Translate this page into:
Synthesis and characterization of Chitosan–silica hybrid aerogel using sol-gel method
⁎Corresponding author. kelebis@yahoo.com (Kelechi Ebisike)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Chitosan–silica hybrid aerogel [(CS)hA] was prepared using sol-gel method by combining an inorganic network in the presence of an organic polymer. Chitosan biopolymer and silica (SiO2) which are obtained from agricultural waste of crab shell and bamboo leaves are the primary precursors used in the sol-gel reaction and hexamethyldisiloxane applied for hydrophobicity. The derived aerogel has been characterized using Fourier transform infrared (FTIR), scanning electron microscope (SEM), energy dispersive X-ray (EDX), and thermogravimetric analysis (TGA). Sears method was used to determine surface area. The FTIR spectrum shows presence of organic and inorganic entities which are also seen on the EDX chart. TGA provides information on the thermal stability of the derived aerogel. The SEM analysis shows clearly the surface morphology and roughness of (CS)hA. The calculated pore volume, bulk density, surface area, and porosity is 0.286 cm3 g−1, 0.441 g cm−3, 237.4 m2 g−1 and 0.126.
Keywords
Chitosan–silica hybrid aerogel
Hexamethyldisiloxane
Hydrophobicity
Sol-gel
Agricultural waste
1 Introduction
Many researches and industries have shown increased interest in using developed advanced materials by combining macromolecules with inorganics to form organic-inorganic hybrid materials (Silva et al., 2005). Sol-gel technology is a simple and convenient method for preparing organic-inorganic materials based on molecular level hybridization (Shin et al., 2005). There are two methods of preparing these hybrid materials. One is the formation of composite materials on which organic material is supported. The second method is the use of sol-gel technique in forming the hybrid material. The formation of the organic-inorganic material is achievable by the hybridization of organic constituent with inorganic constituent. It was reported that silica is a good supporting material because of its large surface area and excellent mechanical properties (Li et al., 2007).
Several preparatory methods of hybrid materials based on polysaccharides and inorganic materials meant for different applications have been studied (Zhou et al., 2015; Budnyak et al., 2014; Kavitha et al., 2013; Spirk et al., 2013; Puchol et al., 2009; Ganji and Abdekhodaie, 2008). Studies have been extensively carried out for decades on sol-gel reaction for ceramic precursor and inorganic glass preparation at relatively low temperature (Budnyak et al., 2015).
Sol-gel technique has been used to form biopolymer-silica hybrid materials with potential properties, living matter compatibility and applicability in biomimetic processes (Prerna and Ramanand, 2012). The preparation of these hybrid materials follows a controlled sequence of hydrolyses and condensation starting from utilizing an alkoxysilane like tetraethylsilane in alcohol and other polar solvents which leads to a solution or colloidal suspension of siloxane polymer (Brinker and Scherer, 1990). It is possible to introduce a soluble organic polymer in the sol at this stage or at the beginning of the process (Prerna and Ramanand, 2012).
Naturally occurring polymer usage is becoming very attractive and vital to the growing need for sustainable and environmental development. Chitosan is one of the natural polymers with good characteristics such as biocompatibility, biodegradability, hydrophilicity, low toxicity and availability from renewable resources for preparation of aerogel (Xu et al., 2016).
Chitosan is obtained by alkaline deacetylation of chitin. It is a biodegradable, non-toxic, biocompatible polymer with the potential to form film and resist heat (Kurita, 1998). The amino and hydroxyl group in chitosan make chemical modifications possible and attractive for hybrid material preparation (Cao et al., 2006; Molvinger et al., 2004; Yeh et al., 2007; Kurita, 1998). The potential scientific and technical applications which have provided the combination of both organic and inorganic species properties have made chitosan-inorganic materials of great interest. Chitosan-silica hybrid materials have been applied in various fields where they find application due to their characteristic properties (Xu et al., 2010; Al-Sagheer and Muslim, 2010).
Aerogel is a lightweight porous material in which the pores have been replaced by gas. Aerogel finds application as catalyst supports, particle filters, particle trappers and heat insulators because of its large inner surface area and low heat conductivity (Gan et al., 2017). Sol-gel method was used to produce aerogel in which the textural properties of the gel was preserved when removing the solvent. Aerogel production using supercritical drying is very expensive hence the need for a low cost drying technique i.e. Ambient pressure drying (APD). Moreover there is disparity in information on the production of chitosan-silica hybrid aerogel which arises from the types of precursor, drying methods, conditions of the process, chemicals and hydrophobic agents being applied.
This work presents the production of Chitosan–silica hybrid aerogel whose primary precursors (chitosan and silica) were extracted from agricultural waste using sol-gel method at ambient pressure drying technique and its characterization to identify the functional group, morphology, elemental composition, thermal stability, surface area, bulk density, pore volume and porosity.
2 Materials
The silica was extracted from bamboo grass by utilizing alkaline method. The chitosan was extracted from crab shell. Glacial acetic acid was supplied by BDH Chemical Ltd. Ammonia solution was supplied by Griffin and George Fiscon Pls Scientific Equipment Division. Absolute Ethanol, Pentane and Hydrochloric acid were supplied by Guangdong Guadgua Chemical Factory Co Ltd. Sodium hydroxide pellets were supplied by Kem Light Laboratories PVT Ltd. Sodium chloride was supplied by Guangdong Guanghua Si-Tech CO. Ltd. Hexamethyldisiloxane was supplied by Sigma Aldrich Co.
3 Methodology
3.1 Chitosan–silica hybrid aerogel [(CS)hA] preparation
Silica (30 g) was added to water (1080 ml) and ammonia (120 ml) solution was also added to the mixture and stirred to dissolve the silica. Chitosan (30 g) was dissolved in 3% acetic acid (900 ml), stirred and filtered. The filtrate from the chitosan solution was then added to the silica solution and stirred. Heat was applied at 60 °C for 3 mins to catalyze the hydrolysis and condensation of the reaction. The resulting hydrogel was washed three times in ethanol and then in heptane and put in 10% hexamethyldisiloxane solution. This was then heated to dryness at 80 °C. The derived aerogel, which is a white opaque light mass, was characterized using FTIR, SEM-EDX, and TGA.
3.2 Fourier transform infra-red spectroscopy
Perkin Elmer FTIR Spectrometer was used to characterize the functional groups of the chitosan–silica hybrid aerogel in the region from 4000 to 400 cm−1. The samples were ground and pressed with KBr.
3.3 Scanning electron microscopy-energy dispersive X-ray spectroscopy
Phillips XL 30S FEG device at accelerating voltage of 20 kV was used to obtain the morphology of (CS)hA, whereas the EDX identifies the elemental composition.
3.4 Thermogravimetric analysis
Shimadzu-TGA0H Thermobalance was used for thermal gravimetric analysis under synthetic air (50 ml min−1) at a heating rate of 10 °C min−1 to characterize their thermal behavior.
3.5 XRD analysis
Shimadzu XRD-7000 X-ray diffractometer was used to obtain the X-ray diffraction pattern with Cu Kα radiation being the source of X-ray at a setting of 30 kV and 30 mA. The 2 θ for the XRD pattern is recorded within the range of 10°–80° at a scan rate of 2° per min.
3.6 Surface area determination
Surface area per gram of the chitosan–silica hybrid aerogel was obtained using Sears method (Jemal et al., 2014; Sears, 1956). A 1.5 g sample of the derived aerogel was mixed with 100 ml of water and 30 g NaCl. The mixture was stirred for five minutes. To this mixture 0.1 N HCl was added to make a final volume of 150 ml and final pH of 4.0. It was then titrated with 0.1 N NaOH. The volume of 0.1 N NaOH required to raise the pH from 4.0 to 9.0 was noted.
3.7 Determination of bulk density
The density of chitosan–silica hybrid aerogel was determined by filling the derived aerogel in a 10 ml measuring cylinder. This was weighed using an analytical weighing balance. The recorded weight which is also known as the bulk weight was divided by the measuring cylinder volume used.
3.8 Determination of pore (void) volume
Chitosan–silica hybrid aerogel (2 g) was immersed in 30 ml of distilled water; weighed using an analytical weighing balance, and then heated at 100 °C for 25 mins, so as to displace the air contained in the pores. The derived aerogel was reweighed after being retrieved from the water. It was then superficially dried on a filter paper. The weight increase per unit mass of the derived aerogel divided by the density of water gave the pore volume as reported (Agwogie, 2014; Okafor et al., 2015).
3.9 Porosity
The porosity or void fraction (Po), of the chitosan–silica hybrid aerogel was calculated using the Eq. (1);
4 Results and discussion
Sol-gel method can be a two-network forming process in which the hydrolysis of silica is the first stage followed by a poly-condensation reaction. The in situ formation of inorganic network in the presence of preformed organic polymer possess strong chemical bonds and weak phase interaction such as hydrogen bond between their phases (Budnyak et al., 2016). Hydroxyl groups from chitosan could form hydrogen bond or react with silanol groups during condensation reaction produced from hydrolysis of the precursor thereby leading to silica nucleation on macro-molecules (Zou et al., 2008). Amino groups in chitosan molecule aid the hydrolysis of the siloxane and the condensation of the formed silanol groups as well as the silanol groups of the silica reaction with the polymer carbonyl groups.
Fig. 1 shows FTIR spectrum of the derived chitosan–silica hybrid aerogel. IR band in 3700–3200 cm−1 is assigned to the overlapping of Si-OH stretch and amine band. Likewise, the IR bands in 2100–2360 cm−1 is due to Si-H stretch which overlaps ⚌C⚌O vibration. There appears a —C⚌O band at 1636 cm−1 (Prerna and Ramanand, 2012). The adsorption peak at 1439 cm−1 is related to in-plane OH bending. The peak observed at 1093 cm−1 is related to Si—O—Si stretch (Prerna and Ramanand, 2012) while the peak at 800 cm−1 is due to the overlapping of Si—C stretch and NH2 wag. The peak at 467 cm−1 is related to O-Si- bending (Azadeh et al., 2013). The large intensity of the peak at 1093 cm-1 is due mainly to the overlapping of the Si—O—Si and the —C—O—C— of glycosidic linkage (Prerna and Ramanand, 2012; Yeh et al., 2007). Chitosan-silica hybrid was also reported to have Si—OH and Si—O—Si with bands in the regions of 3300–3370 cm−1 and 1000–1250 cm−1 respectively (Al-Sagheer and Muslim, 2010).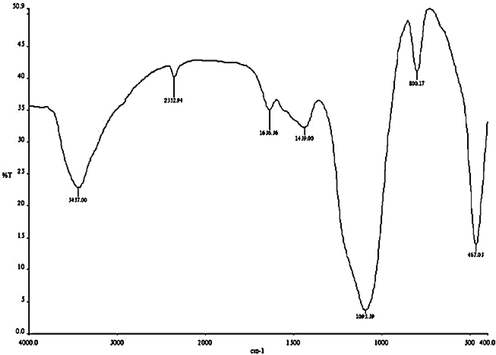
FTIR spectrum of chitosan – silica hybrid aerogel.
Fig. 2 is a presentation of the XRD analysis carried out to characterize the phase structure of chitosan-silica hybrid aerogel. From the diffractogram, the peak most prominent amorphous curve is observed at 22.9° in the 2θ angle which shows the amorphous state of the silica network whereas the chitosan exist as an anhydrous crystalline phase. Todorova et al. (2014) and Kow et al. (2014) reported anhydrous crystalline chitosan and amorphous silica respectively at 2θ = 23°. A highly discrete crystalline projection found at 2θ angle of 29.3° shows the contribution of the silica phase.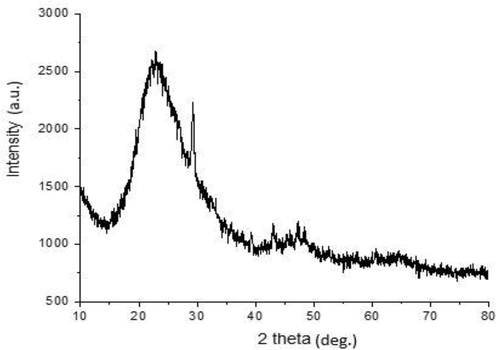
X-ray pattern of chitosan–silica hybrid aerogel.
Hybrid materials prepared by sol-gel method have a rough and irregular surface when viewed from SEM images. This could portray a high specific surface area due to plenty of holes and canals on the hybrid material (Budnyak et al., 2016). Fig. 3 presents the scanning electron micrograph, which reveals the surface texture and morphology of the Chitosan–Silica Hybrid Aerogel produced. It is observed that the chitosan-silica hybrid aerogel has a rough surface texture which appears as agglomerates of varied sizes and shapes of microfibrils and leaf-like structure. This could be an indication of high surface area that is required to promote adherence of metal ions (Gong et al., 2012). Perdigoto et al. (2012) reported that aerogels with high surface area, have enhanced capacity for metal ion adsorption.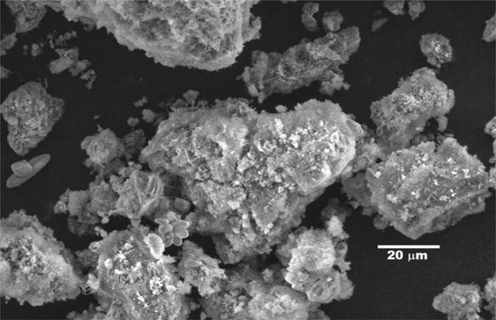
SEM analysis of chitosan–silica hybrid aerogel.
The energy dispersive x-ray pattern of the derived Chitosan–Silica Hybrid Aerogel is presented in Fig. 4. It is observed that the aerogel consists essentially of Silicon (Si), Carbon (C), Oxygen (O), and Calcium (Ca). The very high peak of Si in the EDX spectrum suggests that the hybrid aerogel consists predominantly of silica. The carbon is from the chitosan as well as a substrate material used during the SEM-EDX analysis. The calcium is from the chitosan which was extracted from a calcium-rich Crab shell.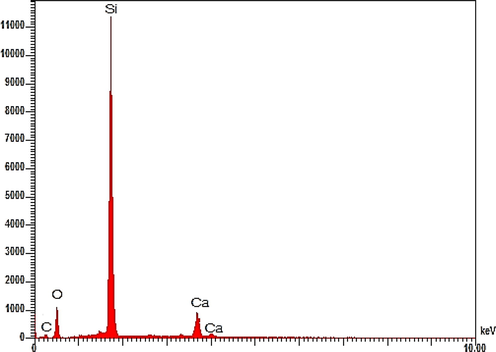
EDX analysis of chitosan–silica hybrid aerogel.
Thermogravimetric analysis was performed under nitrogen atmosphere from room temperature to 700 °C as shown in Fig. 5. The TGA and DTG curves shows that at about 60 °C there is a first weight loss which could be attributed to loss of water molecules present in the chitosan–silica hybrid aerogel. This loss of water continues to around 100 °C. The second weight loss which appears at around 440 °C can be attributed to the loss of hydrophobicity. At 540 °C, there is the decomposition of chitosan. Above this temperature to around 630 °C is the oxidation of methyl group which is responsible for the hydrophobicity of the derived aerogel and the complete decomposition of the backbone of the derived aerogel matrix. This thermal stability could be as a result of the silane used as the hydrophobic agent during the derived aerogel formation. High content of silylating agent increases the thermal stability of chitosan (Xi et al., 2006). Also the silica in the aerogel becomes a protective material which impedes the thermal degradation of chitosan and hence, becomes a hindrance on thermal degradation which enhances char formation (Liu et al., 2004). A thermal degradation of this nature could indicate organic – inorganic hybridization which the FTIR chart obtained supports. It is likely for the process of condensation and elimination of hydroxyl groups to take place at higher temperatures (380 °C to 700 °C) (Budnyak et al., 2015). The DTA curve shows that the loss of weight process gave endothermic reaction.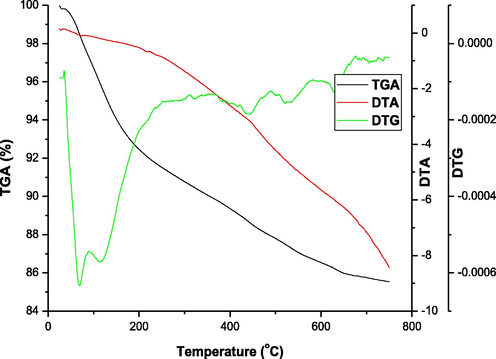
TGA – DTA analysis of chitosan–silica hybrid aerogel.
The calculated pore volume is 0.286 cm3 g−1. The larger the pore volume the larger the amount of waste water the materials produced will hold which also implies that the drying process for the produced material will be slow. The bulk density is 0.441 g cm−3. The specific area (i.e. area per gram) was obtained using Eq. (2);
5 Conclusion
Chitosan–silica hybrid aerogel was successfully synthesized using sol-gel and ambient drying methods. The morphology shows it is rough with irregular shape. The peaks of the FTIR spectrum shows the inclusion of Si-O-Si polymeric network in the chitosan of the derived aerogel.
6 Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7 Declarations of interest
None.
8 Authors contributions
This work was carried out in collaboration between all authors. Authors Kelechi Ebisike and Afamefuna Elvis Okoronkwo designed the study, managed the literature searches and managed the analyses of the study. Authors Kelechi Ebisike, Afamefuna Elvis Okoronkwo and Kenneth Kanayo Alaneme wrote the protocol and the first draft of the manuscript. All authors read and approved the final manuscript.
References
- The Potentials of Adansonia Digitate Root and Stem Powders and Stem Activated Carbon as Low-Cost Adsorbents for the Removal of Heavy Metals from Aqueous Solutions. Nsukka, Nigeria: University of Nigeria Nsukka; 2014. (Master’s thesis)
- Thermal and mechanical properties of chitosan/SiO2 hybrid composites. J. Nanomater.. 2010;1–7
- [CrossRef] [Google Scholar]
- Synthesis and characterization of hydrophobic silica aerogel by two step (acid-base) sol-gel process. JNS. 2013;3:181-189.
- [CrossRef] [Google Scholar]
- Sol-gel science. In: The Physics and Chemistry of Sol-Gel Processing. NY, USA: Academic Press; 1990.
- [Google Scholar]
- Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res. Lett.. 2015;10(87):1-10.
- [CrossRef] [Google Scholar]
- Chitosan immobilized on saponite surface in extraction of V(V), Mo(VI) and Cr(VI) oxoanions. Chem. Phys. Tech. Surf.. 2014;5:445-453.
- [Google Scholar]
- Preparation and properties of organomineral adsorbent obtained by sol-gel technology. J. Therm. Anal. Calorim.. 2016;125:1335-1351.
- [CrossRef] [Google Scholar]
- Chitosan as a polymer for pH-induced DNA capture in a totally aqueous system. Anal. Chem.. 2006;78(20):7222-7228.
- [CrossRef] [Google Scholar]
- Highly porous regenerated cellulose hydrogel and aerogel prepared from hydrothermal synthesized cellulose carbamate. PLoS One. 2017;12(3):1-13.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of a new thermosensitive chitosan-PEG diblock copolymer. Carbohydr. Polym.. 2008;74:435-441.
- [CrossRef] [Google Scholar]
- Adsorption of heavy metal ions by hierarchically structured magnetite-carbonaceous spheres. Talanta. 2012;101:45-52.
- [CrossRef] [Google Scholar]
- Kinetics and Equilibrium Study of Adsorption of Phenol Red on TEFF (Eragrostis teff) Husk Activated Carbon. Int. J. Innov. Sci. Res. 2351-8014 (11):471-476.
- [Google Scholar]
- In situ synthesized novel biocompatible titania-chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr. Polym.. 2013;93:731-739.
- [CrossRef] [Google Scholar]
- From bamboo leaf to aerogel: Preparation of water glass as a precursor. J. Non-Cryst. Solids. 2014;386:76-84.
- [Google Scholar]
- Chemistry and application of Chitin and Chitosan. Polym. Degrad. Stab.. 1998;59(2):117-120.
- [CrossRef] [Google Scholar]
- Preparation of silica-supported porous sorbent for heavy metal ions removal in wastewater treatment by organic-inorganic hybridization combined with sucrose and polyethylene glycol imprinting. Anal. Chim. Acta.. 2007;585(2):211-218.
- [CrossRef] [Google Scholar]
- In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using gamma-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer. 2004;45(20):6831-6837.
- [CrossRef] [Google Scholar]
- Porous Chitosan-Silica. Hybrid microspheres as a potential catalyst. Chem. Mater.. 2004;16:3367-3372.
- [CrossRef] [Google Scholar]
- Studies on the adsorption of heavy metals in a paint industry effluent using activated Maize cob. Jmest. 2015;2(2):1-8.
- [Google Scholar]
- Application of hydrophobic silica based aerogels and xerogels for removal of toxic organic compounds from aqueous solutions. J. Colloid Interface Sci.. 2012;380:134-140.
- [CrossRef] [Google Scholar]
- Comparative study of physical and thermal properties of Chitosan – Silica hybrid coatings prepared by sol-gel method. Pelagia Reseach Library, Der Chemica Sinica. 2012;3(3):589-601.
- [Google Scholar]
- Biomimetic chitosan-mediated synthesis in heterogeneous phase of bulk and mesoporous silica nanoparticle. Chem. Commun. 2009:2694-2696.
- [CrossRef] [Google Scholar]
- Determination of specific surface area of colloidal silica by titration with sodium hydroxide. Anal. Chem.. 1956;28(12):1981-1983.
- [CrossRef] [Google Scholar]
- Sol-gel derived amperometric nitric oxide microsensor. Anal. Chem.. 2005;77(11):3494-3501.
- [CrossRef] [Google Scholar]
- Functional nanostructured chitosan/siloxane hybrids. J. Mater. Chem.. 2005;15:3952-3961.
- [CrossRef] [Google Scholar]
- Chitosan-silane sol-gel hybrid thin films with controllable layer thickness and morphology. Carbohydr. Polym.. 2013;93:285-290.
- [CrossRef] [Google Scholar]
- Structure and properties of functionalized porous silica hybrid materials. Open J. Inorg. Non-Metal. Mater.. 2014;4:35-43.
- [CrossRef] [Google Scholar]
- Novel nylon-supported organic-inorganic hybrid membrane with hierarchical pores as a potential immobilized metal affinity adsorbent. J. Chromatogr. A. 2006;1125(1):38-51.
- [CrossRef] [Google Scholar]
- Porous cellulose aerogels with high mechanical performance and their absorption behaviors. BioRes.. 2016;11(1):8-20.
- [CrossRef] [Google Scholar]
- A simple strategy for preparation of spherical silica-supported porous chitosan matrix based on sol-gel reaction and simple treatment with ammonia solution. Anal. Methods. 2010;2:546-551.
- [CrossRef] [Google Scholar]
- Synthesis and Properties of Chitosan/ SiO2 Hybrid Materials. Mater. Lett.. 2007;61:1292-1295.
- [CrossRef] [Google Scholar]
- Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym.. 2015;117:524-536.
- [CrossRef] [Google Scholar]
- Polymer/silica nanocomposites, preparation, characterization, properties, and applications. Chem. Rev.. 2008;108(9):3893-3957.
- [CrossRef] [Google Scholar]







