuged at 10,000 rpm for 10 min to separate the nanoparticles. TiO2 OGNPs were dried at 100 °C for one night and then calcined in a muffle furnace for three hours at 570 °C. As a result, TiO2 OGNPs were gathered and stored for later research Fig. 1.
. TiO2 Onion Garlic nanoparticles (TiO2 OGNPs) made synthetically.
2.4 Characterization of TiO2 OGNPs
Several analytical techniques, including UV–vis spectroscopy, scanning electron-energy dispersive X-ray spectroscopy (SEM-EDS), X-ray powder diffraction (XRD) analysis, and Fourier-transform infrared spectroscopy (FT-IR), were utilised to analyse the nanoparticles. The hydrolysis of TTIP brought on by the successive addition of mixed extracts of onion and garlic was measured using an ultraviolet spectrophotometer with a resolution of 1 nm between 350 nm and 750 nm. A SEM was used to look at the surface morphology of TiO2 OGNPs. The powdered sample was mounted on carbon tape and placed on top of the sample holder for SEM analysis. Platinum was then sputter coated onto it for 120 s in an ion coater. From an expanded SEM image, the size distribution of TiO2 OGNPs was determined by counting roughly 100 particles. Using an XRD device set to 30 kV and 40 mA with Cu Ka radians at an angle of 2 h, the crystalline structure of powdered TiO2 OGNPs was studied. Using an FT-IR spectrophotometer, the produced TiO2 OGNPs were studied in the 400–4000 cm-1 wavelength range. After combining the sample in a 1:100 ratio with potassium bromide (KBr), it was compressed to a 2 mm semi-transparent disc using a specially made screw knot. The existence of various functional groups from the onion and garlic combined extract that are involved in the production and stability of TiO2 OGNPs was then examined using various modes of vibration. The average hydrodynamic size and zeta potential of Cobalt nanoparticles at a concentration of 100 g/ml in double-distilled water were discovered by dynamic light scattering (DLS) investigation.
2.5 Biological application
Using the disc diffusion method, the antibacterial activity of the aforementioned combination of crude onion and garlic peel extract and green TiO2 OGNPs was examined. In order to do this, 50 cc of the aforementioned combined extract of onion and garlic peel were used at concentrations of 10%, 8%, 6%, and 4%, respectively. Gram positive S. aureus, S. cohnii, and Gramme negative E. coli, Proteus, and K. pneumonea were tested against the crude extract and TiO2 OGNPs. In order to assess the antibacterial activity of the crude extract and the green-produced TiO2 nanoparticles, the diameter of the inhibition zone surrounding the disc against the test organisms was measured using a ruler.
2.6 Statistical analysis
Data were evaluated using the programme Primer. P0.05 was regarded as statistically significant following the analysis of variance between the different concentrations. Mean and Standard Deviation (SD) were used to determine the results.
3 Results and discussion
The identification of the secondary metabolites necessary for the formation of nanoparticles was the main goal of the phytochemical study of numerous solvent samples of the combined extract of onion and garlic peel. Many primary and secondary metabolites in plant extracts have lowering effects. The group of major metabolites includes proteins and carbohydrates. Flavonoids, phenolic acids, tannins, vitamins, essential oils, and other secondary metabolites are some examples of reducers. The findings demonstrated that all samples of mixed onion and garlic peel extracts made with various solvents (water, methanol, ethanol, acetone) contained polyphenols. Fig. 2 shows that the overall polyphenol concentration was highest in the watery sample. In the sample made using acetone, the concentration was lowest. The outcomes imply that polar polyphenols with a polar character are present in the combined onion and garlic peel extract. Solubilizing them can be made simpler by using polar solvents like water. The polyphenols' polarity caused greater concentrations of polar groups like hydroxyl and carboxyl, as well as connections with polar mono- and disaccharides (McRae, 2007). Tannins were only observed to be present in the onion and garlic aqueous extracts. According to other researchers, the primary source of phenolic acids is aqueous extracts (Rehman et al., 2022, Hsueh et al., 2022).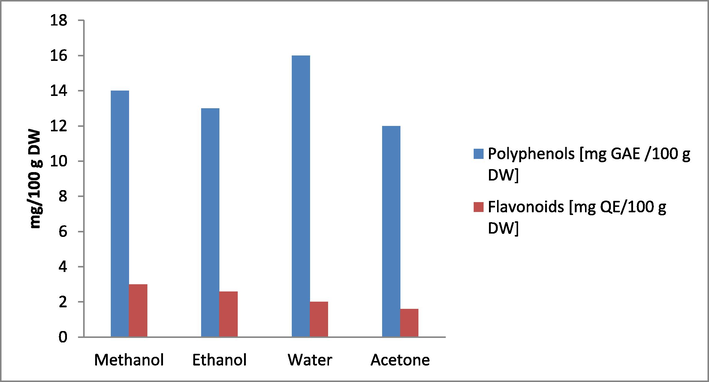
. Phytochemical analysis of different sample of crude combine extract of Onion and Garlic peel.
The secondary metabolites tannins, flavonoids, phenolic acids, anthocyanins, and coumarins are all included in the large category of secondary metabolites known as polyphenols. The content of flavonoids may have been larger than it should have been as a result. The amount of polyphenol was really ten times lower. The total flavonoid content decreased in the combined extract of onion and garlic peel in the following order: methanol, ethanol, aqueous, and acetone. This kind of result was attained as a result of the synergistic interactions between the two unique components that are combined to generate the combination extract.
There must be a synergistic effect since, according to a variety of experts, combined outcomes must be preferable to solo conclusions. Synergy is the capacity for two or more agents to collaborate and create an influence that is greater than the sum of each agent's individual effects (Ma et al., 2021). In the context of medical research, synergy might be difficult to describe. Based on the methods of action, some researchers divided the idea of synergy into two broad groups: pharmacodynamic synergy and pharmacokinetic synergy (Inui et al., 2007). The first type of synergy occurs when two or more drugs that target the same receptors or biological targets work well together to increase the efficacy of therapy. The second type of synergy results from interactions between two or more substances throughout their pharmacokinetic processes (absorption, distribution, metabolism, and elimination). These interactions change the quantities of the substances in the body and, as a result, their therapeutic effects (Ottarel et al., 2007).
UV-spectroscopy analysis: In this investigation, the production of a milky white colloidal solution served as a demonstration of the transition of titanium nitrate tetrahydrate Ti(NO3)4·4H2O into nanosized TiO2 colloidal particles. There is a discernible peak at 349 nm in the UV absorbance range of 200–600 nm, which supports the creation of green TiO2 OGNPs. The direct recombination of holes in the valence band and electrons in the conduction band was illustrated by the UV–vis peaks (Vijayalakshmi et al., 2012).
X-ray powder diffraction analysis: TiO2 OGNPs were analysed using X-ray powder diffraction to determine their crystalline phase, crystal structure, purity, and average crystal size. The XRD pattern of the green-produced TiO2 OGNPs is shown in Fig. 3. The Braggs reflection planes of (1 1 0), (0 0 4), (2 1 0), (1 0 5), and (3 0 1), which are located at 27.45°, 37.97°, 44.07°, 54.40°, and 69.85°, respectively, correspond to the diffraction angle (θ). The obtained angle of 27.45° accurately depicts the TiO2 OGNPs' highly crystalline structure (1 0 1). The XRD pattern of TiO2 OGNPs reveals a tetragonal crystal structure. The usual TiO2 crystal size can be calculated using the Debye Scherer formula. It was demonstrated that the crystal diameters of TiO2 NPs were typically between 10 and 22 nm. The average crystalline size values that were observed agreed pretty well with past observations. Due to the synthesis process, there is a minor variation in peak strength, phase shift, and average crystalline size. The polyphenolic and organosulfur compounds in the aforementioned extract gave the green TiO2 nanoparticles stronger TiO2 peaks.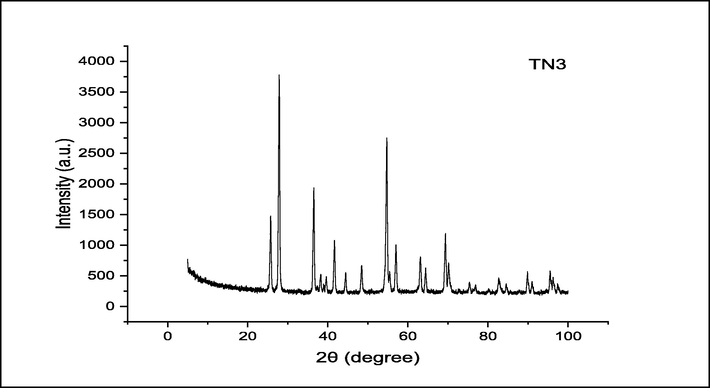
. XRD pattern of synthesized TiO2 OGNPs.
FTIR analysis: The various functional groups involved in the synthesis of nanoparticles were identified using the FTIR spectra of TiO2 NPs made in a green environment. As seen in Fig. 4, the hydroxyl group and surface-adsorbed water led the green-produced TiO2 NPs to have two distinct peaks, one at 3354 cm−1 and the other at 1628 cm−1. The FTIR spectrum of the green-produced nanoparticles showed unique bands, which suggested that various functional groups had experienced C-O stretching. Ether is detectable at 1024 cm−1, the peak. Peak values for the Ti-O stretching and Ti-O-Ti bridging stretching modes were 1024 and 493 cm−1, respectively (Ghaly et al., 2011, Peiro et al., 2011, Yu et al., 2006).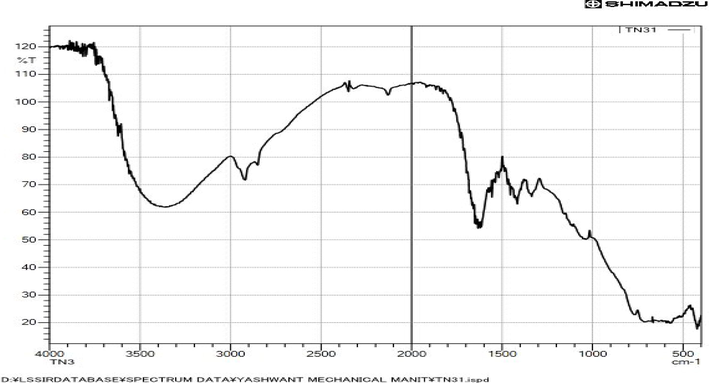
. FTIR spectrum of a combined onion-and-garlic peel extract-derived TiO2 nanoparticle.
Analysis using an energy dispersive X-ray spectrometer and a scanning electron microscope: The SEM image of the green-produced TiO2 nanoparticles in Fig. 5(A) shows that the chemically synthesised TiO2 nanoparticles have sphere-like surface forms. Spherical TiO2 NPs were found to have an average particle size between 10 and 22 nm. There is a strong correlation between the estimated particle size from SEM data and the average crystalline size reported by XRD. The volume of the material's total surface is inversely associated with the reduction in particle size. As a result, as soon as the smaller particle size material penetrates the bacterial surface and potentially harmful substances, the breakdown process is quickly started (Tulip et al., 2012, Pal et al., 2007). The elemental analyses of the chemical components were completed using EDS spectra. Fig. 5(B) displays the green TiO2 OGNPs' EDS spectra. The elements that make up the TiO2 NPs that are formed are titanium (Ti) and oxygen (O) (Venkatesh et al., 2020). In bio-mediated TiO2 NPs, the titanium element predominates over the oxygen concentration. The atomic and weight compositions of TiO2 NPs are displayed in Table 1. The suspension of Cobalt nanoparticles was prepared in Mille Q water and their size was determined using a zeta sizer, size and zeta potential were found to be 120 ± 1.0 nm and −12.4 ± 0.2 mV.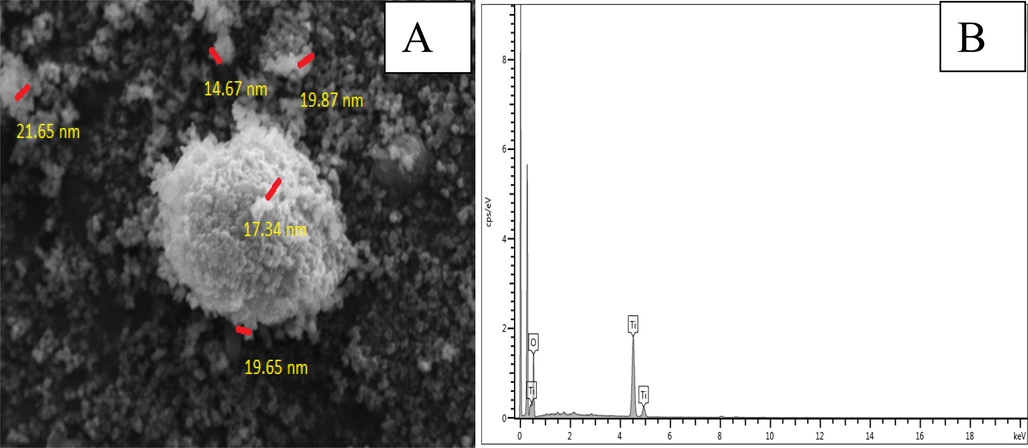
. (A) SEM image and (B) EDS analysis of green synthesized TiO2 OGNPs.
S.No.
Element
Weight (%)
1
Ti
61.95
2
O
38.05
Antimicrobial analysis: On a number of bacterial strains, strong inhibitory effects of nanomaterials have been noted. The metal oxides transmit a positive charge, whereas the microorganisms carry a negative charge. Therefore, oxidation which results from electromagnetic interactions between bacteria and metal oxides causes the death of microbes. Crude extracts of several medical plant parts, such as the root, stem, flower, fruit, and twigs, were frequently used to cure a variety of human illnesses (Ibrahim, 2015). Flavonoids, alkaloids, tannins, and terpenoids are only a few of the phytochemicals found in medicinal plants that have antibacterial and antioxidant properties. The antibacterial capabilities of specific plant species have been the subject of numerous investigations. Many herbs, including those from cinnamon, garlic, basil, curry, ginger, sage, and mustard, for example, exhibit antibacterial effects on a range of Gram-positive and Gram-negative bacteria (Shrivastava, 2007).
The antibacterial effect of TiO2 NPs is due to the breakdown of bacterial outer membranes by reactive oxygen species (ROS), particularly hydroxyl radicals (OH), which leads to phospholipid peroxidation and ultimately cell death (Roy et al., 2010). Photocatalytic oxidation processes begin when a photon with an energy level greater than or equal to the band gap energy is absorbed by a TiO2 catalyst. This reaction moves an electron (e) from the valence band to the conduction band while also producing a positive hole (h + ) in the valence band. The positive hole in TiO2 (OH) breaks the water molecule, releasing hydrogen gas (H2) and hydroxyl radicals. When a negative electron comes into contact with an airborne oxygen molecule, super oxide ions are produced. Hydroxyl peroxide (H2O2) is produced when these hydroxyl radicals come into contact. These ROS are capable of degrading organic molecules and halting biological activity. The photocatalytic action of TiO2 results in significant ROS damage in cell membranes, which is followed by the loss of essential functions.
In a study using a crude mixed extract of onion and garlic peel, the zones of inhibition for Staphylococcus aureus, Staphylococcus cohnii, Escherichia coli, Proteus, and Klebsiella pneumonia were found to be 18, 18, 14, and 16 mm, respectively. While the zone of inhibition for the same gram-positive bacteria (Staphylococcus aureus, Staphylococcus cohnii) was found to be 29, 26, 29, 28 and 27 mm in the green-synthesised TiO2 OGNPs Table 2, the crude combined extract of onion and garlic peel in methanol has the strongest antibacterial effect. It was shown that both Gram-positive and Gram-negative bacteria responded favourably to the green TiO2 OGNPs that were synthesised. Line regression curve for the determination of IC50 of biosynthesized TiO2 nanoparticles is shown in Fig. 6, and it was found be 0.1 mg/ml against all micro-organism This suggests that Gram-negative bacteria were less sensitive than the crude extract (Malarkodi et al., 2013). Gram-negative bacteria were suppressed by crude methanolic mixed onion and garlic peel extract in regions where they were less prevalent than gram-positive bacteria. Results were calculated using Mean and Standard Deviation (SD). *p less than 0.05 is considered statistically significant.
Name of
Micro-organisms
Zone of inhibition of different concentration (mm in diameter)
Crude combined extract of Onion and Garlic peel
Green synthesized TiO2 OGNPs
10%
8%
6%
4%
10%
8%
6%
4%
Proteus
16.0
±0.12*15.0
±1.3413.0±
0.2312.0
±0.3428.0
±0.8427.0
±1.3226.0
±0.5627.0
±0.12
Escherichia coli
14.0
±0.34*12.0
±0.6312.0
±0.4511.0
±1.1227.0
±0.3227.0
±0.7826.0
±0.4825.0
±0.34
Klebsiella pneumonia
15.0
±1.42*14.0
±0.3815.0
±0.6013.0
±0.2329.0
±1.5629.0
±0.8628.0
±0.9727.0
±0.17
Staphylococcus cohnii
18.0
±1.45*17.0
±0.8517.0
±1.5916.0
±0.1626.0
±0.6025.0
±1.3025.0
±0.2324.0
±0.23
Staphylococcus aureus
18.0
±0.76*18.0
±2.1317.0
±1.0317.0
±0.2329.0
±0.5428.0
±1.3428.0
±0.1926.0
±0.35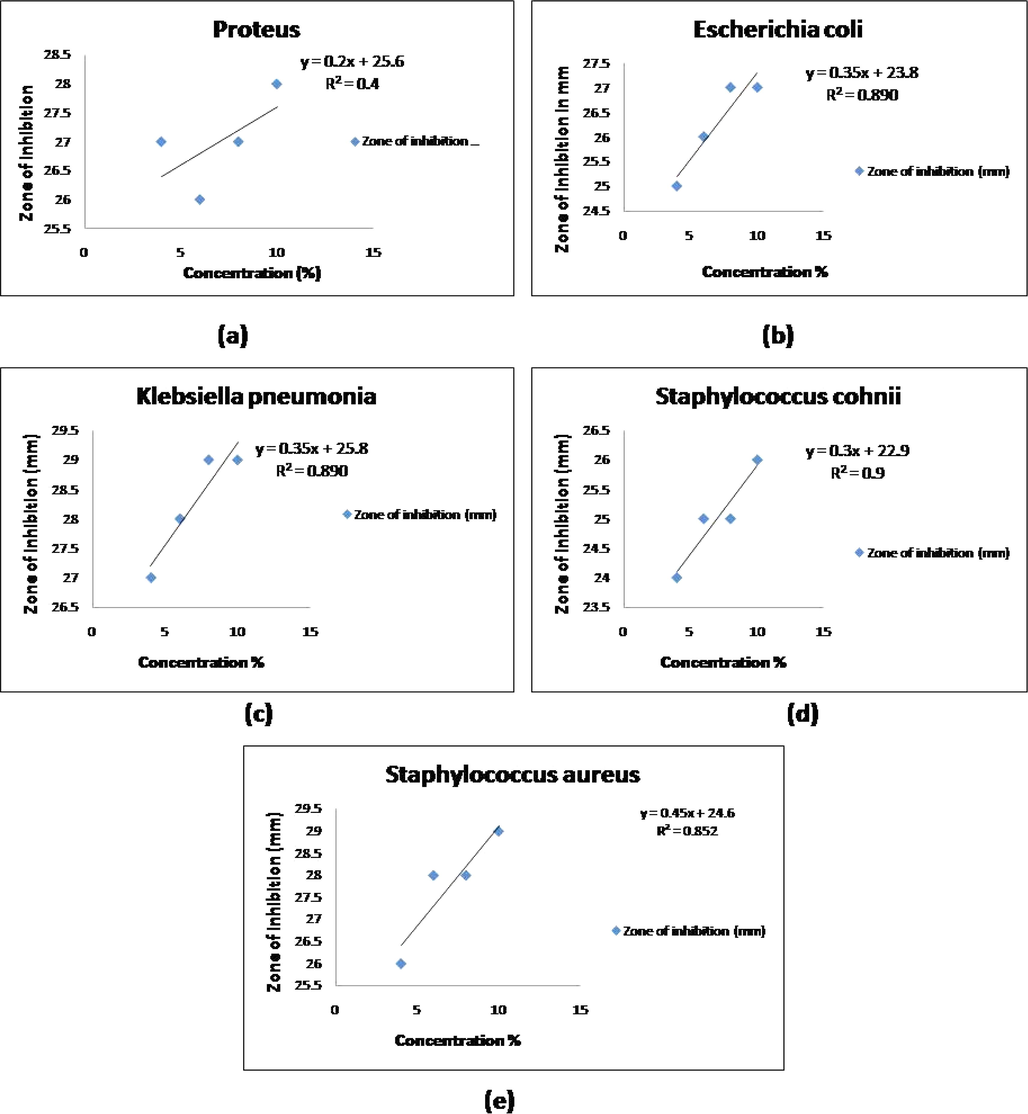
Line regression curve for the determination of IC50 of biosynthesized TiO2 nanoparticles against(a) Proteus (b) Escherichia coli (c) Klebsiella pneumonea (d) Staphylococcus cohnii (e) Staphylococcus aureus.
According to the results of the current work, TiO2 NPs of the right size could one day function as effective antibacterial and antifungal agents. The current experimental work is also conducted in daylight, making it significant for prospective uses of these NPs for antibacterial surface modification. TiO2 NPs could be employed as the appropriate disinfectant in hospital settings where resistant strains could spread quickly and infect patients with surgical incisions and burns. To further reduce the rate of infection in patients, cotton textiles containing antibacterial TiO2 might be used to make sutures or wound bands.
4 Conclusion
In the realm of nanotechnology, the creation of a dependable and environmentally friendly method for producing metallic nanoparticles is essential. Nanotechnology is believed to be impossible without nanoparticles. TiO2 nanoparticles are important in biology and medicine because of their appealing physiochemical characteristics. In this paper, we describe a green nanochemistry method that fabricates metal nanostructures without the use of hazardous waste or toxic solvents, using a natural, inexpensive biological reducing agent and a combined extract of onion and garlic peel. The biosynthesised TiO2 nanoparticles were highly effective against microorganisms. To show biological activity, employ the disc diffusion technique. This research revealed a straightforward, efficient, and inexpensive way to make TiO2 nanoparticles. Prepared nanoparticles can be used in many different medical specialties and as bactericides.
Acknowledgement
The author thanks the Researchers Supporting Project Number (RSP2023R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- TiO2 nanoparticles as an antibacterial agent against E. coli. Int. J. Innov. Res. Technol. Sci. Eng.. 2013;2:3569-3574.
- [Google Scholar]
- Green synthesis of TiO2 nanoparticles using orange peel extract for antibacterial, cytotoxicity and humidity sensor applications. Mater. Today. 2019;8:323-331.
- [Google Scholar]
- A biological approach to the synthesis of TiO2 nanoparticles with Streptomyces sp JAR1 and its antimicrobial activity. Sci. Pharm.. 2013;81:607-621.
- [Google Scholar]
- Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J. Nanopart. Res.. 2011;13:921-930.
- [Google Scholar]
- Treatment of highly polluted paper mill wastewater by solar photocatalytic oxidation with synthesized nano TiO2. Chem. Eng. J.. 2011;168:446-454.
- [Google Scholar]
- The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int. J. Mol. Sci.. 2022;23(3):1255.
- [Google Scholar]
- Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. J. Chromatogr. A. 2007;1151:211-215.
- [Google Scholar]
- TiO2 nanoparticles mediated by Callistemon citrinus extracts and their antimalaria, antitrypanosoma and antibacterial efficacy. J. Mol. Liq.. 2019;273:615-625.
- [Google Scholar]
- The emerging roles of arthropods and their metabolites in the green synthesis of metallic nanoparticles. Nanotechnol. Rev.. 2016;5:601-622.
- [Google Scholar]
- Chemical Composition, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Activities of Essential Oils from the Leaves and Rhizomes of Acorusmacro spadiceus (Yamamoto) J. Essent. Oil Bear.. 2021;24:1323-1332.
- [Google Scholar]
- Novel eco-friendly synthesis of titanium oxide nanoparticles by using Planomicrobium sp. and its antimicrobial evaluation. Der Pharmacia Sinica. 2013;4:59-66.
- [Google Scholar]
- Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med.. 2013;13:60118-160112.
- [Google Scholar]
- Green synthesis of mesoporousanatase TiO2 nanoparticles and their photocatalytic activities. RSC Adv.. 2017;7:48083-48094.
- [Google Scholar]
- Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol.. 2007;12:547-555.
- [Google Scholar]
- Size-controlled synthesis of spherical TiO2 nanoparticles: morphology, crystallization, and phase transition. J PhysChem C.. 2007;111(1):96-102.
- [Google Scholar]
- Lowtemperature deposition of TiO2 thin films with photocatalytic activity from colloidal anatase aqueous solutions. Chem. Mater.. 2011;13:2567-2573.
- [Google Scholar]
- Green biosynthesis of TiO2 nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloids Surf. B Biointerfaces. 2017;158:408-415.
- [Google Scholar]
- Enzymes inhibition and antioxidant potential of medicinal plants growing in Oman. Biomed Res. Int.. 2022;2022:9.
- [Google Scholar]
- Effect of nanotitanium dioxide with different antibiotics against methicillin-resistant staphylococcus aureus. J Biomater Nanobiotechnol.. 2010;01(01):37-41.
- [CrossRef] [Google Scholar]
- Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18(22):225103
- [Google Scholar]
- Extracellular biosynthesis of iron oxide nanoparticles by Bacillus subtilis strains isolated from rhizosphere soil. Biotechnol. Bioprocess Eng.. 2012;17:835-840.
- [Google Scholar]
- Biosynthesis of TiO2 nanoparticles using Morindacitrifolia L. as capping and reducing agents. IJETT.. 2012;3:24-34.
- [Google Scholar]
- Enhanced photocatalytic activity of reduced graphene oxide/SrSnO3nanocomposite for aqueous organic pollutant degradation. Optik. 2020;206:164055
- [Google Scholar]
- Scholar Research Library. 2012;4(2):1183-1190.
- Phytochemical screening and in vitro evaluation of the antioxidant potential of dichloromethane extracts of Strtchnos henningsii Gilg. and Ficus sycomorus L. Scientific World Journal. 2023;12
- [Google Scholar]
- Effects of pH on the microstructures and photocatalytic activity of mesoporousnano crystalline titania powders prepared via hydrothermal method. J. Mol. Catal. A-Chem.. 2006;258:104-112.
- [Google Scholar]
- 3D structured TiO 2-based aerogel photocatalyst for the high-efficiency degradation of toluene gas. New J. Chem.. 2022;5:2272-2281.
- [Google Scholar]







