Translate this page into:
Synchronization of arbuscular mycorrhizae fungi inoculation with different zinc application methods for improvement in BASMATI rice growth and yield in alkaline calcareous soil
⁎Corresponding authors at: Department of Soil Science, Bahauddin Zakariya University, Multan, Punjab 60800, Pakistan (S. Danish and M. Arif Ali); Department of Geology and Pedology, Faculty of Forestry and Wood Technology, Mendel University in Brno, Zemedelska1, 61300 Brno, Czech Republic (R. Datta). arif1056@bzu.edu.pk (Muhammad Arif Ali), rahulmedcure@gmail.com (Rahul Datta), sd96850@gmail.com (Subhan Danish)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Management of zinc (Zn) in calcareous soils is a major problem for higher rice growth and yield. Need of time is to introduce environmentally friendly approach for the management of Zn. Inoculation of arbuscular mycorrhizae fungi (AMF) is one of such efficacious technique for enhancing the availability of Zn in these soils. The present study investigated the effect of different Zn application methods i.e., seed coating (SC; 1.25 g Zn/kg seed), seed priming (SP; 0.5 M solution) and soil application (SA; 4 mg ZnSO4/kg soil) alone or in combination with AMF applied in completely randomized design (CRD) on morpho-physiological growth and productivity of rice. Results showed that Zn SA + AMF remained significantly the best treatment for the improvement in germination, plant height, spike length and number of spikes than control + AMF and control with no AMF. A significant enhancement in 1000 grains weight (71.11%), total chlorophyll (40.38%), photosynthetic rate (16.17%) and transpiration rate (41.48%) validated the efficacious role of Zn SA + AMF over control + AMF. Significant increase in rice grains N (25.68 and 40.11%), P (29.41 and 25.00%), K (42.86 and 47.37%) and Zn (10.42 and 59.03%) signified the imperative functioning of Zn SA + AMF and Zn SA over control + AMF and control without AMF respectively. In conclusion, Zn SA has the potential to improve the rice growth nutrients uptake. However, Zn SA + AMF is better to approach than the sole application of Zn SA for rice. More investigations at the field level under different soil textures are suggested to declare Zn SA + AMF as the best treatment for improvement in the productivity of rice.
Keywords
Symbiosis
Micronutrient
Fertilization methods
Oryza sativa L.
Nutrients uptake
Gas exchange attributes
1 Introduction
The deficiency of zinc is one of the major problems in the arid and semi-arid areas of the world (Akça et al., 2022; Saboor et al., 2021a; Sher et al., 2020). The presence of optimum Zn in soil ranges from 10 to 300 mg kg−1. However, most of Zn is present in the forms of complexes with organic matter (OM) and soil collides. That’s why a small fraction of Zn is normally available for crops (Hussain et al., 2020; Raza et al., 2021; Saboor et al., 2021a).
High soil pH in arid and semi-arid areas immobilizes Zn in soil (He et al., 2021), and decrease its availability to plants (Saboor et al., 2021b). It has also been observed that the imbalance application of phosphorus caused the formation of Zn-P complex in soil. This Zn-P is insoluble in water and thus decrease Zn uptake in the plants (Bibi et al., 2020). High CaCO3 and low organic matter are other allied factors that also decrease the availability of Zn to the plants (Iratkar et al., 2014). Nevertheless, the balanced uptake of Zn not only enhance the production of the crop but also improve the quality (Rehman et al., 1999; Shivay et al., 2005; Stino et al., 2011). In biochemical processes, it is not only a structural component, but it also acts as a cofactor for the proper functioning of many enzymes (Alloway, 2008). Some of the important Zn regulated processes in plants include photosynthesis, protein metabolism, carbohydrate metabolism, pollen formation, membrane integrity and auxin metabolism (Alloway, 2008; Hassan et al., 2020). However, deficiency of Zn usually resulted in disturbance of plants physiological functioning which can be tackled through development of symbiosis (Jabborova et al., 2020; Luh Suriani et al., 2020; Parasuraman et al., 2020) i.e., plant roots – beneficial microorganism relationship development (Ahmed et al., 2021; Jabborova et al., 2021; Kapadia et al., 2021; Sadeghzadeh, 2013).
In recent years, research focus has also been given on the management of Zn through synchronization of application methods with the rate of application (Saboor et al., 2021b, Saboor et al., 2021a, Saboor et al., 2021c). There are four major zinc application methods which include soil application, foliar, seed priming and seed coating (Tondey et al., 2021; Zajaczkowska et al., 2020). Both seed priming and seeds coating have been known to play an imperative role in the provision of Zn at the earlier seedling stage. It has been observed that the provision of nutrients by seed coating or priming not only improve the germination rate but also enhance the vigour of seedlings (Zajaczkowska et al., 2020). Soil application is a conventional method of nutrient application. It not only provides nutrients to the crops but also fulfils the deficient status of nutrients in the soil. Similarly, many studies also showed that foliar application of micronutrients to plants is more economic and efficacious. It significantly improves the productivity of the crop but also decreases the loss of nutrients comparative to soil application (Brennan, 1991; Rafie et al., 2017; Rehman et al., 1999; Zajaczkowska et al., 2020).
On the other hand, inoculation of arbuscular mycorrhizae fungi (AMF) is mostly recommended in this regard (Adesemoye et al., 2008; Saboor et al., 2021b; Saboor et al., 2021a). The AMF facilitates the uptake of nutrients and water in plants. Extraradical hyphae formed due to plant and AMF symbiosis increase the root elongation. This increase in rhizosphere area helps plants in obtaining nutrients away from the rhizosphere (Smith and Read, 2008).
Therefore, to cover this knowledge gap regarding the best application methods for Zn in the presence and absence of AMF, the current study was planned. The aim of the study was the assessment of different Zn application methods i.e., seed coating, seed priming and soil application on rice growth in the presence and absence of AMF. There were 2 hypotheses proposed and assessed in the current study. 1. Soil application may be a better approach compared to seed coating and seed priming. Inoculation of AMF with soil application of Zn may be a better strategy to improve rice cultivation over sole inoculation of AMF or Zn application.
2 Material and methodology
2.1 Experimental site and design
A pot experiment (natural open air conditions) was done in the research area of the Department of Soil Science (71.43° E, 30.2° N and 122 m above sea level), Bahauddin Zakariya University Multan. The experiment was laid out as a completely randomized design (CRD) under two factorial arrangements of treatments.
2.2 Treatment plan
There were 8 treatments and 3 replications. Three Zn application methods were tested including seed priming, seed coating and soil application with and without AMF. The treatment plan includes control, Zn seed priming (Zn SP = 0.5 Molar solution of Zn with 1:5 seed to solution ratio), Zn seed coating (Zn SC = 1.25-gram Zn/kg seed), Zn soil application (Zn SA = 4 mg ZnSO4/kg soil), AMF, Zn SP + AMF, Zn SC + AMF and Zn SA + AMF.
2.3 Pots preparation and soil characterization
Clay pots with dimensions 60 cm depth and 45 cm width were used in the experiment. The soil was collected from the experimental site and sieved from a 2 mm sieve to remove residues and stones. After 8 kg soil was filled in each pot. A composite sample was also taken for the characterization of soil attributes. The texture was analyzed by using a hydrometer. After the determination of sand, silt and clay, the textural triangle of USDA was used for the determination of final soil texture (Bouyouces, 1962). For determination of pH, 1:1 ratio soil and deionized water were mixed for paste formation. After that pH was computed by using a pre-calibrated pH meter (Page et al., 1983). For EC, soil and distilled water were mixed in 1:10 ratio. Extraction was done and then EC of the extract was noted on pre-calibrated (1/100 N KCl) EC meter (Rhoades, 1996). Potassium dichromate and ferrous ammonium sulphate were used for the assessment of soil organic matter according to (Sparks et al., 1996). Digestion of soil sample was done at 380 ◦C on the hot plate for the examination of total nitrogen in the soil (Bremner, 1996). For analysis of available phosphorus, Olsen extraction was performed, and final P was computed on a spectrophotometer at 880 nm wavelength (Kuo, 1996). Potassium was examined in the ammonium acetate soil extract on a flame photometer by following the protocol of (Pratt, 1965). For analysis of Zn in soil, DTPA extract was done. The final analysis was performed on atomic absorption spectrophotometer (AAS) according to (Estefan et al., 2013). The pre-experimental soil attributes are provided in Table 1. Values are showing means of three replicates ± SE. Different letters are showing significant change computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Attributes
Units
Values
Sand
%
55
Silt
15
Clay
30
Texture
Sandy Clay Loam
pHs
–
8.41
ECe
dS/m
4.22
Organic matter
%
0.40
Total Nitrogen
%
0.02
Available phosphorus
mg kg−1
4.33
Extractable potassium
151
Extractable zinc
0.19
Inoculation
Zinc
Total Chlorophyll (mg g−1)
Electrolyte Leakage (%)
Mean
SE
Labelling
Mean
SE
Labelling
AMF
Control
0.52
0.009
Bcd
20.67
0.34
c
AMF
Zn SP
0.52
0.012
Bcd
13.68
0.66
f
AMF
Zn SA
0.73
0.012
A
10.68
0.34
g
AMF
Zn SC
0.60
0.006
Ab
15.68
0.34
e
No AMF
Control
0.30
0.058
E
30.70
0.35
a
No AMF
Zn SP
0.45
0.012
Cd
22.67
0.34
b
No AMF
Zn SA
0.40
0.115
De
14.39
0.31
f
No AMF
Zn SC
0.54
0.012
Be
17.36
0.32
d
ECe = EC of soil extract.
2.4 Seeds purchasing and nursery development
Seeds of SUPER BASMATI rice variety was purchased from the certified seed dealer of the government of Punjab, Pakistan. The nursery was planted for 25 days. The purpose of establishing the rice nursery was to develop AMF association/colonization with rice roots before transplanting. Seedlings were ready to transplant in the pots after 25 days of germination.
2.5 AMF inoculation
During pot filling, 10 kg soil was inoculated with 2.5 g mycorrhizal inoculum Clonex® (Root Maximizer; 5711 Enterprise Drive, Lansing, MI, USA). The commercial product has 158 propagule gram−1. Glomus species were major constituents of the product. At the time of transplantation, AMF inoculation was again applied to the seedling in treatment with AMF application for maximum colonization.
2.6 Zinc priming and coating
In priming of Zn, seeds of rice were soaked in an aerated solution of zinc sulphate (ZnSO4) (0.5 M Zn solution) for 12 h. The seeds to solution ratio for soaking will be kept at 1:5 (w/v). After soaking, seeds will be re-dried to their original weight and will be stored in the refrigerator at 4 °C in plastic sealed polythene bags until used for sowing (Keshri et al., 2017). In the coating of the seeds with Zn, the sticky slurry will be prepared with inert Arabic gum in distilled water. Zinc sulphate at the rate of 1.25 g Zn kg−1 of rice seed will be used in the slurry for coating. Bags of polythene will be used to store the air-dried coated seed in the refrigerator at 4 °C until used for sowing (Rehman and Farooq, 2016). For soil application, Zn will be directly applied to the soil at the rate of 4 mg ZnSO4 kg−1 of soil.
2.7 Fertilizer application and irrigation
In each pot, macronutrients i.e., N, P and K were added at the rate of 0.84, 0.54 and 0.36 g as urea, sulfate of potash (SOP) and diammonium phosphate (DAP). For N application, the addition of fertilizer was done in 3 splits as half at the time of sowing, while half remaining in 2 splits, at tillering and spike initiation (Rehman et al., 2020). In each pot, 100% FC of water was maintained throughout the experiment (Wang et al., 2008).
2.8 Gas exchange attributes
Gas exchange attributes i.e., photosynthetic rate, transpiration rate and stomatal conductance were determined by utilizing IRGA [CI-340 Photosynthesis system, CID, Inc. USA] as described by Danish and Zafar-ul-Hye (2019). The readings were collected in 40 days old rice plants on a sunny day with an intensity of light saturation between 10:33 and 11:10 AM.
2.9 Chlorophyll contents and electrolyte leakage
Determination of chlorophyll was done according to the method of Arnon (1949). The extract was collected from leaves using 80% acetone. For assessment of chlorophyll a and chlorophyll b, the absorbance was taken at 663 and 645 nm wavelength.
OD = Optical density (wavelength); V = Final volume made; W = Fresh leaf weight (g).
Electrolyte leakage (EL) was computed according to the methodology of (Saeed et al., 2014). The leaves samples were washed and then cut into 1 cm diameter. Uniform sized leaf pieces of 1 g were immersed in a test tube containing deionized water (20 ml) and incubated at 25 °C for 24 h. After that electrical conductivity (EC1) was noted using EC meter. The second EC (EC2) was noted heating the test tubes in a water bath at 120 °C for 20 min. The final value of EL was calculated using the equation.
2.10 Yield and agronomic traits
Plants were harvested manually at the time of maturity. Morphological attributes i.e., plant height, number of spikes and spike length were computed soon after harvesting of the crop. Straw and grains were separated manually. For 1000 grains weight analytical grade electrical balance was used.
2.10.1 Nutrient accumulation
Nitrogen in grains and straw was assessed by digestion of sample with H2SO4 at 400 °C on the hot plate. After digestion distillation was done on Kjeldhal’s distillation apparatus (Chapman and Pratt, 1961; Donald and Miller, 1998). For phosphorus (P) and potassium (K) in grains and straw, digestion was done with the di-acid mixture (HNO3:HClO4 = 2:1) (Miller, 1998). The yellow colour method was used for the final examination of P on the spectrophotometer (Estefan et al., 2013) while a flamephotometer was used for K determination (Donald and Hanson, 1998). Atomic absorption spectrophotometer was used for the final assessment of Zn in the digested samples (Seregin et al., 2011).
2.11 Statistical analysis
The standard statistical procedure was adopted for statistical analysis (Steel et al., 1997). Two factorial ANOVA was applied for the assessment of treatments significant. A comparison of each treatment was made by applying the Fisher LSD test (p ≤ 0.05). OriginPro2021 was used for the application of statistics. Pearson correlation was also computed, and probability graphs were made by using OriginPro2021 (OriginLab Corporation, 2021).
3 Results
Results showed that the effects of zinc (Zn) and AMF were significant on AMF colonization of roots in rice. Application of Zn SA + AMF and Zn SC + AMF differed significantly over control + AMF for AMF colonization of roots in rice. No significant change was noted in AMF colonization of roots in rice in Zn SP + AMF than control + AMF. A significant enhancement in AMF colonization of roots in rice was observed in Zn SP, Zn SA and Zn SC compared to control without AMF. Zn SA showed significantly better AMF colonization of roots in rice than Zn SP and Zn SC (Fig. 1). A maximum increase of 9.8 and 32.1% AMF colonization of roots in rice was noted in Zn SA + AMF and Zn SA compared to with and without AMF control respectively.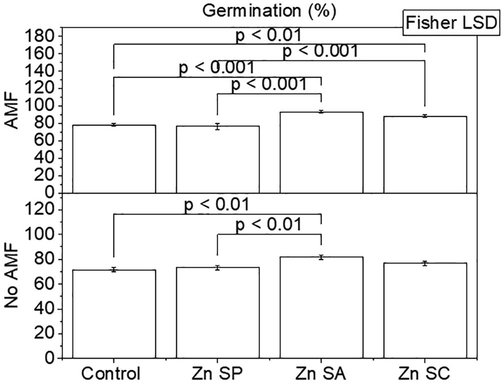
Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on rice seeds germination. Bars are showing means of three replicates ± SE. Different values on bars are showing p-values computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Influences of Zn and AMF were significant on rice seeds germination. Treatments Zn SA + AMF and Zn SC + AMF remained significantly different compared to control + AMF for AMF colonization of roots in rice. A significant difference was also observed in rice seeds germination in Zn SP + AMF over control + AMF. Rice seeds germination was significantly better in Zn SP, ZnSA and Zn SC than in control without AMF. Zn SA differed significantly better for improvement in rice seeds germination than Zn SP and Zn SC (Fig. 2). A maximum increase of 19.2 and 14.1% in rice seeds germination was noted in Zn SA + AMF and Zn SA compared to with and without AMF control respectively.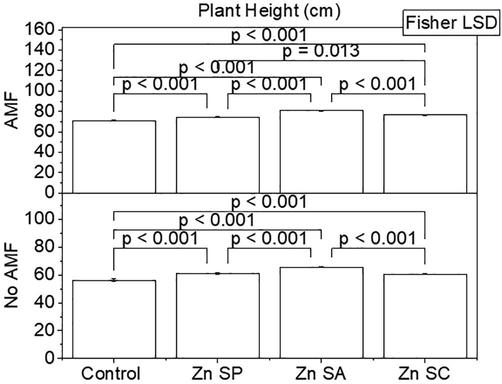
Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on rice plant height. Bars are showing means of three replicates ± SE. Different values on bars are showing p-values computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Zn and AMF were significantly different for rice plant height. Plant height was significantly higher in Zn SA + AMF, Zn SP + AMF and Zn SC + AMF than control + AMF. It was also observed that Zn SP, Zn SA and Zn SC remained significantly different from the control without AMF. Zn SA performance was significantly best for enhancement in plant height over Zn SP and Zn SC (Fig. 3). A maximum increase of 13.6 and 16.57% in plant height was noted where Zn SA + AMF and Zn SA was applied over with and without AMF control respectively.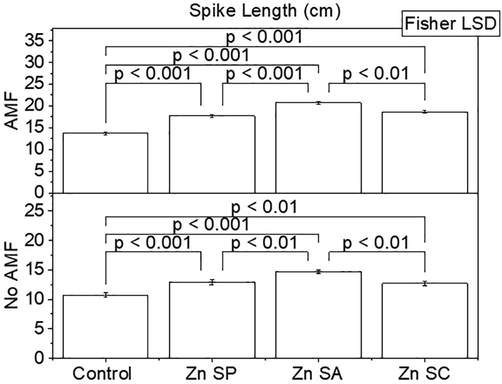
Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on rice spike length. Bars are showing means of three replicates ± SE. Different values on bars are showing p-values computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
A significant change was noted in spike length by application of Zn and AMF in rice plants. Results showed that spike length was significantly higher in Zn SA + AMF, Zn SP + AMF and Zn SC + AMF compared to control + AMF. Application of Zn SP, Zn SA and Zn SC differed significantly better over control without AMF. The addition of Zn SA was significantly better for an increase in spike length of rice plants than Zn SP and Zn SC (Fig. 4). A maximum increase of 51.13 and 37.19% in spike length was noted where Zn SP + AMF and Zn SA was applied over with and without AMF control respectively.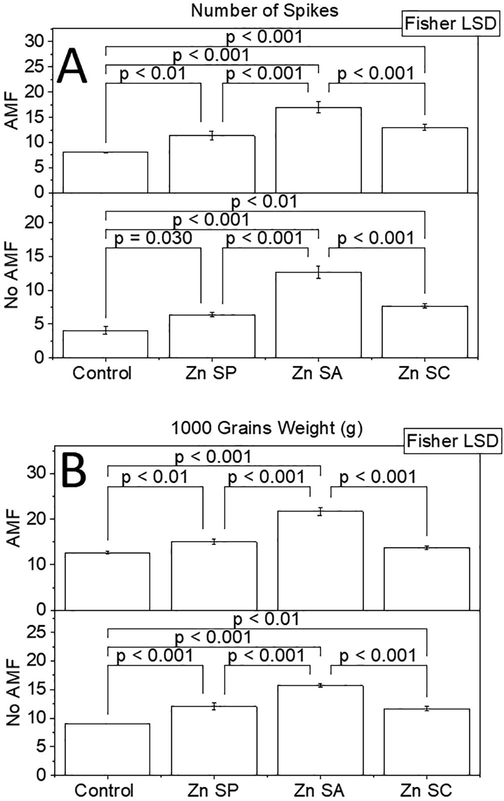
Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on rice number of spikes. Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on rice 1000 grains weight (A). Different values on bars are showing p-values computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating (B).
Number of spikes was significantly changed by Zn and AMF application in rice plants. Number of spikes remained significantly higher in Zn SA + AMF, Zn SP + AMF and Zn SC + AMF than control + AMF. The addition of Zn SP, Zn SA and Zn SC caused a significant increase in number of spikes over control without AMF. Treatment Zn SA remained significantly better for an increase in number of spikes of rice plants compared to Zn SP and Zn SC. No significant change in number of spikes was noted between Zn SP and Zn SC with and without AMF (Fig. 5).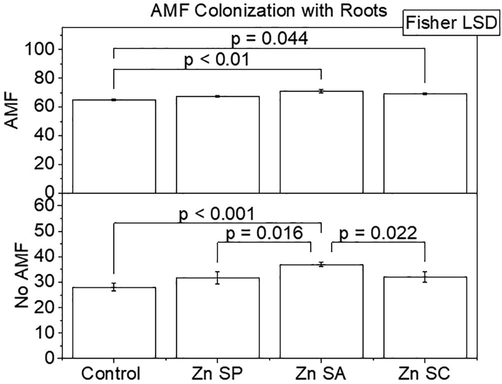
Effect of zinc seed priming, seed coating and soil application with and without AMF inoculation on AMF colonization (%) with rice roots. Bars are showing means of three replicates ± SE. Different values on bars are showing p-values computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Application of Zn and AMF treatments significantly enhanced 1000 grains weight of rice. Compared to control + AMF, 1000 grains weight was significantly enhanced in Zn SA + AMF, Zn SP + AMF and Zn SC + AMF. Over control without AMF, Zn SP, Zn SA and Zn SC performed significantly better for improvement in 1000 grains weight. Among treatments, Zn SA caused a more significant increase in 1000 grains weight of rice than Zn SP and Zn SC (Fig. 5). A maximum increase of 71.11 and 71.04% in 1000 grains weight was noted in Zn SA + AMF and Zn SA treatments than control with and without AMF respectively.
Results showed that the effect of Zn and AMF was significant on total chlorophyll and electrolyte leakage in rice leaves. Compared to control + AMF, total chlorophyll was significantly enhanced in Zn SA + AMF. No significant change was noted in total chlorophyll where Zn SP + AMF and Zn SC + AMF were applied over control with AMF. Among treatments, Zn SA caused a more significant increase in total chlorophyll than Zn SP. However, Zn SC + AMF and Zn SA + AMF remained statistically alike to each other for change in total chlorophyll in rice leaves (Table 1). It was noted that treatment Zn SP and Zn SC caused a significant increase in total chlorophyll over control without AMF. No significant change was observed in total chlorophyll of Zn SA and control without AMF. A maximum increase of 40.38 and 80.00% in total chlorophyll was noted in Zn SP + AMF and Zn SC treatments than control with and without AMF respectively. For electrolyte leakage, treatments Zn and AMF caused a significant decrease. Over control + AMF, electrolyte leakage was significantly decreased in Zn SA + AMF, Zn SP + AMF and Zn SC + AMF. Compared to control without AMF, Zn SP, Zn SA and Zn SC performed significantly better for minimization in electrolyte leakage. Among treatments, Zn SA caused a more significant decrease in electrolyte leakage than Zn SP and Zn SC (Table 1). A maximum decrease of 48.33 and 53.12% in electrolyte leakage was noted in Zn SA + AMF and Zn SA treatments than control with and without AMF respectively.
Treatments Zn and AMF significantly changed photosynthetic rate, transpiration rate and gas exchange attributes in rice leaves. The photosynthetic rate was significantly high in Zn SA + AMF over control + AMF. No significant change was noted in the photosynthetic rate was observed where Zn SP + AMF and Zn SC + AMF were applied compared to control with AMF (Table 2). It was noted that treatment Zn SP and Zn SC caused significant improvement in photosynthetic rate than the control without AMF. A significant change was observed in photosynthetic rate was noted in Zn SA and control without AMF. A maximum increase of 16.17 and 39.82% in photosynthetic rate was noted in Zn SA + AMF and Zn SA treatments than control with and without AMF respectively. For transpiration rate, treatments Zn and AMF caused significant enhancement. Over control + AMF, transpiration rate was significantly decreased in Zn SA + AMF and Zn SC + AMF. Treatments Zn SP, Zn SA and Zn SC performed significantly better for an increase in transpiration rate than the control without AMF. Among treatments, Zn SA caused a more significant increase in transpiration rate than Zn SP and Zn SC (Table 2). Maximum improvement of 41.48 and 58.61% in transpiration rate was noted in Zn SA + AMF and Zn SA treatments over control with and without AMF respectively. In the case of stomatal conductance, Zn SA + AMF, Zn SP + AMF and Zn SC + AMF caused significant improvement over control + AMF. Treatments Zn SA and Zn SC different significantly better than the control without AMF for stomatal conductance. No significant change was noted in stomatal conductance where control without AMF and Zn SP were applied (Table 2). Values are showing means of three replicates ± SE. Different letters are showing significant change computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Inoculation
Zinc
Photosynthetic Rate (µmol CO2/m2/s)
Transpiration Rate (mmol H2O/m2/s)
Stomatal Conductance (µmol CO2/m2/s)
Mean
SE
Labelling
Mean
SE
Labelling
Mean
SE
Labelling
AMF
No-Zn
5.69
0.34
b
5.81
0.01
c
0.06
0.009
c
AMF
Zn SP
5.71
0.35
b
6.11
0.01
bc
0.11
0.006
b
AMF
Zn SA
6.61
0.06
a
8.22
0.01
a
0.16
0.009
a
AMF
Zn SC
5.42
0.05
bc
7.62
0.01
a
0.13
0.009
b
No AMF
Control
3.44
0.03
e
4.18
0.04
e
0.04
0.006
c
No AMF
Zn SP
4.37
0.08
d
5.00
0.58
d
0.06
0.015
c
No AMF
Zn SA
4.81
0.39
cd
6.63
0.02
b
0.14
0.009
ab
No AMF
Zn SC
4.66
0.03
d
5.18
0.11
d
0.11
0.006
b
For grains nitrogen, Zn SA + AMF, Zn SP + AMF and Zn SC + AMF remained significantly better than control + AMF. Treatments Zn SA, Zn SP and Zn SC also differed significantly for improvement in grains nitrogen than the control without AMF. Maximum increase of 25.68 and 40.11% in grains nitrogen was noted in Zn SA + AMF and Zn SA treatments compared to control with and without AMF respectively (Table 3). In the case of grains phosphorus, treatments Zn SA + AMF, Zn SP + AMF and Zn SC + AMF performed significantly better compared to control + AMF. Over control without AMF, Zn SA cause significant enhancement in grains phosphorus, however, Zn SP and Zn SC did not differ significantly. Maximum increase of 29.41 and 25.00% in grains phosphorus was observed in Zn SA + AMF and Zn SA over control with and without AMF respectively (Table 3). In the case of grains potassium, Zn SA + AMF, Zn SP + AMF and Zn SC + AMF differed significantly for enhancement than control + AMF. The addition of Zn SA, Zn SP and Zn SC caused a significant increase in grains potassium compared to control without AMF. Maximum increase of 42.86 and 47.37% in grains potassium was noted in Zn SA + AMF and Zn SA treatments than in control with and without AMF respectively (Table 3). Regarding significant improvement in grains zinc, Zn SA + AMF and Zn SC + AMF performances were better over control + AMF. The addition of Zn SP + AMF did not differ significantly from control + AMF for grains zinc. Treatments Zn SA, Zn SP and Zn SC caused significant enhancement in grains zinc than the control without AMF. Maximum enhancement of 10.42 and 59.03% in grains zinc was noted in Zn SA + AMF and Zn SA treatments than control with and without AMF respectively (Table 3). Values are showing means of three replicates ± SE. Different letters are showing significant change computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Inoculation
Zinc
Grains Nitrogen (mg g−1)
Grains Phosphorus (%)
Mean
SE of Mean
Labelling
Mean
SE of Mean
Labelling
AMF
Control
11.68
0.34
c
0.17
0.006
c
AMF
Zn SP
12.68
0.34
b
0.19
0.006
b
AMF
Zn SA
14.68
0.34
a
0.22
0.006
a
AMF
Zn SC
12.67
0.34
b
0.20
0.006
b
No AMF
Control
9.05
0.03
e
0.16
0.006
c
No AMF
Zn SP
10.69
0.35
d
0.17
0.006
c
No AMF
Zn SA
12.68
0.34
b
0.20
0.006
b
No AMF
Zn SC
11.69
0.34
c
0.16
0.009
c
Inoculation
Zinc
Grains Potassium (%)
Grains Zinc (µg g−1)
AMF
Control
0.21
0.009
e
28.69
0.35
cd
AMF
Zn SP
0.25
0.006
c
29.38
0.69
c
AMF
Zn SA
0.30
0.006
a
31.67
0.34
a
AMF
Zn SC
0.27
0.006
b
30.67
0.34
ab
No AMF
Control
0.19
0.006
f
18.67
0.34
f
No AMF
Zn SP
0.23
0.006
de
24.69
0.35
e
No AMF
Zn SA
0.28
0.006
b
29.69
0.35
bc
No AMF
Zn SC
0.25
0.009
cd
27.68
0.34
d
In the case of straw nitrogen, Zn SA + AMF and Zn SC + AMF differed significantly better than control + AMF. No significant change in straw nitrogen was observed between Zn SP + AMF and control + AMF. A significant increase in straw nitrogen was noted where Zn SA, Zn SP and Zn SC were applied over control without AMF. Maximum increase of 15.07 and 16.39% in straw nitrogen was noted in Zn SA + AMF and Zn SA treatments compared to control with and without AMF respectively (Table 4). For straw phosphorus, treatments Zn SA + AMF was significantly better over control + AMF. Treatments Zn SP + AMF and Zn SC + AMF did not differ significantly for straw phosphorus than control + AMF. Compared to control without AMF, the addition of Zn SA and Zn SC cause a significant increase in straw phosphorus. Treatment Zn SP caused no significant change in straw phosphorus than the control without AMF. Maximum increase of 66.67 and 33.33% in straw phosphorus was noted in Zn SA + AMF and Zn SA over control with and without AMF respectively (Table 4). Regarding improvement in straw potassium, Zn SA + AMF, Zn SP + AMF and Zn SC + AMF caused significant change compared to control + AMF. Application of Zn SA and Zn SC significantly increase in straw potassium over control without AMF. No significant improvement was noted in straw potassium where Zn SP was applied than the control without AMF. Maximum increase of 18.75 and 23.29% in straw potassium was observed in Zn SA + AMF and Zn SA treatments than in control with and without AMF respectively (Table 4). Values are showing means of three replicates ± SE. Different letters are showing significant change computed by Fisher LSD at p ≤ 0.05. Zn = zinc; SP = seed priming; SA = soil application; SC = seed coating.
Inoculation
Zinc
Straw Nitrogen (%)
Straw Phosphorus (%)
Mean
SE
Labelling
Mean
SE
Labelling
AMF
Control
0.73
0.009
cd
0.06
0.01
bc
AMF
Zn SP
0.74
0.015
c
0.07
0.01
b
AMF
Zn SA
0.84
0.006
a
0.10
0.01
a
AMF
Zn SC
0.79
0.009
b
0.07
0.01
b
No AMF
Control
0.61
0.006
f
0.04
0.01
d
No AMF
Zn SP
0.65
0.006
e
0.05
0.01
cd
No AMF
Zn SA
0.71
0.012
d
0.06
0.01
bc
No AMF
Zn SC
0.68
0.009
e
0.06
0.01
bc
Inoculation
Zinc
Straw Potassium (%)
Straw Zinc (µg g−1)
AMF
Control
2.08
0.08
e
11.69
0.35
d
AMF
Zn SP
2.60
0.01
abc
13.72
0.36
c
AMF
Zn SA
2.47
0.29
bcd
16.71
0.35
a
AMF
Zn SC
2.81
0.01
a
15.71
0.36
ab
No AMF
Control
2.19
0.01
de
10.72
0.36
d
No AMF
Zn SP
2.31
0.01
cde
11.67
0.34
d
No AMF
Zn SA
2.70
0.00
ab
14.69
0.35
bc
No AMF
Zn SC
2.51
0.01
abc
13.67
0.34
c
For straw zinc, Zn SA + AMF and Zn SC + AMF caused a significant increase compared to control + AMF. Treatments Zn SA and Zn SC differed significantly for straw zinc than the control without AMF. No significant change was noted in Zn SP over control without AMF for straw zinc. Maximum enhancement of 42.94 and 37.03% in straw zinc was noted in Zn SA + AMF and Zn SA treatments than control with and without AMF respectively (Table 4).
Pearson correlation showed that electrolyte leakage was significant negative in correlation with growth attributes of rice. AMF colonization was significantly positive in correlation with all the growth attributes, nutrients uptake and gas exchange attributes except straw potassium concentration. Although the effect of straw potassium concentration was positive, yet change was not significant with the rice studied attributes except the number of spikes, 1000 grains weight, transpiration rate, stomatal conductance, straw yield, grains N, P, K and Zn (Fig. 6).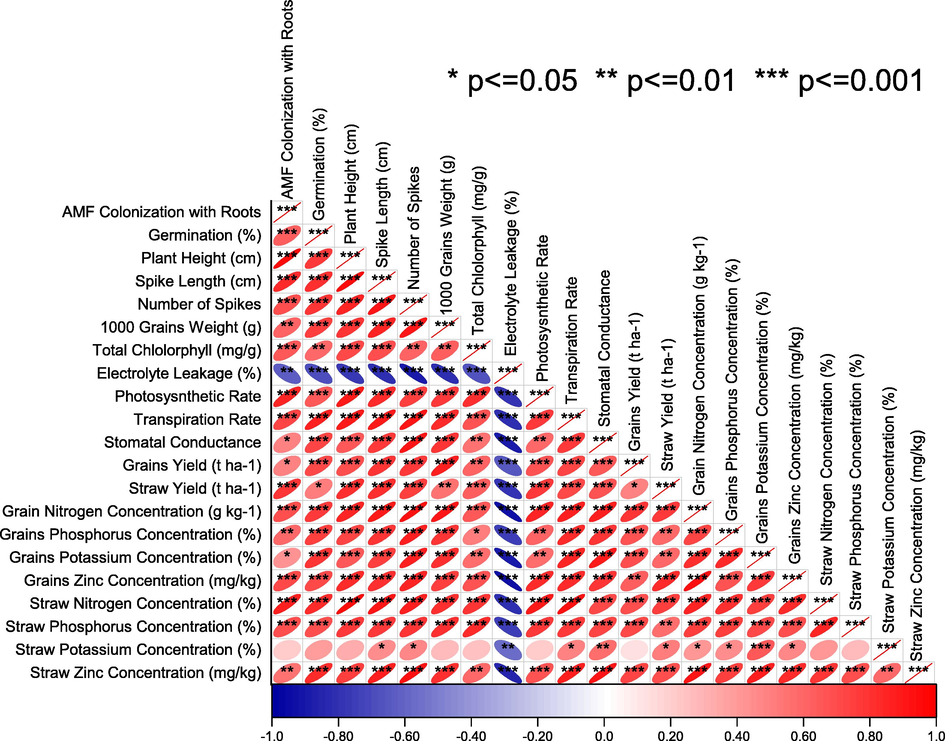
Pearson correlation of different rice attributes cultivated with and without AMF inoculation under zinc seed priming, seed coating and soil application. The red colour is indicating a positive and the blue colour is signifying a negative correlation. Ellipse having no star is an indication of a non-significant correlation.
4 Discussion
In the current study, the application of Zn SA with and without AMF performed significantly better compared to all other treatments for the improvement of rice growth. This improvement in different growth attributes was significantly positive in correlation with better uptake of N, P, K and Zn in grains and straw of rice. Better uptake of N promotes mesophyll cell division and epidermal cell elongation actively in the plants. As both cells are key components of plants growth, their division and elongation resulted in improvement of morphological growth attributes (MacAdam et al., 1989; Zeiger and Taiz, 2010). In plants, balance N also regulates biomass accumulation and cell cycle progression under kinase activity. Such improvements eventually played an imperative role in the enhancement of growth in plants (Jüppner et al., 2018). It is also well documented that improvement in N uptake, positively influence the photosynthetic process via smoothing the Rubisco synthesis (Heckathorn et al., 1996). This improvement might be one of the major causes of enhancement in the photosynthetic rate of rice plants in the current study. According to Singh et al. (2016), improvement in plant K uptake play a critical role in the maintenance of cells turgor and stomatal conductance through osmoregulation (Shabala, 2003; Wilkinson and Davies, 2002). Stomatal guard cells in the presence of K become swollen which caused the opening of stomata. This opening of stomata allows gaseous exchange between the environment and plants which improve evapotranspiration (ET) of water via stomatal pores (Cochrane and Cochrane, 2009). Balance K concentration in plants also decreased ROS synthesis through restriction of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases activity. On the other hand, maintenance of photosynthetic electron transport activity is also associated with the presence of the optimum concentration K in plants (Waraich et al., 2012). In addition to the above, photosynthesis, carbohydrate translocation and metabolism that eventually increase the crop productivity and improve grain quality are also regulated by balance uptake of K (Lu et al., 2016; Pettigrew, 2008; Zörb et al., 2014). The improvement in stomatal conductance and transpiration rate in the current study was also linked with better uptake of K where Zn SA was applied with and without AMF. The role of Zn is also vital in the regulation of gas exchange attributes and chlorophyll contents. Tobin (Tobin, 1970) argued that carbonic anhydrase (CA) is one of the crucial parts of chloroplast in the cytoplasm. After Rubisco, the presence of CA is most abundant in the chloroplast (Escudero-Almanza et al., 2012). Zinc act as a cofactor in CA enzyme. This enzyme is also involved in CO2 fixation, respiration, ion exchange, pH regulation, and photosynthetic fixation (Xing et al., 2016). Under deficiency of Zn in plants activity of CA become limited. As controls CO2 diffusion in cell lipid, the restrictions in its activity significantly decrease the photosynthesis in the plants. It also minimized the stomatal conductance and transpiration rate when Zn become deficient (Sharma et al., 1995). According to Sagardoy et al. (2010), the deficiency of Zn has the potential to decrease 76% stomatal conductance which eventually resulted in a greater disturbance in physiological and chemical processes in plants. Our findings are also in line with the above arguments, better uptake of Zn in Zn SA with and without AMF compared to other application methods might play a critical role in the improvement of CA activity. This improvement in CA might be associated with improvement in chlorophyll a, chlorophyll b, total chlorophyll, transpiration rate, stomatal conductance and photosynthetic rate in the plants. It was also noted that AMF played an imperative role in the N, P, K and Zn better uptake in rice. Mostly Zn and P showed an antagonistic relationship with each other. An increase in concertation of P minimizes the uptake of Zn in the plants (Saboor et al., 2021c). However, AMF inoculation has the potential to regulate and maintain both Zn and P in the soil. In the current study, it was also clear that an increase in Zn did not decrease P in rice plants where AMF was inoculated. Our findings are in line with Saboor et al. (2021a). Better root elongation due to symbiotic association of AMF with rice roots was major mechanism for the improvement in root and shoot fresh and dry weight in the current study. Inoculation of AMF increases the root elongation and rhizospheric area of plants (Plassard and Dell, 2010). Plants get a better chance to uptake the nutrients present in the soil due to the activity of AMF (i.e., Glomalin-related soil protein (GRSP)) and root secretions in the rhizosphere (Sharma et al., 2017). Furthermore, better uptake of water due to inoculation of AMF also played an imperative role in the enhancement of fresh weight biomass of plants along with nutrient uptake (Jiang et al., 2017).
5 Conclusion
It is concluded that sole inoculation of AMF and soil application of Zn are potential strategies for enhancement in rice growth and nutrient uptake. However, the combined application of AMF and soil-applied Zn (4 mg ZnSO4/kg soil) is a better technique for the achievement of maximum rice productivity. Seed priming and seed coating are also efficacious methods for improvement in rice grains weight, but soil application of Zn potential is significantly better than both methods with and without AMF. Growers are recommended to apply Zn as soil application (4 mg ZnSO4/kg soil) with AMF to achieve maximum benefits from rice production. More investigations are suggested at the field level in variable agro climates to declare Zn SA + AMF as the best strategy for rice productivity improvement.
Acknowledgements
This work was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R317), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Canadian journal of microbiology. 2008;54:876-886.
- [CrossRef] [Google Scholar]
- Drought Tolerant Enterobacter sp./Leclercia adecarboxylata Secretes Indole-3-acetic Acid and Other Biomolecules and Enhances the Biological Attributes of Vigna radiata (L.) R. Wilczek in Water Deficit Conditions. Biology. 2021;10:1149.
- [CrossRef] [Google Scholar]
- Akça, H., Danish, S., Younis, U., Babar, S.K., Taban, S., 2022. Soil and foliar application of zinc-methionine and zinc sulfate effects on growth and micronutrients enrichment in maize cultivated in lime-rich and poor soils. Journal of Plant Nutrition 10.1080/01904167.2022.2046077. https://doi.org/10.1080/01904167.2022.2046077.
- Alloway, B.J., 2008. Zinc in soils and crop nutrition. International Zinc Association, Brussels, International Fertilizer Industry Association, Paris. https://doi.org/10.1016/S0065-2113(06)94003-6.
- Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant physiology. 1949;24:1-15.
- [CrossRef] [Google Scholar]
- Effect of various application rates of phosphorus combined with different zinc rates and time of zinc application on phytic acid concentration and zinc bioavailability in wheat. Agriculture and Natural Resources. 2020;54:265-272.
- [Google Scholar]
- Hydrometer method improved for making particle size analysis of soil. Agronomy Journal. 1962;53:464-465.
- [Google Scholar]
- Bremner, M., 1996. Nitrogen-Total, in: Sumner, D.L., A.L., S., P.A., P., R.H., H., N., L.P., A., S.M., T., T.C., E., J.M. (Eds.), Methods of Soil Analysis Part 3. Chemical Methods-SSSA Book Series 5. John Wiley & Sons, Inc., Madison, WI, USA, pp. 1085–1121.
- Effectiveness of zinc sulfate and zinc chelate as foliar sprays in alleviating zinc deficiency of wheat grown on zinc-deficient soils in Western Australia. Animal Production Science. 1991;31:831-834.
- [CrossRef] [Google Scholar]
- Methods of analysis for soils, plants and water. Division of Agricultural Sciences, Berkeley, CA, USA: University of California; 1961.
- The vital role of potassium in the osmotic mechanism of stomata aperture modulation and its link with potassium deficiency. Plant signaling & behavior. 2009;4(3):240-243.
- [Google Scholar]
- Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Scientific Reports. 2019;9:5999.
- [CrossRef] [Google Scholar]
- Determination of potassium and sodium by flame emmision spectrophotometery. In: Kalra Y., ed. Handbook of Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :153-155.
- [Google Scholar]
- Determination of total nitrogen in plant tissue. In: Kalra Y., ed. Handbook of Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :75-83.
- [Google Scholar]
- Carbonic Anhydrase and Zinc in Plant Physiology. Chilean journal of agricultural research. 2012;72:140-146.
- [CrossRef] [Google Scholar]
- Methods of Soil, Plant, and Water Analysis : A manual for the West Asia and North Africa region (3rd ed.). Beirut, Lebanon: International Center for Agricultural Research in Dry Areas; 2013.
- Hassan, M.U., Aamer, M., Chattha, M.U., Haiying, T., Shahzad, B., Barbanti, L., Nawaz, M., Rasheed, A., Afzal, A., Liu, Y., Guoqin, H., 2020. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, Vol. 10, Page 396 10, 396. https://doi.org/10.3390/AGRICULTURE10090396.
- Strong phosphorus (P)-zinc (Zn) interactions in a calcareous soil-alfalfa system suggest that rational P fertilization should be considered for Zn biofortification on Zn-deficient soils and phytoremediation of Zn-contaminated soils. Plant and Soil. 2021;461(1-2):119-134.
- [Google Scholar]
- Potassium enhanced grain zinc accumulation in wheat grown on a calcareous saline-sodic soil. Pak. J. Bot. 2020;52:69-74.
- [Google Scholar]
- Distribution of DTPA extractable micronutrients and their relationship with soil properties in soil of Parsori watershed of Nagpur district of Maharashtra. Asian Journal of Soil Science. 2014;9:297-299.
- [Google Scholar]
- Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Scientific Reports. 2021;11:22081.
- [CrossRef] [Google Scholar]
- Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy. 2020;10:1142.
- [CrossRef] [Google Scholar]
- Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science. 2017;356:1172-1173.
- [CrossRef] [Google Scholar]
- Halotolerant Microbial Consortia for Sustainable Mitigation of Salinity Stress, Growth Promotion, and Mineral Uptake in Tomato Plants and Soil Nutrient Enrichment. Sustainability. 2021;13:8369.
- [CrossRef] [Google Scholar]
- To find out the effect of seed priming on growth and yield parameter of rice. IJCS. 2017;5:1620-1623.
- [Google Scholar]
- Kuo, S., 1996. Phosphorus, in: Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E. (Eds.), Methods of Soil Analysis Part 3: Chemical Methods. John Wiley & Sons, Ltd, SSSA, Madison, Wisconsin, pp. 869–919. https://doi.org/10.2136/sssabookser5.3.c32.
- Lu, Z., Lu, J., Pan, Y., Lu, P., Li, X., Cong, R., Ren, T., 2016. Anatomical variation of mesophyll conductance under potassium deficiency has a vital role in determining leaf photosynthesis. Plant, Cell \& Environment 39, 2428–2439.
- A Mixture of Piper Leaves Extracts and Rhizobacteria for Sustainable Plant Growth Promotion and Bio-Control of Blast Pathogen of Organic Bali Rice. Sustainability. 2020;12(20):8490.
- [Google Scholar]
- Nitric-Perchloric Acid Wet Digestion In an Open Vessel. In: Kalra Y., ed. Reference Methods for Plant Analysis. Washington, D.C.: CRC Press; 1998. p. :57-62.
- [Google Scholar]
- OriginLab Corporation. 2021. OriginPro. OriginLab, Northampton, MA, USA.
- Page, A.L., Miller, R.H., Keeny, D.R., 1983. Soil pH and lime requirement, in: Page, A.L. (Ed.), Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2/Agronomy Monographs. American Society of Agronomy, Inc. and Soil Science Society of America, Inc., Madison, pp. 199–208. https://doi.org/10.2134/agronmonogr9.2.2ed.
- Isolation and characterization of plant growth promoting rhizobacteria and their biocontrol efficacy against phytopathogens of tomato (Solanum lycopersicum L.). Plant Biosystems – An International Journal Dealing with all Aspects of. Plant Biology 2020:1-7.
- [CrossRef] [Google Scholar]
- Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiologia Plantarum. 2008:670-681.
- [CrossRef] [Google Scholar]
- Pratt, P.F., 1965. Potassium, in: Norman, A.G. (Ed.), Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2. John Wiley & Sons, Ltd, pp. 1022–1030. https://doi.org/10.2134/agronmonogr9.2.c20.
- Influence of foliar-applied zinc in the form of mineral and complexed with amino acids on yield and nutritional quality of onion under field conditions. Scientia Horticulturae. 2017;216:160-168.
- [CrossRef] [Google Scholar]
- Potassium and zinc co-fertilization provide new insights to improve maize (Zea mays L.) physiology and productivity. Pakistan Journal of Botany. 2021;53:2059-2065.
- [Google Scholar]
- Zinc seed coating improves the growth, grain yield and grain biofortification of bread wheat. Acta Physiologiae Plantarum. 2016;38:238.
- [CrossRef] [Google Scholar]
- Effect of foliar applied zinc, manganese and boron on sweet orange quality. Pakistan Journal of Soil Science. 1999;17:113-117.
- [Google Scholar]
- Effect of acidified biochar on bioaccumulation of cadmium (Cd) and rice growth in contaminated soil. Environmental Technology and Innovation. 2020;19:101015.
- [Google Scholar]
- Salinity: Electrical Conductivity and Total Dissolved Solids. In: Sparks D.L., Page A.L., Helmke P.A., Loeppert R.H., Soltanpour P.N., Tabatabai M.A., Johnston C.T., Sumner M.E., eds. Methods of Soil Analysis, Part 3, Chemical Methods. Madison, WI, USA: Soil Science Society of America; 1996. p. :417-435.
- [CrossRef] [Google Scholar]
- Biofertilizer-Based Zinc Application Enhances Maize Growth, Gas Exchange Attributes, and Yield in Zinc-Deficient Soil. Agriculture. 2021;11:310.
- [CrossRef] [Google Scholar]
- Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Scientific Reports. 2021;11:18468.
- [CrossRef] [Google Scholar]
- Zinc nutrition and arbuscular mycorrhizal symbiosis effects on maize (Zea mays L.) growth and productivity. Saudi Journal of Biological Sciences. 2021;28:6339-6351.
- [CrossRef] [Google Scholar]
- A review of zinc nutrition and plant breeding. Journal of soil science and plant nutrition. 2013;13:905-927.
- [CrossRef] [Google Scholar]
- Electrolyte leakage and relative water content as affected by organic mulch in okra plant (Abelmoschus esculentus (L.) Moench) grown under salinity. FUUAST J. Biology. 2014
- [Google Scholar]
- Stomatal and mesophyll conductances to {CO}2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytologist. 2010;187(1):145-158.
- [Google Scholar]
- Tissue zinc distribution in maize seedling roots and its action on growth. Russian Journal of Plant Physiology. 2011;58:109-117.
- [CrossRef] [Google Scholar]
- Regulation of potassium transport in leaves: from molecular to tissue level. Annals of Botany. 2003;95:627-634.
- [Google Scholar]
- Zinc requirement for stomatal opening in cauliflower. Plant Physiology. 1995;107:751-756.
- [CrossRef] [Google Scholar]
- Glycoprotein Associated with Funneliformis coronatum, Gigaspora margarita and Acaulospora scrobiculata Suppress the Plant Pathogens In vitro. Asian Journal of Plant Pathology. 2017;11:199-202.
- [CrossRef] [Google Scholar]
- Grain zinc and iron enrichment through foliar application augments wheat yield under varying nitrogen regimes. Pakistan Journal of Botany. 2020;52:85-94.
- [Google Scholar]
- Effects of zinc fertilization on physical grain quality of basmati rice. International Rice Research Notes (IRRI, Philippines). 2005;32:41-42.
- [Google Scholar]
- Impact of Addition of Biochar Along with PGPR on Rice Yield, Availability of Nutrients and their Uptake in Alluvial Soil. Journal of Pure and Applied Microbiology. 2016;10:2181-2188.
- [Google Scholar]
- Mycorrhizal Symbiosis (3rd ed). Elsevier, New York City, NY, USA: Soil Science Society of America Journal. Academic Press Inc.; 2008. https://doi.org/https://doi.org/10.2136/sssaj2008.0015br
- Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Nelson, D.W., Sommers, L.E., 1996. Total Carbon, Organic Carbon, and Organic Matter, in: Methods of Soil Analysis Part 3—Chemical Methods. Soil Science Society of America, American Society of Agronomy, pp. 961–1010. https://doi.org/10.2136/sssabookser5.3.c34.
- Steel, R.G., Torrie, J.H., Dickey, D.A., 1997. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed. McGraw Hill Book International Co., Singapore.
- Productivity and fruit quality of three mango cultivars in relation to foliar sprays of calcium, Zinc, Boron or potassium. Journal of Horticultural Science & Ornamental Plants. 2011;3:91-98.
- [Google Scholar]
- Carbonic anhydrase from parsley leaves. Journal of Biological Chemistry. 1970;245(10):2656-2666.
- [Google Scholar]
- Tondey, M., Kalia, A., Singh, A., Dheri, G.S., Taggar, M.S., Nepovimova, E., Krejcar, O., Kuca, K., 2021. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronomy 2021, Vol. 11, Page 729 11, 729. https://doi.org/10.3390/AGRONOMY11040729.
- Wang, Y.Y., Zhu, B., Shi, Y., Hu, C.S., 2008. Effects of nitrogen fertilization on upland rice based on pot experiments. Communications in Soil Science and Plant Analysis 39, 1733–1749. https://doi.org/Doi 10.1080/00103620802073743.
- Alleviation of temperature stress by nutrient management in crop plants: A review. Journal of Soil Science and Plant Nutrition. 2012;12:221-244.
- [CrossRef] [Google Scholar]
- ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant, Cell & Environment. 2002;25:195-210.
- [Google Scholar]
- Physiological changes and expression characteristics of ZIP family genes under zinc deficiency in navel orange (Citrus sinensis) Journal of Integrative Agriculture. 2016;15:803-811.
- [CrossRef] [Google Scholar]
- Zajaczkowska, A., Korzeniowska, J., Sienkiewicz-Cholewa, U., 2020. Effect of Soil and Foliar Silicon Application on the Reduction of Zinc Toxicity in Wheat. Agriculture 2020, Vol. 10, Page 522 10, 522. https://doi.org/10.3390/AGRICULTURE10110522.
- Zörb, C., Senbayram, M., Peiter, E., 2014. Potassium in agriculture--status and perspectives. Journal of plant physiology 171, 656–669.







