Translate this page into:
Sulforaphane (4-methylsulfnylbutyl isothiocyanate) mitigates gold nanoparticle induced brain toxicity in male albino rats
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Gold nanoparticles (GNPs) have shown great promise for a variety of biomedical applications; however, emerging evidence suggests that they exhibit toxic effects on different organs including the brain. Sulforaphane (SFN) is a naturally occurring compound derived from plants, which has been recognized for its impressive antioxidant, anticancer, and anti-inflammatory characteristics. The neuroprotective potential of SFN was assessed in this study to determine its effectiveness against GNP-induced toxicity in brain tissues.
Methods
Male Wistar rats were administered GNPs daily, either alone or in combination with SFN, over a 7-day period. Inflammatory and oxidative stress markers including serotonin and Nrf2 levels were measured in brain tissues. Histological changes were assessed for GNP-mediated tissue damage.
Results
Compared with the control, GNP-treated rats exhibited a significant increase in brain inflammatory and oxidative stress (OS) markers including MDA, 8OHdG, and IL-6 and a significant decrease in Glutathione S-transferase, Glutathione reductase, SOD, Total antioxidant capacity, Nrf2, brain parameters (serotonin, dopamine, Gama Amino Butyric Acid) and Brain-derived neurotrophic factor. GNP treatment also resulted in marked histopathological changes in brain tissue. In addition, rats administered with combined GNP and SFN showed a significant reversal in the levels of these biomarkers. SFN also protected brain tissue from GNP-induced histopathological changes. Molecular docking studies confirmed the competitive binding of SFN with GNPs to amino acid receptor proteins, 1MAH and 1KU6, which supports the experimental data.
Conclusion
Collectively, our findings indicate the neuroprotective capacity of SFN against GNP-induced brain damage, presumably by blocking OS and inflammation.
Keywords
Gold nanoparticles
Sulforaphane
Brain toxicity
Oxidative stress
Antioxidant
1 Introduction
Nanotechnology has bridged the gap between biological and physical sciences by incorporating nanostructures into various disciplines, particularly in nanomedicine and nano-based drug delivery systems (Puri et al., 2024). Nanoparticles are objects that have dimensions between 1 and 100 nm, exhibiting distinct physicochemical and biological attributes that set them apart. Because of the small dimensions and large surface areas NPs possesses high absorption and reactivity and due to their high surface area they penetrate the cell membrane and affect various biochemical functions (Biswas et al., 2023). There are various ways that drug materials can be attached, dissolved, encapsulated, or entrapped in NPs' matrix. Delivery of drugs to different parts of the body can be achieved with the help of NPs for short-term and sustained dosages (Panao Costa et al., 2019). Gold nanoparticles (GNPs) are one of the most widely explored NP types because of their wide-ranging applications. GNPs are utilized as carriers for the targeted drug delivery in cancer treatment They are also used in photothermal radiotherapy, wound healing and as biosensors (Stavropoulou et al., 2022). GNPs are primarily used in the pharmaceutical, microelectronics, and food packaging industries. Although GNPs are chemically inert, they may be functionalized with biologically active organic molecules and directly conjugated with drugs, nucleic acids, proteins, and enzymes (Hu et al., 2020). GNPs are known for a low rate of clearance from the circulatory system and tissues and thus may be detrimental to tissue and organ damage. GNPs are capable of generating free radicals and disrupting the antioxidant defense system, thereby inducing oxidative stress (OS) and inflammation (Tedesco et al., 2010). GNPs functionalized with different ligands may cause oxidative stress-induced DNA damage in cultured human hepatocellular carcinoma and lung epithelial cells (Llewellyn et al., 2022). In a recent study, nephrotoxicity was attributed to increased OS and inflammation in rats exposed to gold nanoparticles (Abdelhalim et al., 2020). Histopathological changes, increased inflammatory mediators were observed in different organs of mice treated with GNPs (Khan et al., 2020). The brain tends to be more susceptible to oxidative stress-induced damage resulting from its higher concentrations of transition metal ion. Because of their smaller size, GNPs can cross the blood–brain barrier, which may cause tissue damage by disturbing the redox balance in the brain (Kang et al., 2010). Consistently, GNP-induced OS and inflammation suppressed antioxidant and neurotransmitter levels in the brain tissues of mice (Siddiqi et al., 2012). GNPs caused OS induced DNA damage, cell cycle disruption, and apoptosis in human neural precursor cells (Söderstjerna et al., 2013). These findings indicate the vulnerability of various organs, particularly the brain, to GNP-induced toxicity, which appears to be due to increased OS and inflammation.
Sulforaphane (SFN) is a well-known constituent of the isothiocyanate group, which is commonly found in cruciferous vegetables. A number of studies have demonstrated the neuroprotective effects of SFN using cell and animal based systems. Of importance, SFN has been found to cross blood–brain barrier, which is crucial for its neuroprotective effects, reduced oxidative stress and accumulation of misfolded proteins and exerted detoxification and antioxidant effects against several neurological disorders (Uddin et al., 2020). SFN with its antioxidant and anti-inflammatory properties is known to induces the expression of phase 2 detoxification and antioxidant enzymes (Janczewski, 2022). In mice, SFN prevented brain damage caused by hypoxia by facilitating Nrf2 nuclear translocation and autophagy. In a similar manner, SFN exhibited protective effects on cortical neurons and significantly decreased carbon monoxide-induced neurodegeneration by inhibiting apoptosis and activating AMPK (Yue et al., 2024). Inhibition of abnormal C/EBPβ/α-Syn signaling pathway through activation of Nrf2 ameliorated Parkinson's disease-like pathology in SFN treated mice (Lin et al., 2023). Sulforaphane demonstrated its ability to attenuate microglia-mediated neuronal damage by down-regulating the ROS/autophagy/NLRP3 signal axis in fibrillar Abeta-activated microglia (Yang et al., 2023). It also exerted acute antioxidant and cytoprotective effects in brain endothelial cells and astrocytes and increased antioxidative ability in brain tissue in response to focal cerebral ischemia, inflammation, and intracerebral hemorrhage (Guerrero-Beltrán et al., 2012). Because of the reported pro-oxidant and proinflammatory properties of GNPs in the brain and other organs, we aimed to investigate the potential advantages of SFN in alleviating oxidative stress (OS) and inflammation in the brain tissue of rats exposed to GNP.
2 Materials and methods
2.1 Reagents
GNPs, with a (196.97 molecular weight and 10 nm a diameter, stabilized suspension in citrate buffer), were supplied from Sigma-Aldrich (USA). The optical density, concentration, polydispersity index, and wavelength of the GNPs were 1.0–6.0 × 1012 particles/mL, <0.2, and 510–525 nm, respectively. SFN (broccoli seed extract) was obtained from Swanson GreenFoods Supplements (USA).
2.2 Animals
Animal studies were carried out in accordance to the guidelines of the Biomedical Ethics Unit of the KFMRC, Jeddah, Saudi Arabia. Approval number (177–19). Male albino rats were allowed to acclimatize to animal facility for 15 days and had free access to water and standard diet. In total, 40 rats weighing approximately 170 g were equally distributed into four groups, designated as group 1, 2, 3, and 4, with each group consisting of 10 rats. Group 1, rats served as control and received DMSO as vehicle control. Group 2, rats received intraperitoneal injections of 10 nm GNP suspension at 20 μg/kg body weight for 7 days (Abdelhalim and Moussa, 2013). Group 3, rats supplemented with SFN dissolved in DMSO daily for 7 days at 5 mg/kg body weight through oral gavage (Zhao et al., 2016). Group 4, healthy rats received intraperitoneal injections with a 10 nm GNP suspension and oral supplementation of SFN dissolved in DMSO by oral gavage for 7 days at 5 mg/kg body weight daily (Zhao et al., 2016).
Diethyl ether was used to sacrifice the rats after they had completed treatment. Immediately after collecting the blood, the serum was centrifuged for 10 min at 3000 rpm and stored at 20 °C until needed. Brain samples were collected and processed appropriately for biochemical and histological examination.
2.3 Brain homogenates
A Teflon homogenizer (Ultra Turrax T18 Basic) was used to homogenize brain tissue in 0.1 M Tris–HCl buffer (pH 7.4), followed by centrifugation at 5,000 g for 15 min.the clear supernatant was separated and stored until needed.
2.4 Oxidative stress parameters
2.4.1 Lipid peroxidation assay
The determination of Thiobarbituric acid-reactive substances (TBARS) were quantified using a spectrophotometric method to determine lipid peroxidation in the brain tissues (Nagababu et al., 2010).
2.4.2 Glutathione reductase activity assay
An analysis of glutathione reductase activity (GR) was conducted as described previously (Beutler 1963). Incubation at 37 °C for 5 min was performed after mixing 0.1 mL of sodium azide, 0.5 mL of potassium phosphate buffer (pH 7.4), 0.1 mL of H2O2, 0.2 mL of test solution, and 0.5 mL of the sample. After adding 0.5 ml of TCA, the mixture was centrifuged, and the absorbance at 412 nm was measured. GR activity is presented as nmol/min/mg protein.
2.4.3 8-Hydroxydeoxyguanosine (8-OHdG) assay
The brain 8-OHdG (8-hydroxydeoxyguanosine) content was quantified by ELISA (Kamiya Biomedical Company, USA). Quantification method was strictly based on the kit supplier.
2.4.4 Glutathione S-transferase activity assay
The evaluation of Glutathione S-transferase (GST) activity was conducted by utilizing a kit from (Cloud-Clone Corp. USA) in accordance with the provided guidelines.
2.4.5 Total antioxidant capacity activity assay
TAC was estimated using FRAP (ferric reducing antioxidant power) solution. Briefly, a combination of TPTZ solution and acetate buffer was prepared in HCl. Brain tissue homogenate (8 µl) was added to the FRAP solution (240 µl). The absorption change was measured at 532 nm after a10 min incubation of the mixture at room temperature (Benzie and Strain, 1999).
2.4.6 SOD activity assay
Brain SOD (superoxide dismutase) activity was quantified according to the method described by Oyagbemi et al. (2017). Briefly, a mixture consisting of 2.5 mL of 0.05 M carbonate buffer, 100 ml of epinephrine (100 mg) solution, and 30 µl of tissue sample were mixed; subsequently, 300 µl of 0.3 mM adrenaline was added, and the absorbance was recorded every 30 s at 480 nm.
2.4.7 Assessment of inflammatory markers (Nrf2 and IL-6)
Nuclear-related factor 2 (Nrf2) and IL-6 (interleukin-6) contents were assayed in brain tissues as detailed by the manufacturer (LifeSpan Biosciences, USA). In brief, the wells, precoated with Nrf2 and IL-6 antibodies, were incubated for 2 h. at ambient temperature with standards or brain tissue homogenates. Wells were washed and incubated for 1 h with corresponding biotin-labeled antibodies at room temperature. Further incubation with secondary antibodies and TMB medium were performed sequentially. The absorbance of each well was read at 450 nm. These data are presented as picograms per milligram protein (pg/mg protein).
2.5 Measurement of brain parameters
2.5.1 Serotonin
Serotonin levels were assessed by employing a serotonin ELISA Kit (Abcam, Cambridge, MA, USA), following the guidelines provided by the manufacturer. Samples were evaluated in triplicate, and the data are presented as nanograms of serotonin per milligram of protein.
2.5.2 Dopamine
Dopamine was determined using ELISA based kits following the method detailed by the supplier (Biomatic, USA). The competitive inhibition enzyme immunoassay technique utilized in this assay demonstrates a negative relationship between sample concentration and the intensity of the assay signal.
2.5.3 Gama amino Butyric Acid
Levels of Gamma Amino Butyric Acid were quantified using commercially available ELISA based kits, following the guidelines provided by the manufacturer (Lifespan Biosciences, USA). The competitive inhibition enzyme immunoassay technique utilized in this assay demonstrates an inverse relationship between the concentration of Gama Amino Butyric Acid in the sample and the intensity of the assay signal.
2.5.4 Brain-derived neurotrophic factor (BDNF)
The determination of Brain-derived neurotrophic factor (BDNF) level was assessed by ELISA based commercially available kit (Invitrogen Corporation Camarillo, California, USA) following the company’s instructions.
2.6 Histopathological analysis
The brain tissue samples were immersed in formalin buffered with natural substances for a duration of 12 h, then encased in paraffin, sliced into sections approximately 5 μm in thickness, and finally dyed using hematoxylin and eosin (H&E). The light microscope revealed sections with staining.
2.7 Assessment of molecular docking
Molecular docking was performed to determine the selective binding of sulforaphane to its receptor (to assess the anticancer properties and toxicity of the ligand) Zayed et al., 2018. The AutoDock vina 4.2 software was used to explore the docking studies and the determination of the binding affinities of sulforaphane to receptors. Nonpolar hydrogen atoms were conjoined, and rotatable bonds were clarified. Upon adding the Kollman United Atom charges, the hydrogen atoms and salvation parameters were created (Zayed et al., 2018). The auto distance-dependent dielectric function and dock parameter set were used to establish electrostatic and Van der Waals interactions. A simulated docking procedure was implemented using Solis and Wets’ local search and Lamarckian’s genetic algorithm (Pietro and Hehre, 1983). A determination was made of the ligand's initial position, orientation, and rotation.
2.8 Statistical analysis
The SPSS software program, developed by SPSS Inc. in Chicago, IL, United States, was employed for data analysis. The mean values along with standard deviation (SD) were used to express the results. A one-way ANOVA (LSD) was employed to compare the outcomes among various experimental groups. A level of statistical significance was determined with a p < 0. 01.
3 Results
3.1 Brain oxidative stress markers
The effects of GNP and SFN on brain OS markers are presented in Figs. 1 and 2. Brain MDA and 8OHdG levels were found to be significantly increased in rats treated with GNP treated rats compared to control. Rats administered with GNP exhibited significantly lower GR, GST, SOD, and TAC levels compared with control (p < 0.01). SFN treatment significantly reversed GNP induced effects on MDA, 8OHdG GST, GR, SOD, and TAC (p < 0.01). SFN alone had no significant effects on any of the aforementioned markers.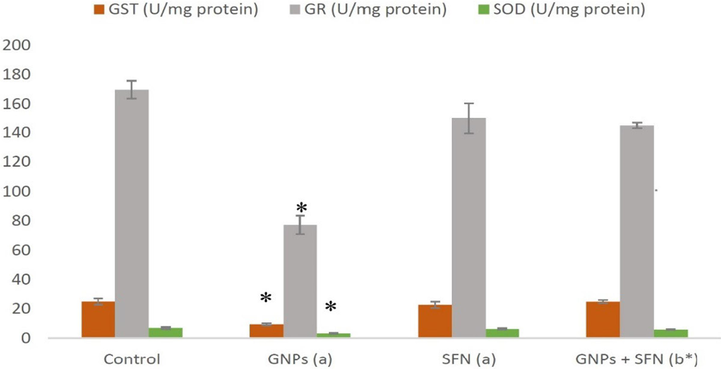
GST, GR and SOD marker levels in brain rats. GNPs, gold nanoparticles; SFN, sulforaphane; GST, glutathione S-transferase; GR, glutathione reductase; SOD, superoxide dismutase, (a) compared to control, (b) compared to GNP group, *p < 0.01. Data are presented as the mean ± SD (N = 10).
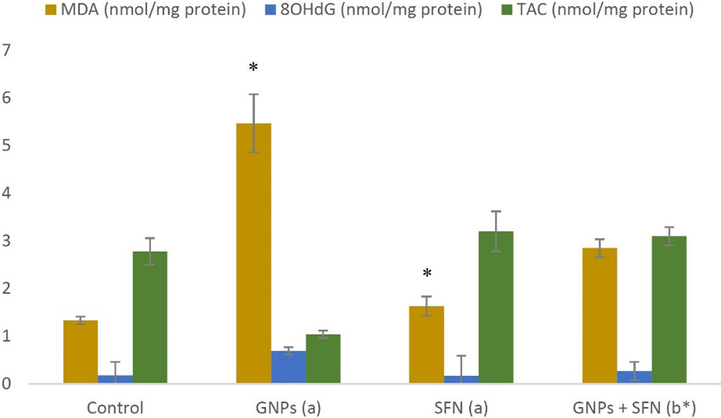
MDA, 8OHdG and TAC marker levels in brain rats. GNPs, gold nanoparticles; SFN, sulforaphane; MDA, malondialdehyde; 8OHdG, 8-hydroxy deoxyguanosine; TAC, total antioxidant capacity, (a) compared to control, (b) compared to GNP group, *p < 0.01. Data are presented as the mean ± SD (N = 10).
3.2 Inflammatory markers and brain parameters
Effects of GNPs and SFN on inflammatory markers serotonin, dopamine, GABA and BDNF) are presented in Fig. 3 and Fig. 4 A significant decrease in Nrf2, and a significant increase in IL-6 levels were noted in GNP treated rats (p < 0.01). Co-administration of GNPs and SFN led to a significant elevate in Nrf2 and a significant decline in IL-6 (p < 0.01). Nrf2, or IL-6 levels in rats treated with SFN alone were comparable to the control group (p < 0.01). GNPs treatment significantly decreased serotonin, dopamine, GABA and BDNF while co-treatment with SFN led to their significant rise (p < 0.01). Treatment with SFN alone exerted no significant effect on these parameters.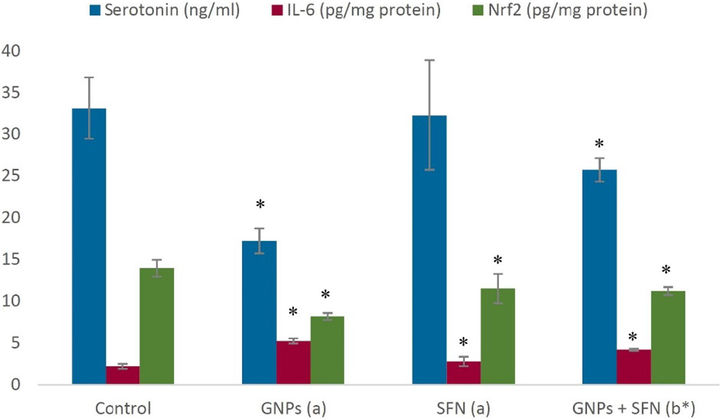
Brain serotonin, IL-6, and Nrf2 levels in different treatment groups. GNPs, gold nanoparticles; SFN, sulforaphane; Nrf2, nuclear-related factor 2, IL-6, interleukin-6. (a)compared to control, (b)compared to SFN group, *p < 0.01. Data are expressed as the mean ± SD (N = 10).
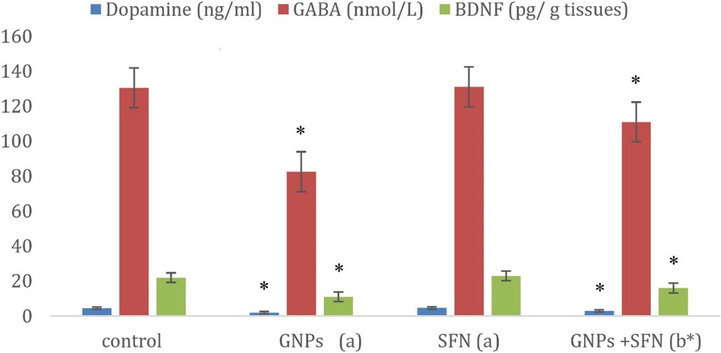
Dopamine, Gama Amino Butyric Acid (GABA), Brain-derived neurotrophic factor (BDNF) levels in control and different treatment groups. GNPs, gold nanoparticles; SFN, sulforaphane; (a)compared to control, (b)compared to SFN group, *p < 0.01. Data are expressed as the mean ± SD (N = 10).
3.3 Histology of the brain tissue
Histological changes in response to GNPs and SFN are presented in Fig. 5. Compared to normal healthy control, GNP-treated group displayed disorganized tissue architecture with distorted pyramidal cells containing darkly stained nuclei. Few cells were surrounded by clear halos, and vascular dilatation in the neuropil was noted. In contrast, rats co-treated with GNPs and SFN exhibited predominantly normal cells with dilated blood vessels. Histology of the brain tissue of SFN only treated rats was comparable to that of controls.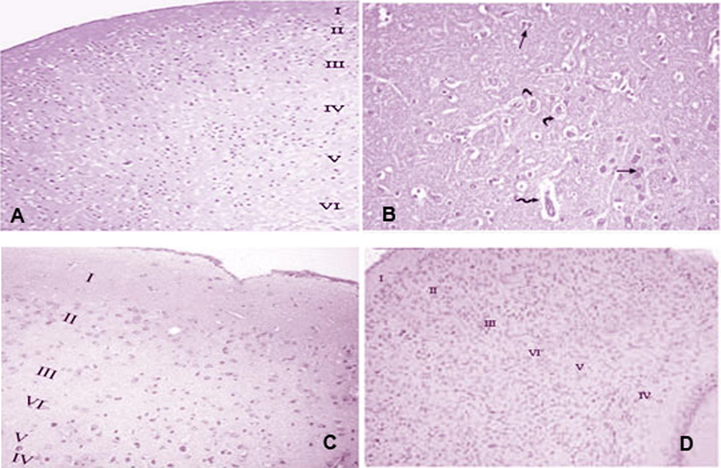
Photomicrographs of cerebral cortex sections prepared for all the four treatment groups. (A) G1 (control): the cerebral cortex is labeled as follows: molecular layer (I), outer granular layer (II), outer pyramidal layer (III), inner granular layer (IV), inner pyramidal layer (V), and polymorphic layer (VI). (B) G2 (GNPs): disorganization of the normal architecture – most pyramidal cells are distorted with darkly stained nuclei (arrow); some cells are surrounded by clear pericellular halos (curved arrow), and vascular dilatation occurred in the neuropil (zigzag arrow). (C) G3 (SFN): normal histological appearance. (D) G4 (SFN + GNPs): normal histological appearance of the cerebral cortex, (H&E 20).
3.4 Molecular docking studies
Fasciculin 2-mouse acetylcholinesterase complex (PDB: 1MAH, 1KU6) crystal structures were used as templates for docking. Isothiocyanato-4-methylsulfinylbutane was used as a ligand for docking, and a pictorial representation of the best possible binding sites is shown in Fig. 6A and B. The results indicated that the compounds interacted favorably with the amino acids in the binding sites of the proteins.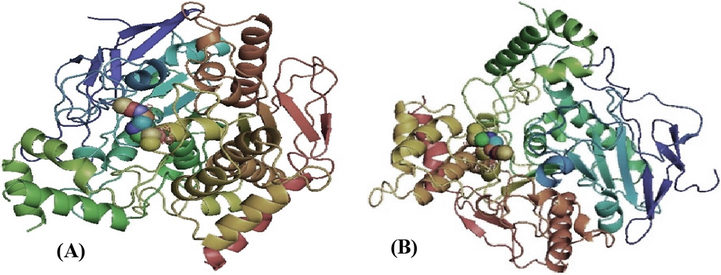
Three-dimensional schematic of isothiocyanato-4 methylsulfinylbutane ligand binding to the (A) 1MAH receptor or (B) 1KU6 receptor.
For the protein PDB code, the calculated free energy for the binding of isothiocyanato-4-methylsulfinylbutane to the receptor 1MAH was −2.9 kcal/mol and −3.1 kcal/mol with 1KU6 receptor. The binding between the amino acids of the proteins, 1MAH and 1KU6, with isothiocyanato-4-methylsulfinylbutane is shown below. The results indicated that the toxicity can be reduced or released by this compound. There were several factors to consider when choosing proteins, including the (X, Y, Z) cavity, the rmsd value, crystallography, method, resolution, and others. A good connection or binding between the amino acids of the two proteins with the isothiocyanato-4-methylsulfinylbutane compound is shown, indicating that the toxicity may be reduced or released by this compound.
4 Discussion
The study was assessed the protective effects of SFN against the brain injury caused by GNP. Because of their low rate of clearance from the circulatory system and tissues, GNPs are considered toxicy to different organs including the brain (Abdelhalim and Moussa, 2013). GNPs can pass through the blood–brain barrier because of their similarity in size with cell components and proteins. After inhalation, GNPs can enter brain through trans-synaptic transmission, olfactory epithelia or during lung-to-blood translocation, which leads to systemic brain exposure. Importantly, for the induction of toxicity, peritoneal administration was preferable over oral administration, and GNPs of 10 nm in size were found to be safer (Tedesco et al., 2010). Transcription factor, Nrf2 counteract OS by upregulates antioxidant genes. Selective induction of Nrf2 and the subsequent transactivation of antioxidant genes blocked oxidative stress-induced brain damage. Inline, impaired antioxidant defense led to production of the 8OHdG and induction of inflammation and cell apoptosis (Xiong et al., 2011). This is consistent with our finding of decreased Nrf2 expression, increased 8OHdG and inflammatory markers in brain tissue of rats treated with GNPs. Increased serotonin levels in brain tissues demonstrate the ability of GNPs to cross blood–brain barrier and to cause functional dysregulation. The histopathological alteration in brain tissue of GNP treated rats demonstrates the tissue damaging effect of GNPs. A number of studies have reported augmented OS and inflammation in response to GNPs in brain tissue. For example, Au-TiO (2) NPs increased oxidative stress and neurotoxicity by altering neurotransmitter enzyme activity (Mezni et al., 2021). Increased inflammation and OS and suppressed antioxidants were noted in the brains of mice exposed to GNPs (Siddiqi et al., 2012). Consistently, in this study, rats administered 10 nm GNPs showed increased OS markers and decreased antioxidants in the brain tissue, demonstrating their pro-oxidant nature. In contrast to our observation of downregulated SOD values in response to GNPs, study by Mehanna et al. (2022) found increased SOD in rabbits treated with gold nano rods and nano spheres. These contrasting observations may have stemmed from differences in species, dosage and the source of nanomaterials. GNPs also reported to cause DNA damage, disruption of the cell cycle, and induction of apoptosis in human neural precursor cells exposed to GNPs (Soderstjerna et al., 2013). Collectively, above findings including this study indicate the sensitivity of the brain to GNP-induced toxicity, and these effects appear to be mediated through the induction of OS and inflammation.
SFN is a natural substance extracted from plants that possesses antioxidant and anti-inflammatory properties. It was shown to induce the expression of phase 2 detoxification and antioxidant enzymes (Janczewski, 2022). Importantly, pharmacokinetic studies have indicated that SFN crosses the blood–brain barrier after oral ingestion (Huang et al., 2019). In this study, SFN treatment of GNP-exposed rats resulted in a significant decrease in OS and inflammatory markers, which resulted in a major significant increase of antioxidants and the Nrf2 transcription factor. Furthermore, a significant recovery was evident in the histology of brain tissues from GNP-administered rats treated with SFN. These data highlight the potential of SFN to prevent GNP-induced brain damage through the abrogation of OS and inflammation and by augmenting the expression of antioxidant gene expression through an increase in Nrf2 expression.
Our findings are consistent with the number of studies, where neuroprotective effects of SFN were reported. For example, SFN administration to GNP-exposed rats dowsnregulated the oxidative state and reduced brain damage by decreasing the levels of inflammatory biomarkers (Hussein et al., 2021). Administration of SFN rescued cholinergic neurons in the hippocampus and the septum pellucidum. In addition, SFN reduced OS caused by aggregated proteins in Alzheimer’s disease and protected cultivated neural cells from toxicity (Angeloni et al., 2015). The availability of reduced GSH is needed to avoid damage caused by free radicals. SFN was found to conjugate with glutathione (GSH) by GSTs and enhanced the release of GSH in cultured astrocytes and decreased OS under multiple conditions (Sun et al., 2017). Importantly, SFN increased the amount of GSH in human brain after 7 days of administration demonstrating SFN’s antioxidant pathways in humans. The antioxidant effect of SFN prevented neuronal toxicity caused by hydrogen peroxide and attenuated the production of reactive oxygen species by proinflammatory stimuli (Townsend and Johnson, 2016). SFN also lowered the number of infarcts in adult ischemic rat brains and was able to maintain cognitive function. This occurred through its ability to protect neurons and other cell types from OS and reduced injury produced by ischemia–reperfusion of the brain and prevented histological alterations (Yoon et al., 2008).
A neurotransmitter known as GABA, mediates a wide range of functional responses in non-neuronal tissue, making it a significant inhibitory in the nervous system. BDNF, a member of the neurotrophin family in the brain, plays a crucial role in various functions such as plasticity, neuronal survival, synapse formation, dendritic branching, and regulation of neurotransmitter profiles. Reduction in BDNF expression was correlated with neuronal atrophy and neurological disorders (Soden et al., 2020). Results in the present study showed that GNPs induced a significant reduction in neurotransmitters, GABA, BDNF, serotonin and dopamine as compared to control group. Similar observations were made in a previous study (Siddiqi et al., 2012) where GNPs caused a significant decrease in dopamine and serotonin levels, indicating a possible behavioral change in the treated animals. In line Lebda et al. (2018) revealed the neurotoxic side effects of GNPs, with a concomitant reduction in monoamine serotonin and dopamine levels. Importantly, SFN treatment significantly restored the levels of serotonin and dopamine, demonstrating the neuroprotective effects of SFN. In correlation with this finding, SFN administration protected neurons and reduced death of nigrostriatal dopaminergic neurons (Qin et al., 2018). Pharmacological inhibitors of AChE, also known as anti-cholinesterases, effectively hinder the breakdown of ACh by the cholinesterase enzyme, thereby enhancing the level and duration of the neurotransmitter's action. Acetylcholinesterase inhibitors are well documented to confer neuroprotection against various neurodegenerative diseases and brain injuries (Pathak and Kabra, 2024). However, the use of these pharmacological inhibitors was found to be associated with toxicity thereby limiting their usage (Colovic et al., 2013). Sulforaphane, on the other hand is naturally occurring compound and shown to exert neuroprotection. For instance, the administration of sulforaphane-loaded iron oxide nanoparticles intranasally provided neuroprotection against Cisplatin-Induced Neurotoxicity in mice (Ibrahim Fouad et al., 2022). In line, sulforaphane enhanced cognitive performance and mitigated the decline of cholinergic neurons in the medial septal and hippocampal CA1 areas in mice exhibiting Alzheimer's disease characteristics (Zhang et al., 2014). Consistently, our finding of alleviation of GNP-induced brain damage substantiates above findings. Moreover, docking studies suggest that neuroprotective action of SFN presumably mediated through AChE inhibition by sulforaphane and thereby effectively preventing the GNP-induced brain tissue damage in the rats. Moreover, brain histology of SFN alone treated rats was comparable to that of control rats, highlighting the safety and efficacy of this natural compound. SFN is a major inducer of Nrf2-ARE pathway, activation of which upregulates antioxidants involved in protection against oxidative stress, a major causal factor in tissue damage and cell death. NRF2-ARE pathway was found to be involved in the protective effective SFN against several neurodegenerative diseases (Zhou et al., 2016). bSFN is also a reported to downregulate the expression of proinflammatory mediators and protect neurons against immune response (Qin et al., 2018). Given that SFN treatment reduced oxidative stress and inflammation, induced Nrf2, antioxidants, and neurotransmitters and prevented tissue damage, we propose that neuroprotective effects of SFN against GNPs presumable mediated by its ability to induce Nrf2-ARE antioxidant pathway blunt oxidative stress, inflammation and improve neuronal function.
5 Conclusions
Findings of this study revealed that GNPs can induce OS and inflammation and thus may cause significant injury to brain tissues. GNPs also reduced the expression of Nrf2, which resulted in reduced antioxidant levels. SFN significantly abrogated oxidative stress and inflammation and improved antioxidant levels by activating Nrf2-mediated gene expression. SFN also prevented GNP-induced histopathological changes in the brain tissues. Thus, regular consumption of an SFN-rich diet appears to have a beneficial effect on the brain.
Funding
The author did not receive support from any organization for the submitted work.
References
- The gold nanoparticle size and exposure duration effect on the liver and kidney function of rats: In vivo. Saudi J. Biol. Sci.. 2013;20(2):177-181.
- [CrossRef] [Google Scholar]
- The protective roles of vitamin E and α-lipoic acid against nephrotoxicity, lipid peroxidation, and inflammatory damage induced by gold Nanoparticles. Int. J. Nanomed.. 2020;15:729.
- [CrossRef] [Google Scholar]
- Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol.. 2015;28(6):1234-1245.
- [CrossRef] [Google Scholar]
- [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Meth. Enzymol.. 1999;299:15-27. Academic press
- [CrossRef] [Google Scholar]
- Improved method for the determination of blood glutathione. J. Lab. Clin. Med.. 1963;61:882-888.
- [Google Scholar]
- Advanced implications of nanotechnology in disease control and environmental perspectives. Biomed. Pharmacother.. 2023;158:114172
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol.. 2013;11(3):315-335.
- [CrossRef] [Google Scholar]
- Protective effect of sulforaphane against oxidative stress: recent advances. Exp. Toxicol. Pathol.. 2012;64(5):503-508.
- [CrossRef] [Google Scholar]
- Multifunctional gold nanoparticles: a novel nanomaterial for various medical applications and biological activities. Front. Bioeng. Biotechnol.. 2020;990
- [CrossRef] [Google Scholar]
- Effects of sulforaphane in the central nervous system. Eur. J. Pharmacol.. 2019;853:153-168.
- [CrossRef] [Google Scholar]
- Eco-friendly microwave synthesis of gold nanoparticles for attenuation of brain dysfunction in diabetic rats. J. Clust. Sci.. 2021;32(2):423-435.
- [CrossRef] [Google Scholar]
- Neuroprotective potential of intranasally delivered sulforaphane-loaded iron oxide nanoparticles against cisplatin-induced neurotoxicity. Neurotox. Res.. 2022;40(5):1479-1498.
- [CrossRef] [Google Scholar]
- Sulforaphane and its bifunctional analogs: synthesis and biological activity. Molecules. 2022;27(5):1750.
- [CrossRef] [Google Scholar]
- Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc.. 2010;132(5):1517-1519.
- [CrossRef] [Google Scholar]
- Immunohistochemistry of IL-1β, IL-6 and TNF-α in spleens of mice treated with gold nanoparticles. Saudi J. Biol. Sci.. 2020;27(4):1163-1168.
- [CrossRef] [Google Scholar]
- Potential role of α-lipoic acid and Ginkgo biloba against silver nanoparticles-induced neuronal apoptosis and blood-brain barrier impairments in rats. Life Sci.. 2018;212:251-260.
- [CrossRef] [Google Scholar]
- Inhibition of abnormal C/EBPβ/α-Syn signaling pathway through activation of Nrf2 ameliorates Parkinson's disease-like pathology. Aging Cell. 2023;22(10):e13958. Epub 2023 Aug 23
- [Google Scholar]
- Deducing the cellular mechanisms associated with the potential genotoxic impact of gold and silver engineered nanoparticles upon different lung epithelial cell lines in vitro. Nanotoxicology. 2022;1–21
- [CrossRef] [Google Scholar]
- Effect of gold nanoparticles shape and dose on immunological, hematological, inflammatory, and antioxidants parameters in male rabbit. Veterinary World. 2022;15(1):65.
- [CrossRef] [Google Scholar]
- Au-TiO2 nanoparticles exposure induced oxidative stress and neurotoxicity in rat. Biomarkers. 2021;26(3):240-247.
- [CrossRef] [Google Scholar]
- Nagababu, E., Rifkind, J.M., Boindala, S., Nakka, L., 2010. Assessment of antioxidant activity of eugenol in vitro and in vivo. In Free radicals and antioxidant protocols, 165-180. Humana Press. Doi: 10.1007/978-1-60327-029-8_10.
- Effect of arsenic acid withdrawal on hepatotoxicity and disruption of erythrocyte antioxidant defense system. Toxicol. Rep.. 2017;4:521-529.
- [CrossRef] [Google Scholar]
- Optimization of chitosan-α-casein nanoparticles for improved gene delivery: characterization, stability, and transfection efficiency. AAPS Pharm Sci Tech. 2019;20:1-12.
- [CrossRef] [Google Scholar]
- A comprehensive review of multi-target directed ligands in the treatment of Alzheimer’s disease. Bioorg Chem.. 2024;107152
- [CrossRef] [Google Scholar]
- Pietro, W.J., Hehre, W.J., 1983. Molecular orbital theory of the properties of inorganic and organometallic compounds. 3. STO‐3G basis sets for first‐and second‐row transition metals. J. Comput. Chem., 4(2), 241-251. Doi: 10.1002/jcc.540040215.
- From nature to nanotechnology: The interplay of traditional medicine, green chemistry, and biogenic metallic phytonanoparticles in modern healthcare innovation and sustainability. Biomed. Pharmacother.. 2024;170:116083
- [CrossRef] [Google Scholar]
- Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res.. 2018;133:218-235.
- [CrossRef] [Google Scholar]
- Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflammation. 2012;9(1):1-7.
- [CrossRef] [Google Scholar]
- Anatomic resolution of neurotransmitter-specific projections to the VTA reveals diversity of GABAergic inputs. Nat. Neurosci.. 2020;23(8):968-980.
- [CrossRef] [Google Scholar]
- Gold-and silver nanoparticles affect the growth characteristics of human embryonic neural precursor cells. PloS One. 2013;8(3):e58211.
- [Google Scholar]
- Bimetallic gold-platinum nanoparticles as a drug delivery system coated with a new drug to target glioblastoma. Colloids Surfaces b: Biointerfaces. 2022;214:112463
- [CrossRef] [Google Scholar]
- Sun, Y., Yang, T., K Leak, R., Chen, J., Zhang, F., 2017. Preventive and protective roles of dietary Nrf2 activators against central nervous system diseases. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 16(3), 326-338..
- Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat. Toxicol.. 2010;100(2):178-186.
- [CrossRef] [Google Scholar]
- Sulforaphane induces Nrf2 target genes and attenuates inflammatory gene expression in microglia from brain of young adult and aged mice. Exp. Gerontol.. 2016;73:42-48.
- [CrossRef] [Google Scholar]
- Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ.. 2020;707:135624
- [CrossRef] [Google Scholar]
- Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci. Total Environ.. 2011;409(8):1444-1452.
- [CrossRef] [Google Scholar]
- Sulforaphane attenuates microglia-mediated neuronal damage by down-regulating the ROS/autophagy/NLRP3 signal axis in fibrillar Aβ-activated microglia. Brain Res.. 2023;1801:148206
- [CrossRef] [Google Scholar]
- Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem. Pharmacol.. 2008;75(11):2214-2223.
- [CrossRef] [Google Scholar]
- Effect and molecular mechanism of Sulforaphane alleviates brain damage caused by acute carbon monoxide poisoning: Network pharmacology analysis, molecular docking, and experimental evidence. Environ. Toxicol.. 2024;39(3):1140-1162. Epub 2023 Oct 20
- [CrossRef] [Google Scholar]
- Synthesis, structural characterization, density functional theory (B3LYP) calculations, thermal behaviour, docking and antimicrobial activity of 4-amino-5-(heptadec-8-en-1-yl)-4H-1, 2, 4-triazole-3-thiol and its metal chelates. Appl Organomet Chem. 2018;32(12):e4535.
- [Google Scholar]
- Neuroprotective effects of sulforaphane on cholinergic neurons in mice with Alzheimer’s disease-like lesions. Int. J. Mol. Sci.. 2014;15(8):14396-14410.
- [CrossRef] [Google Scholar]
- Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev.. 2016;2016
- [CrossRef] [Google Scholar]







