Synergistic anti-bacterial effects of green synthesized zinc oxide nanoparticles with levofloxacin
⁎Corresponding author. hardeep.biotech@gmail.com (Hardeep Singh Tuli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nowadays, the emergence of drug resistance is a major problem due to the limited bioavailability and unintended toxicity of antibiotics because of their non-specific targeting, which makes it difficult to eradicate pathogenic infections. Zinc oxide nanoparticles gain much attention as nanocarriers due to their non-toxic, eco-friendly, and economical cost to treat severe pathogenic infections. The purpose of this research was to produce and analyze zinc oxide nanoparticles (ZnONPs) functions using a green synthesis process to evaluate their anti-bacterial and biofilm inhibitory activities against gram-positive and gram-negative microorganisms. ZnONPs were formed via a green synthetic approach using a seed extract of soybean (Glycine max) and nanoparticle characterization was done using FESEM, X-ray diffraction, UV–VIS spectroscopy, and EDAX. The anti-bacterial and biofilm inhibitory activities of ZnONPs, levofloxacin, and levofloxacin-loaded ZnONPs were determined using an agar disc diffusion assay and a microtiter plate assay, respectively. Levofloaxcin-loaded ZnONPs showed more potent and statistically significant antibacterial activity than ZnONPs alone against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis. The bacterial inhibition zone of levofloxacin-loaded ZnONPs at 80 µg/ml concentrations was higher in comparison to the standard levofloxacin and ZnONPs alone, which showed levofloxacin-loaded ZnONPs possess effective antibacterial activity that prevents the growth of diverse microorganisms. Our findings revealed that levofloxacin-loaded ZnONPs have an effective range of antimicrobial effects and could be used for the eradication of pathogenic infections.
Keywords
ZnO nanoparticles
Soyabean
Levofloxacin
Antibiotic resistance
Antibacterial
Anti-biofilm
1 Introduction
Nanotechnology is a leading field of technology with various biomedical applications. With the increasing advancement in nanotechnology, research has been conducted to synthesize nanoparticles of biomedical importance. Nanoparticles gain attention because they possess advanced physiochemical properties such as small size and a larger surface area by volume ratio. It can be produced through physical, chemical, and biological processes, the latter of which is economical and environment-friendly relative to others (Gomathi et al., 2017; Joshi et al., 2019; El Shafey, 2020; Rzayev et al., 2022; Aman et al., 2023). There have been successful attempts to synthesize nanoparticles using microorganisms or plants, but plant-based synthesis has found its main application as synthesis is more non-toxic, eco-friendly, cheap, easy, and fast than microbe-facilitated synthesis (Cruz et al., 2020).
The inorganic nanoparticles gained scientific attention because of their unique optoelectronic and physiochemical features, which are most required in the fields of sensing, drug delivery, molecular diagnosis, imaging, and catalysis (Shah et al., 2015; Mughal, 2022). The most latent use of zinc oxide (ZnO) appeared to be non-toxic by the Food and Drug Administration (FDA) (21 CFR 182.8991). ZnO nanoparticles (ZnONPs) have applications in diagnostics, drug delivery, and therapeutics due to their multifunctional properties such as magnetic, catalytic, binding, antimicrobial, antifungal, anti-inflammatory, and ultraviolet protective properties (Kalpana and Devi Rajeswari, 2018). ZnONPs have superior antimicrobial, antibacterial, and anti-inflammatory properties (Kalpana and Devi Rajeswari, 2018). ZnONPs can be easily formed by different plant extracts, like Lumina acidissima (leaf), Euphorbia jatropha (stem), Celosia argeniea (leaf), Solanum nigrum (leaves), Allium sativum (bulb), and Jacaranda mimosifolia (flower) (Kalpana and Devi Rajeswari, 2018). Nowadays, several reports have been submitted on the antimicrobial potential of ZnONPs. The ZnONPs formed using a pulsed laser beam in water were shown to have a strong zone of inhibition (ZOI) to suppress the growth of Staphylococcus aureus and E. coli (Khashan et al., 2021). ZnONPs prepared by utilizing the plant extract of Cassia alata demonstrated potent antibacterial properties against the bacteria E. coli, with an IC50 value of 20 µg/ml. It indicates the excellent biocompatibility of ZnONPs. The ZnONPs extracted using drumstick leaves (Moringa oleifera) were confirmed as a photocatalytic agent to reduce the organic dye VISA and also possess antibacterial potential to prevent the growth of Bacillus subtilis and E. coli bacteria (Pal et al., 2018). The ZnONPs prepared by consuming Trifolium pretence flower had shown great bactericidal potential against E. coli, P. aeruginosa, and S. aureus. The synthesized nanoparticles were larger, about 60–70 nm, and formed by the agglomeration of smaller nanoparticles from Trifolium pretence (Dobrucka and Długaszewska, 2016). The ZnONPs created using an extract from the seeds of Murraya koenigii show great promise against pathogenic bacteria and fungi growth (Sundaraselvan and Quine, 2017). The ZnONPs produced by the exhausting leaf extract of Camellia sinesis showed substantial bactericidal effects for diverse microorganisms and also reported inhibitory effect against the strain of fungus. The extreme ZOI was found against P. aeruginosa, and the least ZOI was against S. aureus (Noorjahan et al., 2015). Taken together, these studies revealed the prominent function of ZnONPs as antimicrobial and antifungal agents.
Combinatorial action of nanoparticles with conventional antibiotics could be used against the emerging nature of different antibiotic-resistant bacteria. The increasing usage of antibiotics is due to the advancement of antibiotic resistance in bacteria. Current research reports that metallic nanoparticles, owing to their high antibacterial activity and large specific surface area, are exploited as carriers of antibacterial drugs to advance their efficiency of inhibiting human pathogens. Sharma et al. (2016) investigated the combinatorial potentials of ZnONPs with antibiotics for different pathogens using their fractional inhibitory concentrations in checkboard tests (Sharma et al., 2016). Also, in the class of penicillin antibiotics, amoxicillin loaded with ZnONPs exhibited great antibacterial potential toward various microorganisms, and the highest was obtained for Staphylococcus epidermis (Palanikumar et al., 2013). The ZnONPs were utilized to synergistically potentiate the effect of ciprofloxacin and ceftazidime.
The effectiveness of drugs in the presence of nanoparticles has attracted substantial consideration in research. The synergetic effect can enhance the drug's effect in the presence of metal nanoparticles. As biological sources (plants) have the potential to synthesize metal nanoparticles by acting as reducing agents to the metal salt in abundance, the efficiency of the drug can be enhanced by green nanotechnology by evaluating the combinatorial effect of metal nanoparticles with the drug on bacteria. In this study, we prepared and characterized the ZnONPs as a drug delivery system against gram (+ve) and gram (−ve) bacteria using soybean seeds. Hardly ever is soybean seed extract investigated in the synthesis of ZnONPs. The antibacterial and anti-biofilm effects of levofloxacin, ZnONPs, and Lfx-loaded ZnONPs on different microorganisms were also examined.
2 Materials and methods
2.1 Materials
Infinity Laboratories Private Limited, Dera Bassi District Sahibzada Ajit Singh Nagar, Punjab provided the levofloxacin (purity 97 %) and zinc acetate. Muller-Hinton Agar (MHA), Nutrient Media Broth (NMB), Phosphate-buffering saline (pH 7.2), sodium acetate, crystal violet, deionized water, ethanol, and DMSO were purchased from Himedia. The soybean seeds were purchased from a local vendor in Ambala, Haryana. The bacterial strains S. aureus (ATCC 29213), E. faecalis (ATCC 29212), P. aeruginosa (ATCC 27853), and E. coli (ATCC 25922) were obtained as a gift from Dr. Saurab Gupta, Dean of Research at Mata Gujri College.
2.2 Assortment and preparation of plant material
The seeds of soybean were procured from a local market vendor in Ambala, Haryana. The soybean seeds were cleaned thoroughly with distilled H2O (dH2O) and air-dried for 10 days in the dark. The fine powder was prepared by grinding dried soybean seeds using a mixer grinder.
2.3 Synthesis of extract
1 g of finely powdered soybean seeds were maintained at 60 °C for 40 min in 50 ml of double-dH2O. After heating, the seed extract was centrifuged to remove heavy debris of plant material and further vacuum filtered to obtain a clear extract of soybean seeds.
2.4 Preparation of ZnO nanoparticles
In the process of synthesis, zinc acetate (0.1 M) was used as a precursor in this study. Zinc acetate was freshly prepared and then used for metal ions reduction from Zn2+ into Zn ions. 10 ml extract of soybean seed was blended with 90 ml of zinc acetate solution and maintained at 60 °C for 10 min followed by overnight incubation. Reduction of zinc acetate was monitored by a visual color change.
2.5 Purification of ZnO nanoparticles
After completion of the process of reduction and synthesis, ZnONPs were spin down for 15 min at 10,000 rpm at room temperature (RT). Drying of the collected pellets was conducted in a hot air oven set at 60 °C for 3 h and kept in airtight vials.
2.6 Characterization of biosynthesized ZnONPs
The first preliminary test used for the manufacture of ZnONPs was a color change. The optical parameter was analyzed by UV–VIS spectroscopy in the range of 280–700 nm. The shape and size of ZnONPs were examined by FESEM, and crystallinity was observed using XRD.
2.7 Drug loading
To load the 5 mg/ml of levofloxacin (Lfx) on to 1 mg/ml of ZnONPs, the drug was solubilized in aqueous DMSO (dimethyl sulfoxide). The contents of the levofloxacin and ZnONPs were mixed using a magnetic stirrer at 600 rpm for half an hour at RT. Then the mixture was left undisturbed for an overnight period at RT. After the overnight incubation, the contents were settled down at 10,000 rpm for 10 min. The precipitate and supernatant were collected separately. The separated precipitate was dried in an oven at 120 °C for 30 min. The loaded drug's concentration was determined using a spectrophotometer in the UV–Vis range of max. The percentage of drug loading was determined by finding the variation between the before and after-drug concentrations in the supernatant.
2.8 Antibacterial activity
The antimicrobial function of prepared ZnONPs, Lfx, and Lfx-loaded ZnONPs was assessed using the ZOI method in Muller-Hinton agar. The antimicrobial potential was examined against E. coli, S. aureus, P. aeruginosa, and E. faecalis in BSL-2 laboratories. All samples of nanoparticles and drugs were dissolved in aqueous DMSO to make final concentrations of 1 mg/ml. Dilution of the stock solution made working concentrations from 20 to 100 μg/ml with a 20 μg/ml difference. The sterilized Petri plates were used to pour 25 ml of MHA media onto each plate. The 200 µl of bacterial inoculum were swabbed onto plates for their growth. The test samples (ZnONPs, Lfx, and Lfx-loaded ZnONPs) were well prepared by using a cork borer. The loaded test samples along with the control were maintained at 37 °C for 24 h. Afterward, the ZOI was determined using the antibiotic zone scale.
2.9 Anti-biofilm activity
The anti-biofilm potential of ZnONPs was evaluated by using a microtiter plate assay (MTP) (Basumatari et al., 2021). To determine the percentage of biofilm inhibition for three prepared samples (ZnONPs, Lfx, and Lfx-ZnONPs), we used a 96-well flat-bottom microtiter plate. The pure culture of all four strains of ATCC E. coli, S. aureus, P. aeruginosa, and E. faecalis was inoculated in nutrient media broth and maintained for 24 h at 37 °C. After the period of incubation, the broth medium of bacterial cultures was diluted at 1:100 in sterilized nutrient media broth. For the treatment of biofilm, all samples of ZnONPs, Lfx, and Lfx-ZnONPs were made in concentrations of 0–200 μg/ml in aqueous DMSO. The sterilized nutrient broth media sustained as blank. The 200 µl of bacterial cultures were added to wells for biofilm formation at the surface of wells. The 20 µl of all concentrations of respective samples (ZnONPs, Lfx, and Lfx-ZnONPs) were added to wells except blank. For each bacterial culture and sa,mple three replicates were kept. The microtiter plates of all samples were maintained for 24 h at 37 °C. After the period of incubation, the contents of the plates were discarded and washed three times with PBS (pH 7.2) solution to remove planktonic cells. The titer plates were rinsed further with 2% sodium acetate to enhance the adherence of biofilm to the wells of the titer plate. After the adherence of biofilm, stained with 2% (w/v) crystal violet (CV) and cleaned with deionized water. After washing with deionized water, plates were kept in an inverted position for drying. The optical density (OD) was analyzed at 570 nm wavelength. The average mean of OD was used to calculate the % of biofilm inhibition using the given formula:
2.10 Statistical analysis
Data on the antibacterial and anti-biofilm effects of ZnONPs, the drug Lfx, and the combinatorial Lfx-ZnONPs were statistically observed using the statistical tool ANOVA to test the samples effect and their dilutions on the level of antibacterial activity. The ZOI and % of biofilm inhibition of each microorganism were analyzed using one-way ANOVA. The P value obtained from the ANOVA result was 0.5, indicating a significant test. The explanatory ability of ANOVA provides effectiveness among different concentrations towards antibacterial activity. If the F calculated > F critical value, then the test was significant.
3 Results
3.1 Visual observation
When the solution mixture is heated, white flocculent appears, indicating a preliminary indication of synthesis. On incubation of the solution, white flocculants turn light yellow.
3.2 Uv–visible analysis
The production of ZnONPs was also evident from the presence of characteristic plasmon absorbance spectra. The UV–VIS spectrum revealed distinctive absorption maxima of ZnONPs at 330 nm. The UV spectra was analyzed in a quartz cuvette having a path length of 1 cm at RT. Fig. 1A and 1B depict the UV spectrum absorption at a wavelength from 200 to 800 nm. If the absorption frame switches to a lower wavelength, it depicts the decreasing size of nanoparticles (Gupta et al., 2015).
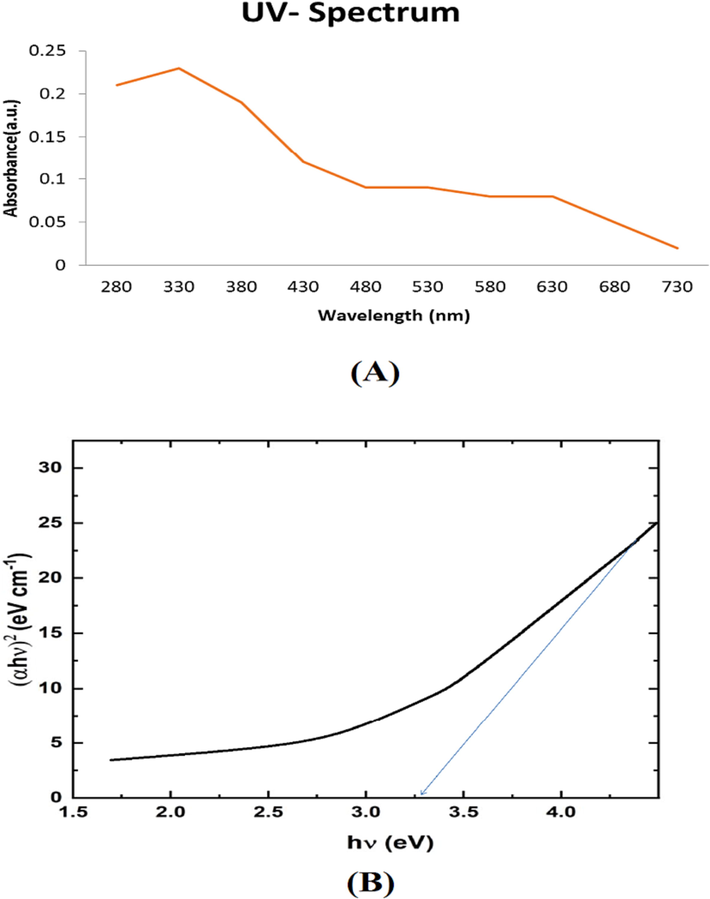
- UV spectrum (A) and the band gap (B) of ZnO nanoparticles.
3.3 FESEM and EDAX
The FESEM and EDAX were done to assess the morphology and purity of formed ZnONPs. Fig. 2 shows the FESEM of manufactured ZnONPs. FESEM images were seen in a different range of magnification ranging from 200 nm to 500 nm, which visibly validated the occurrence of sphere-shaped ZnONPs with a mean diameter of 12 nm obtained by the biosynthesis process. FESEM in combination with EDAX confirmed the synthesis of the oxide form of ZnONPs. Appearance of oxygen in the analysis confirms the construction of ZnONPs. The Fig. 3 depicts the graph of the EDAX of synthesized nanoparticles.
| Element | Series. | C norm. | C Atom. | C Error | (3 Sigma) |
|---|---|---|---|---|---|
| [wt%] | [wt.%] | [at.%] | [wt.%] | ||
| Zinc | K-series | 86.34 | 91.35 | 72.10 | 8.92 |
| Oxygen | K-series | 8.18 | 8.65 | 27.90 | 3.76 |
| Total: | 94.51 | 100.00 | 100.00 |
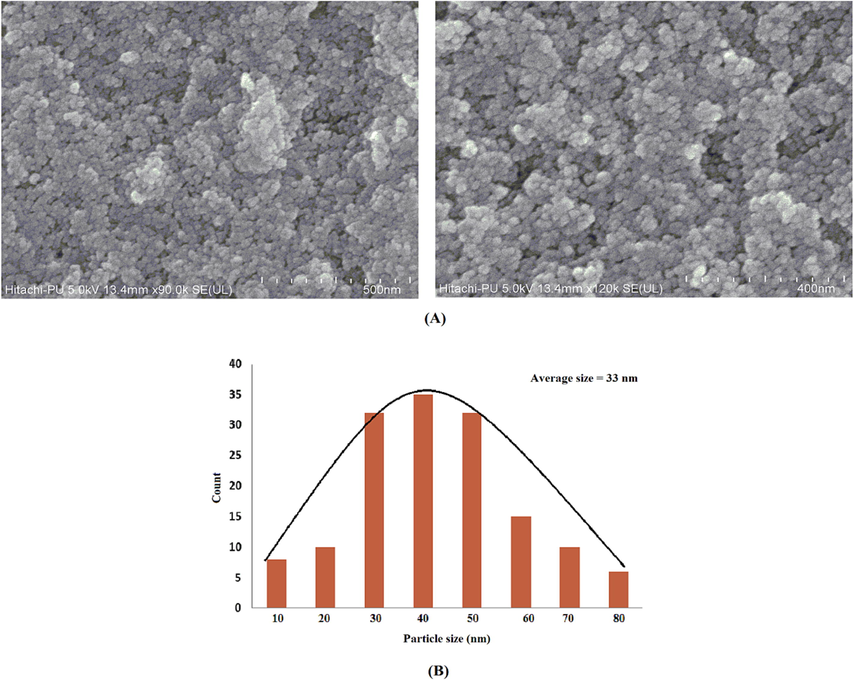
- FESEM of ZnO nanoparticles (A). Histogram of particle size distribution (B).
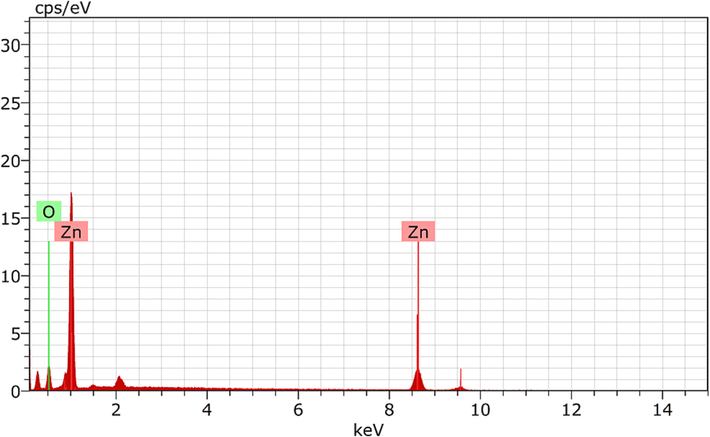
- EDAX of synthesized ZnO nanoparticles.
3.4 X-ray diffraction (XRD)
XRD spectra provide details regarding the crystallinity of nanoparticles. Fig. 4 depicts the XRD spectrum of freshly prepared ZnONPs using seeds of Glycine max. The size of nanoparticles was unwavering with the Debye-Scherrer equation. The peaks found by XRD were 31.7, 34.40, 36.20, 47.50, 56.50, 62.80, 66.80, 66.30, 67.90, and 69.0. These respective peaks relate to the lattice planes of (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 1 2), and (2 0 1), which suggests a face-centered cubic crystal structure of ZnONPs according to the Joint Committee on Powder Diffraction Standards (JCPDS). The obtained value from the XRD laboratory is shown in Table 1, which is used to obtain the crystalline size. The crystalline size obtained from XRD data was 12 nm.
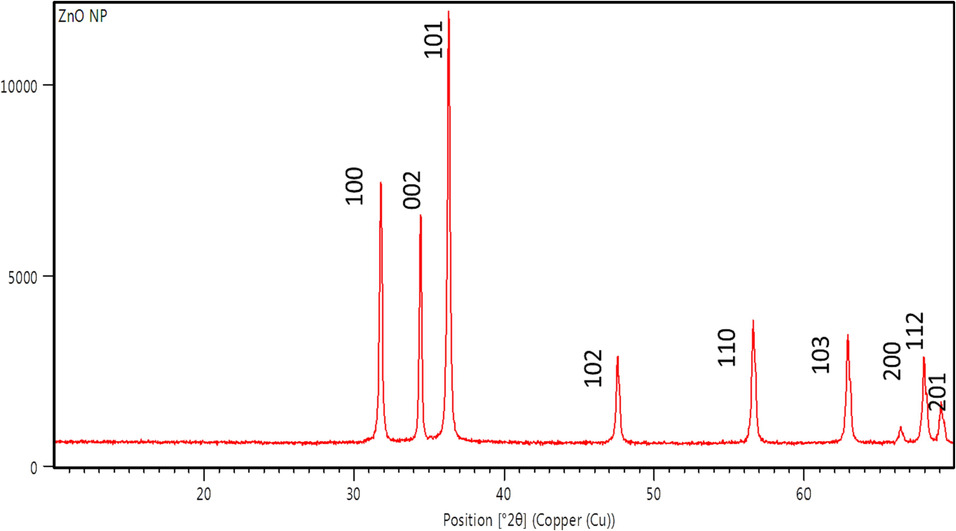
- XRD spectra of ZnO nanoparticles.
| Pos. [°2θ] | WHM Total [°2θ] | d-spacing [Å] | Rel. Int. [%] | Area [cts*°2θ] |
|---|---|---|---|---|
| 31.7681 | 0.1624 | 2.81448 | 60.26 | 1301.35 |
| 34.4204 | 0.1308 | 2.60343 | 55.27 | 981.96 |
| 36.2496 | 0.1694 | 2.47614 | 100.00 | 2284.23 |
| 47.5325 | 0.1862 | 1.91138 | 20.53 | 506.98 |
| 56.5866 | 0.2061 | 1.62515 | 29.70 | 770.84 |
| 62.8506 | 0.1985 | 1.47741 | 26.91 | 718.37 |
| 66.3699 | 0.2265 | 1.40734 | 3.78 | 112.98 |
| 67.9386 | 0.2142 | 1.37861 | 21.49 | 605.48 |
| 69.0763 | 0.2302 | 1.35866 | 9.84 | 263.42 |
Where D- particle size in nm, λ is X-ray wavelength, β is FWHM, and θ is Bragg’s angel of reflection.
3.5 Drug loading
Lfx-loaded ZnONPs were prepared by dissolving both reaction mixtures in DMSO at RT. The contents were stirred using a magnetic stirrer and incubated overnight. The standard curve was prepared using the drug Lfx to analyze the concentration of the drug in our preparation of the mixture. Our compound, Lfx, absorbs the most at 228 nm (max). The graph for Lfx (0.5–8 mg/ml) is shown in Fig. 5B. The loaded nanoparticles were settled down at 12,000 rpm for 12 min for purification. The purified, Lfx-loaded ZnONPs were also taken to the FESEM lab. The FESEM image is shown in Fig. 5A. The supernatant was used to measure the concentration of Lfx. Our test sample had an absorption wavelength of 0.191 nm. The % of loading was determined with the given formula:
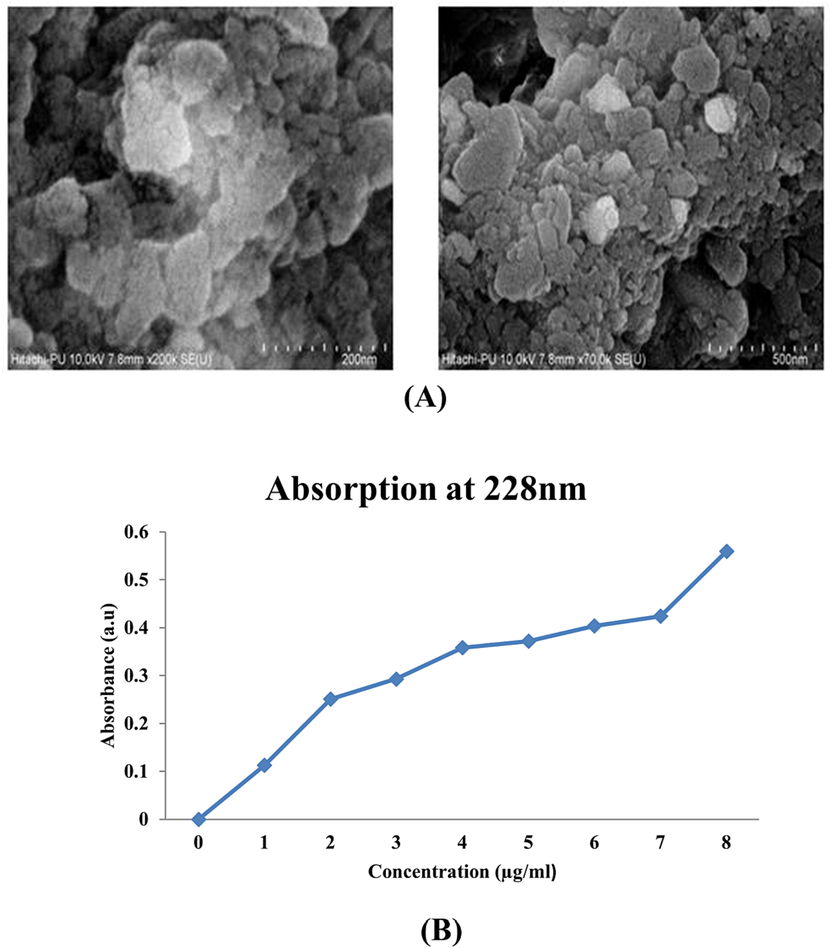
- (A) FESEM of levofloxacin-loaded ZnO nanoparticles. (B) Standard curve of levofloxacin (1–8 mg/ml).
The pellet was then lyophilized in order to dry it. The loading percentage of levofloxacin was found to be 70 % for 5 mg/ml Lfx and 1 mg/ml ZnONPs.
3.6 Antibacterial activity
The antibacterial capability of synthesized nanoparticles and drug-loaded NPs was inspected through an agar diffusion assay. The agar diffusion technique was analyzed against two gram (−ve) and gram (+ve) bacteria. The ZOI formed by microbes around the wells was measured by a zone reader. Both nanoparticles (ZnONPs), the drug levofloxacin (Lfx), and combinatorial nanoparticles (Lfx-ZnONPs) were analyzed to possess inhibitory effects on E. coli, S. aureus, P. aeruginosa, and E. faecalis, as depicted in the Table 2. The antibacterial assay results revealed that the combinatorial combination of drug and nanoparticle has the greatest inhibitory capacity against the respective species of bacteria. The maximum ZOI of 39 mm was shown by combinatorial Lfx-ZnONPs by inhibiting the growth of E. coli and P. aeruginosa. The ZnONPs and drug Lfx both demonstrated antibacterial activity against the mentioned bacteria, as depicted in Table 2.
| Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|
| Sample | Concentration (µg/ml) | Gram-positive | Gram-negative | ||
| S. aureus | E. faecalis | P. aeruginosa | E.coli | ||
| DMSO | 1:01 | 5.6 ± 0.57 | 6.3 ± 1.15 | 5.6 ± 1.52 | 7 ± 0.57 |
| ZnONPs | 20 | 10.3 ± 1.52 | 12 ± 2 | 9.3 ± 2.08 | 10.6 ± 2.08 |
| 40 | 16.3 ± 2.08 | 18.3 ± 2.51 | 16 ± 1 | 16.6 ± 1.52 | |
| 60 | 22 ± 2 | 24 ± 2 | 21 ± 2 | 22.3 ± 2.08 | |
| 80 | 24.6 ± 2.08 | 28.6 ± 1.15 | 24.3 ± 2.08 | 27 ± 3 | |
| 100 | 31 ± 2 | 30 ± 1.73 | 30 ± 2.64 | 30 ± 1.73 | |
| Lfx | 20 | 11 ± 2 | 10.3 ± 1.52 | 12.3 ± 2.88 | 10 ± 2.08 |
| 40 | 15 ± 1.73 | 15 ± 2 | 16 ± 1.73 | 16 ± 2.30 | |
| 60 | 20.3 ± 1.52 | 20.3 ± 2.08 | 20.3 ± 2.30 | 20.6 ± 1.52 | |
| 80 | 25 ± 1 | 24 ± 2 | 24.6 ± 2.51 | 23 ± 2.30 | |
| 100 | 28.3 ± 1.15 | 29 ± 2 | 29.6 ± 2.08 | 27.6 ± 2.51 | |
| Lfx-ZnONPs | 20 | 20 ± 1 | 20 ± 3 | 20.3 ± 3.75 | 19 ± 3.05 |
| 40 | 26.6 ± 2.51 | 26.6 ± 2.08 | 26.6 ± 2.30 | 26 ± 2.64 | |
| 60 | 31 ± 1.73 | 31 ± 1 | 31.6 ± 1.15 | 31 ± 1.73 | |
| 80 | 35.3 ± 2.08 | 35 ± 1.73 | 35.3 ± 1.52 | 35 ± 2.08 | |
| 100 | 38.6 ± 0.57 | 38 ± 2 | 39.3 ± 1.15 | 39 ± 1.73 | |
| n = 3 number of experiments | |||||
3.7 Anti-biofilm assay
In this study, manufactured ZnONPs and their combinatorial effects with levofloxacin were evaluated against E. coli, S. aureus, P. aeruginosa, and E. faecalis. These cultures were known for their significant biofilm generation, which causes huge problems. Therefore, the biofilm generated by these organisms was treated with a concentration ranging from 50-200 μg/ml of ZnONPs, Lfx, and combinatorial Lfx-ZnONPs. Combinatorial Lfx-ZnONPs eradication of biofilm was observed against respective microorganisms. At 200 μg/ml, the % inhibition against E. faecalis was 89.8%. The % of biofilm inhibition in all test samples was represented in Table 3.
| Test Sample | % Biofilm inhibition | ||||
|---|---|---|---|---|---|
| Concentration (µg/ml) | S. aureus | E. faecalis | P. aeruginosa | E. coli | |
| Control | Nutrient broth | 1.71 | 2.88 | 2.13 | 2.44 |
| ZnONPs | 50 | 46 | 16.48 | 19.3 | 34.04 |
| 100 | 55 | 20.53 | 20.15 | 67.7 | |
| 200 | 78 | 87.29 | 84.8 | 88.6 | |
| Levofloxacin | 50 | 20 | 17.784 | 36.8 | 21.2 |
| 100 | 76.6 | 32.05 | 45.5 | 32.4 | |
| 200 | 79 | 82.4 | 81.7 | 86.49 | |
| Lfx-ZnONPs | 50 | 20 | 7.83 | 41.2 | 86.9 |
| 100 | 40 | 46.08 | 48.1 | 87.14 | |
| 200 | 84.2 | 89.8 | 86.8 | 89.617 | |
3.8 Statistical analysis
ANOVA revealed that all three samples had significant Fisher values for their antibacterial activity as well as anti-biofilm inhibition. The determined F calculated > F critical value, which showed a significant test. Thus, a difference exists between the three groups of samples, i.e., ZnONPs, Lfx, and Lfx-ZnONPs. The Tables 4 and 5 represent statistical results of ANOVA of antibacterial and anti-biofilm activity.
| ANOVA: Single Factor ANOVA results | ||||||
|---|---|---|---|---|---|---|
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| S. aureus | 18 | 511 | 28.38889 | 11.54575 | ||
| E. faecalis | 18 | 595 | 33.05556 | 17.70261 | ||
| P. aeruginosa | 18 | 578 | 32.11111 | 27.51634 | ||
| E. coli | 18 | 567 | 31.5 | 22.85294 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 220.4861 | 3 | 73.49537 | 3.692416 | 0.015912 | 2.739502 |
| Within Groups | 1353.5 | 68 | 19.90441 | |||
| Total | 1573.986 | 71 | ||||
| ANOVA: Single Factor for Anti-biofilm Inhibition | ||||||
|---|---|---|---|---|---|---|
| SUMMARY | ||||||
| Groups | Count | Sum | Average | Variance | ||
| Column 1 | 10 | 500.51 | 50.051 | 863.8998 | ||
| Column 2 | 10 | 403.124 | 40.3124 | 1161.329 | ||
| Column 3 | 10 | 466.48 | 46.648 | 873.1556 | ||
| Column 4 | 10 | 596.527 | 59.6527 | 1129.933 | ||
| ANOVA | ||||||
| Source of Variation | SS | df | MS | F | P-value | F crit |
| Between Groups | 1954.807 | 3 | 651.6022 | 0.647022 | 0.589941 | 2.866266 |
| Within Groups | 36254.86 | 36 | 1007.079 | |||
| Total | 38209.66 | 39 | ||||
4 Discussion
Nowadays, some plant-derived extracts have been utilized for the formation of a variety of nanoparticles due to their variety of constituents and non-hazardous nature (Küünal et al., 2018). The variety of constituents present in plant extract could reduce the components to metallic ions, the most probable mechanism known for the development of nanoparticles (Bao et al., 2021). In this case, the extract from the seeds of soybeans is utilized to form ZnONPs by zinc salt reduction. The construction of ZnONPs was first validated by visual observation and UV spectroscopy. The color of reaction mixture was transformed upon heating and incubation, confirming the creation of ZnONPs (Naseer et al., 2020). The maximum absorption peak observed by synthesis via an extract of Glycine max seeds was 330 nm. This result satisfies the standard ZnO absorption pattern (Naseer et al., 2020). The morphology and crystalline structure were observed using FESEM and XRD. FESEM provides information regarding the shape that was observed to be sphere-shaped with 12 nm in size. FESEM in combinatorial analysis with EDAX confirmed the presence of oxygen in zinc nanoparticles, which confirms the oxide form of nanoparticles. According to JCPDS (Naseer et al., 2020), the XRD peaks at 31.7o, 34.40, 36.20, 47.50, 56.50, 62.80, 66.80, 66.30, 67.90, and 69.00 corresponded to the cubic crystal structure that reflects face-centered conformation of ZnONPs.
The spherical-shaped ZnONPs were successfully loaded with the drug Lfx (a fluoroquinolone). It was applicable to cure diverse microbial infections, such as urinary tract infections (UTI), pneumonia, bacterial sinusitis, gastroenteritis, and many more. It has serious, unfavorable side effects that necessitate medical attention. Various species, such as E. coli and K. pneumonia, had developed resistance as a result of multiple uses or first-line treatment for various bacterial infections. In some countries, the resistance of levofloxacin to pseudomonas and staphylococcus was more common (Saleem et al., 2019). To overcome the resistance, the effort was strongly recommended. In our case, Lfx was added to ZnONPs as a companion for anti-bacterial properties. In our view, the adsorption of Lfx on ZnONPs might reduce the dosage or have adverse side effects. The adsorption was confirmed by using UV spectroscopy and seen in the FESEM image.
Nowadays, bacterial infections are becoming resistant to antibiotics, causing health problems and causing thousands of deaths each year. ZnONPs proved significant because of diverse advanced applications. The development of novel modes of action and improvements in bacterial enzyme resistance increased the efficiency of resistant bacterial infections via improved drug delivery. Several reports have evidenced the bacterial killing property of ZnONPs (Agarwal et al., 2018). Recently, ZnONPs were synthesized from palm leaves of Phoenix roebelenii extract for the first time, which exhibit antibacterial properties on gram (+ve) (S. aureus, S. pneumonia) and gram (−ve) (E. coli, S. typhi) bacteria (Aldeen et al., 2022). The ZnONPs manufactured from Salvadora persica leaf extract were 25–40 nm in size and observed that both Ag-coated ZnONPs and only ZnONPs had antibacterial effects against E. coli and S. aureus (Akbarizadeh et al., 2022). In recent research, ZnONPs were synthesised using orange peel, and these nanoparticles have shown potent bactericidal properties against P. aeruginosa, Bacillus subtilis, and S. aureus (Menazea et al., 2021). The ZnONPs from an extract of Cassia auriculata leaves were synthesized by Ramesh et al., 2021. It was analyzed that the antimicrobial effect of ZnONPs on P. aeruginosa, B. subtilis, Proteus mirabilis, and K. pneumonia, and proposed the coating of food materials with these ZnONPs could prevent bacterial contamination (Ramesh et al., 2021). The ZnONPs made utilizing the leaves extract of Acalypha indica were 16 nm in diameter and spherical. These nanoparticles have shown antimicrobial functions against E. coli, S. aureus, B. subtilis, and P. aeruginosa. The maximum ZOI was 25.2 nm against E. coli (Kamarajan et al., 2022). Another approach to kill multidrug resistant bacteria by eradicating biofilm from any surface and that was difficult for healthcare workers due to the inability of drugs to reach bacterial cells through matrix (Pinho et al., 2022). Therefore, ZnONPs in combination with levofloxacin were highly recommended for the eradication of biofilm. Numerous reports confirmed biofilm inhibition using ZnONPs. The ZnONPs made from extract of Plumbago zeylanica impaired the formation of biofilm against E.coli, S. aureus, and P. aeruginosa by 62.8 %, 71.5 %, and 77.6 %, respectively (Husain et al., 2022). The ZnONPs produced by use of Ruellia tuberosa also inhibited biofilm made by Serratia marcescens (Pugazhendhi et al., 2022). In the present research, for the first time, ZnONPs were synthesised using Glycine max seeds. The manufactured ZnONPs were identified using UV spectroscopy, XRD, FESEM, and EDAX. These nanoparticles showed characteristic peaks corresponding to standard ZnONPs. These nanoparticles were loaded with the drug levofloxacin to enhance its antibacterial activity and anti-biofilm inhibition. It was clear from our results that the bactericidal property of combinatorial Lfx-ZnONPs was higher than that of levofloxacin alone against both gram (−ve) and gram (+ve) strains of bacteria. To conclude, antibacterial function and anti-biofilm inhibition tests indicate that the administration of levofloxacin on ZnONPs advances their biocidal property.
5 Conclusion
ZnONPs have gained much attention as a drug delivery system to treat pathogenic infections. The green synthetic approach for the preparation of ZnONPs is nontoxic, economical, environment-friendly, reliable, and low-resource requiring process compared to chemical synthesis. In summary, we have synthesized and characterized the different characteristics of ZnONPs and Lfx-loaded ZnONPs. The antimicrobial and anti-biofilm effects were evaluated on gram (+ve) and gram (−ve) bacteria in this study. The result of our study indicates that ZnONPs and Lfx-loaded ZnONPs have good physicochemical properties. The antibacterial and anti-biofilm effects of Lfx-loaded ZnONPs were more effective compared to the standard drug levofloxacin and ZnONPs. Among the four bacterial strains, the most effective anti-bacterial effect was observed at 100 µg/ml concentration of Lfx-loaded ZnONPs in P. aeruginosa and the least in E. faecalis. Likewise, the most efficient biofilm inhibitory effect of Lfx-loaded ZnONPs was analyzed in E. faecalis and the least in S. aureus.
The outcomes of this study confirm that Lfx-loaded ZnONPs, produced by the green synthetic approach from soybean seed extract, could be applicable as a new antimicrobial and anti-biofilm agent for the eradication of pathogenic infections. The use of these Lfx-loaded ZnONPs may help to reduce antibiotic resistance. This research can be scaled up to produce ZnONPs with levofloxacin, which increases levofloxacin's effectiveness and could combat antibiotic resistance. The combinatorial effects of ZnONPs and levofloxacin can be further explored for in vivo testing as nanomedicine in various diseases. These synthesized nanoparticles could be investigated further for their biocompatibility across the blood–brain barrier for effective delivery in the brain and other applications in human and health welfare.
Author contributions
All co-authors have contributed to this work and are aware of this submission.
Disclosure of funding
The research was funded by Mendel University in Brno, 61300 Brno, Czech Republic.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact.. 2018;286:60-70.
- [Google Scholar]
- Study of antibacterial performance of biosynthesized pure and Ag-doped ZnO nanoparticles. Rendiconti Lincei. Scienze Fisiche e Naturali. 2022;33(3):613-621.
- [Google Scholar]
- ZnO nanoparticles prepared via a green synthesis approach: physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solid. 2022;160:110313
- [Google Scholar]
- Novel biocompatible green silver nanoparticles efficiently eliminates multidrug resistant nosocomial pathogens and mycobacterium species. Indian J. Microbiol. 2023:1-11.
- [Google Scholar]
- Musa balbisiana Colla pseudostem biowaste mediated zinc oxide nanoparticles: their antibiofilm and antibacterial potentiality. Curr. Res. Green Sustain. Chem.. 2021;4:100048
- [Google Scholar]
- Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: a review. J. Phys.: Mater.. 2020;3(3):034005
- [Google Scholar]
- Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci.. 2016;23(4):517-523.
- [Google Scholar]
- Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Process. Synth.. 2020;9(1):304-339.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resour.-Effic. Technol.. 2017;3(3):280-284.
- [Google Scholar]
- Comparison of physical and electrochemical properties of ZnO prepared via different surfactant-assisted precipitation routes. Appl. Nanosci.. 2015;5(7):787-794.
- [Google Scholar]
- Biosynthesized zinc oxide nanoparticles disrupt established biofilms of pathogenic bacteria. Appl. Sci.. 2022;12(2):710.
- [Google Scholar]
- In-vitro detection of phytopathogenic fungal cell wall by polyclonal sera raised against trimethyl chitosan nanoparticles. Int. J. Nanomed. 2019:10023-10033.
- [Google Scholar]
- A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018
- [Google Scholar]
- Green synthesis of ZnO nanoparticles using Acalypha indica leaf extract and their photocatalyst degradation and antibacterial activity. J. Indian Chem. Soc.. 2022;99(10):100695
- [Google Scholar]
- Antibacterial activity of Zinc Oxide nanostructured materials synthesis by laser ablation method. J. Phys. Conf. Ser. 2021
- [Google Scholar]
- Plant extract mediated synthesis of nanoparticles. In: Emerging Applications of Nanoparticles and Architecture Nanostructures. Elsevier; 2018. p. :411-446.
- [Google Scholar]
- Novel green synthesis of zinc oxide nanoparticles using orange waste and its thermal and antibacterial activity. J. Inorg. Organomet. Polym Mater.. 2021;31(11):4250-4259.
- [Google Scholar]
- Diagnosis and Treatment of Diseases by Using Metallic Nanoparticles – A Review. Authorea Preprints; 2022.
- Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep.. 2020;10(1):1-10.
- [Google Scholar]
- Green synthesis and characterization of zinc oxide nanoparticles from Neem (Azadirachta indicia) Int. J. Sci. Eng. Technol. Res.. 2015;4(30):5751-5753.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoparticles using Moringa oleifera leaf extract: investigation of photocatalytic and antibacterial activity. Int. J. Nanosci. Nanotechnol.. 2018;14(2):111-119.
- [Google Scholar]
- Influence of particle size of nano zinc oxide on the controlled delivery of Amoxicillin. Appl. Nanosci.. 2013;3(5):441-451.
- [Google Scholar]
- Helicobacter pylori biofilms are disrupted by nanostructured lipid carriers: a path to eradication? J. Control. Release. 2022;348:489-498.
- [Google Scholar]
- In vitro efficacy of green synthesized ZnO nanoparticles against biofilm and virulence of Serratia marcescens. Prog. Org. Coat.. 2022;166:106781
- [Google Scholar]
- Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens. Bio-Sens. Res.. 2021;31:100399
- [Google Scholar]
- Microscopic characterization of bioaccumulated aluminium nanoparticles in simplified food chain of aquatic ecosystem. J. King Saud Univ.-Sci.. 2022;34(1):101666
- [Google Scholar]
- Point prevalence surveys of health-care-associated infections: a systematic review. Pathogens Global Health. 2019;113(4):191-205.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoparticles using leaf extract of Camellia sinesis and evaluation of their antimicrobial efficacy. Int. J. Curr. Microbiol. App. Sci.. 2015;4(8):444-450.
- [Google Scholar]
- Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, ampicillin, fluconazole and amphotericin B against pathogenic microorganisms. Anais da Academia Brasileira de Ciências. 2016;88:1689-1698.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using seed extract of Murraya koenigii and their antimicrobial activity against some human pathogens. J. Nanosci. Technol. 2017:289-292.
- [Google Scholar]







