Translate this page into:
Studying the effectiveness of Jatropha carcus L. Extract as a repellent, antifeedant, and toxic substance against red palm weevil (Rhynchophorus Ferrugineus) adult insects in Saudi Arabia
⁎Corresponding author. aanalharbi@kau.edu.sa (Asmaa Alharbi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Red palm weevil (RPW), Rhynchophorus ferrugineus, is a highly harmful pest that causes severe damage to date palm trees. The detrimental influence of RPW on date palm trees cannot be ignored and must be addressed. Chemical insecticides are available for controlling RPW; however, the insect population has become resistant to these chemicals, leading to damage and spread to new areas. The present investigation examined the impacts of physic nut (Jatropha curcas L.) seeds essential oils (PNEO) on laboratory-reared RPWs adult insects reared in a controlled laboratory environment.
Methods
The insecticidal, repellent, and antifeedant potentials of different doses (10, 20, 30, 40, 50%) of PNEO were tested against RPW. The insecticidal activity of J. curcas seeds oil extracts was assessed using topical application and feeding methods.
Results
RPW showed a high mortality rate when J. curcas seeds oil was applied topically after 24 h (P < 0.05). The mortality (%) of insects was 8, 76, 92, 96, and 100 for 10 %, 20 %, 30 %, 40 %, and 50 % concentrations, respectively. The LC50 and LC90 were 16.50 %, and 28.14 %, respectively. Results from the feeding method demonstrated a significant increase in mortality with a 100 % mortality rate at higher concentrations of PNEO compared to 10 and 20 % concentrations (P < 0.0001). The LC50 and LC90 were 24.42 % and 31.30 %, respectively. There was no significant decrease in food consumption and the antifeedant index when 10 % and 20 % of J. curcas seeds oil were used on the 3rd and 6th day after application. All J. curcas oil concentrations showed a repellent effect significantly higher than the controls (P < 0.05).
Conclusion
J. curcas seeds oil has shown potential insecticidal and repellent effects against RPW, and it could provide an eco-friendly tool for RPW pest management.
Keywords
Jatropha curcas L.
Rhynchophorus ferrugineus
Phoenix dactylifera L.
Adults insects
Toxicity
1 Introduction
Phoenix dactylifera L., the date palm tree, is a member of the Arecaceae family, which comprises over 2500 species and approximately 200 genera. The date palm tree is a valuable economic resource for numerous communities, especially in North Africa and the Middle East, that provides excellent environmental, social, and cultural importance. Most dates are produced and traded by Arab countries, making them the biggest producers and traders. About 1.35 million hectares of date palms are under cultivation in the Arab Region, accounting for 75 % of the global area. Arab Region provides more than 75 % of the worldwide date production or 8.46 million tons., which includes countries such as Saudi Arabia. Saudi Arabia is considered one of the world's leading producers of various cultivates of date palms: among 400 families cultivated, 25 are considered extremely valuable. The Ministry of Environment, Water, and Agriculture has recently announced that the Kingdom of Saudi Arabia produces more than 300 varieties of dates annually, exceeding 1.6 million tons. However, pest infestations are a problem for the cultivation and distribution of date palms (Al-Alawi et al., 2017; Ali Al-Shuraym, 2022; Ministry of Environment, Water, and Agriculture, 2023).
Red Palm Weevil (RPW), Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae), is a highly destructive pest that targets palm trees. Food and Agriculture Organization of the UN (FAO) has classified it as a category one pest of date palms in the Middle East. Worldwide, it damages about 40 palm species in different agroecosystems. Reports of infestations have been recorded in 45 countries worldwide. RPW was first reported in 1987 in AlQatif, a city in the Eastern Province of Saudi Arabia. Palm production communities across the globe endure considerable detriment due to the repercussions of RPW and the requisite actions to eliminate and manage it. It is estimated that at 1 and 5 % infestation, the annual loss from destroying severely infested palms in six Gulf countries ranges from US$ 5.18 million to US$ 25.92 million, respectively. The Vision 2030 initiative of Saudi Arabia seeks to decrease the RPW prevalence levels to less than 1 % by 2030. The Saudi Ministry of Environment, Water, and Agriculture (MEWA) has initiated a countrywide program to manage RPW(Ali-Bob, 2019).
Typically employed synthetic insecticides to control insect infestations can cause the development of resistance, damage beneficial insects, contaminate the atmosphere, and threaten human health. To address these issues, it is essential to switch to less harmful insecticides that are safer for the environment (Ahmed & Freed, 2021). Researchers have conducted studies on botanical bio-pesticides to replace the toxic pesticides used. Experimental research is still being worked on these botanicals. This means that botanicals could be used to protect local crops (Bashir & El Shafie, 2014).
J. curcas, also called the physic nut and scientifically known as J. curcas L., is a small tree or large shrub belonging to the genus Euphorbiaceae, whose seeds contain oil. This species originated in Central and North America. However, it has spread throughout tropical and subtropical areas worldwide, such as China, Southeast Asia, Africa, and India. Due to its high adaptability to drought and soil conditions, J. curcas can grow well in areas with low soil moisture and high temperatures, such as semi-arid and arid regions. Therefore, J. curcas has recently gained popularity as a potential biofuel plant in countries without oil production (Neupane et al., 2021).
The seeds of J. curcas contain phorbol ester, which is toxic to humans and animals and has fungicidal, molluscicidal, and insecticidal properties. Studies have shown that J. curcas seeds possess anthelmintic properties and can be mixed with palm oil and used as rat poison. J. curcas leaves are used in Ghana to fumigate houses against bedbugs. It has been scientifically proven that extracts from different parts of J. curcas, such as roots, stems, leaves, and seeds, have unique characteristics that are highly effective in controlling pests and diseases in various plants. For example, aqueous leaf extract can be applied to stored grains to control Ceratitis capitata (fruit fly) larvae and insect pests (Rhyzopertha dominica and Sitophilus zeamais). Moreover, ether extract shows antibiotic activity against Escherichia coli and Staphylococcus aureus. In addition, water snails were controlled in Germany with methanol extracts containing biodegradable toxins. Furthermore, oil and aqueous extracts of J. curcas were effective against cotton bull worms the pests of corn, pulses, and potatoes. Also, J. curcas leaf extract effectively controlled Spodoptera litura larvae, resulting in a mortality rate of 60 %. Further, methanol extract from seed oil controlled 100 % of the adult insects of termites (Odontotermes obesus). The anthracnose agent Colletotrichum musae can also be controlled by aqueous leaf extract from J. curcas. More recently, studies found that ethanolic extracts from J. curcas leaves inhibit the mycelial growth of Cercospora coffeicola by 20 % and ultimately reduce the germination of Hemileia vastatrix (Neupane et al., 2021).
Several components contribute to the toxicity of J. curcas seeds, including phorbol esters, saponins, lectins (curcin), phytates, curcalonic acid, and protease inhibitors (Bashir & El Shafie, 2014).
This study evaluated the effectiveness of J. curcas seeds oil against RPW adult insects as a choice candidate in controlling this insect pest in laboratory settings using RPW samples collected from five different provinces in Saudi Arabia. J. curcas seeds oil was evaluated for its insecticidal, repellent, and antifeedant efficacy against RPW adult insects in this study. This is the first study to evaluate the J. curcas seeds oil on RPW adult insects.
2 Materials and methods
2.1 Oil extraction
42 kg of fresh J. Curcas L. Seeds were purchased on the 5th of may 2021 from sinhal herbs (India) and shipped via DHL from India to Saudi Arabia (Shipment reference SH/EXP/61). The seeds were then washed and deshelled, and handly, the grains and shells were separated. The kernels were dried in a 40 °C oven for 24 h and stored in sealed plastic bags until used. To obtain J. Curcas oil, the dried kernels were pressed on a cold press machine (Longxuanyin, China). The extracted oil was filtered and transferred to a sample bottle for immediate use.
2.2 Dilutions assayed
Bioassay tests were conducted using 10 %, 20 %, 30 %, 40 %, and 50 % dilutions of J. curcas oil dissolved in ethanol. Two control groups of RPW adult insects were used; one group was treated with ethanol only, while the other remained untreated.
2.3 Insect collection and rearing
RPW larvae and adults (male and female) were collected from five provinces in Saudi Arabia, i.e., Al-Ahsa (Date Palm Research Center of Excellence, King Faisal University, Hofuf), Hail, Al-Madinah, Tabuk, and Taif. RPW were collected from highly infested palm tree farms that had not received insecticides during this study. Under the sterile glow of a laboratory hood, the field-collected RPW specimens were systematically moved from their temporary field containers into designated sterile plastic cages (35 cm × 25 cm × 14 cm) with pored covers to allow the air to enter the boxes to facilitate normal breathing. RPW adults were cultivated on uncontaminated, fresh, and pristine sugarcane stems (refreshed every five days) in a particular breeding room under the laboratory conditions of 25 ± 5 °C, 60 ± 6 % relative humidity (RH), and 12/12 h L/D photoperiod. Adults were kept for breeding and continuity of the colony. When eggs were hatched (a first-age larva), the larvae were reared individually in plastic dishes (4 cm × 4 cm × 2.5 cm) containing a prepared artificial diet for two weeks. After two weeks of feeding on the artificial diet, the larvae were transferred to larger dishes (9.5 cm × 4 cm) to allow them to reach the pupal stage, and sugarcane was introduced to the larvae at this stage.
2.4 Preparation of artificial diet
The larvae were acclimatized on a prepared artificial diet following Martin and Cabello (2006) method with minor modifications, as shown in Table 1. All ingredients (except the pharmaton capsules and agar) were first mixed with 950 ml of distilled water in a sterilized blender for one minute. Agar was melted in a glass beaker with distilled water (800 ml) after being microwaved at maximum power for 5–8 min. The microwaved agar gel was then added to the main ingredients in the blender and blended for two minutes. While the mixture was being blended, pharmaton capsules were added. The mixture was then poured into sterilized plastic dishes (14 cm × 8 cm × 3 cm) and allowed to harden at room temperature. Once set at room temperature, the dishes were refrigerated at 4 °C until they were used. In order to use the artificial environment, it was left out of the refrigerator for a while to reach room temperature.
Component
Amount
Distilled water
1750 ml
Agar
37 g
Instant yeast
90 g
Wheat grains
90 g
Corn flour
90 g
Sorbic acid
3.2 g
Ascorbic acid
8 g
Aminobenzoic acid
3.2 g
Pharmaton capsule
4 capsules
2.5 Bioassay experiments
The bioassay experiments of J. curcas oil as bio-insecticide to control RPW adult insects were evaluated by topical application (spraying) and feeding techniques, as discussed below.
2.5.1 Evaluation of the topical application method of J. Curcas seeds oil on the mortality rate of RPW adults
For insects, topical application is the most common method of exposure to insecticide. Topical application is a reliable method for evaluating the contact toxicity of insecticides on insects (Yu, 2008).
To prepare different concentrations of the J. curcas oil, the oil was first dissolved in a relatively nontoxic and volatile solvent (ethanol). Then, a known volume of the J. curcas solution was sprayed onto the insect's body surface using an ultra-low volume sprayer. The amount of spread volume was estimated to be 1 ml. This method does not indicate how much insecticide enters the insect's body.
For the bioassay, 175 RPW adults were used. For each treatment, 25 adults were used (each treatment contained five replicates, and each replicate contained five adults). The in vivo testing of the bio-insecticidal activity of J. curcas oil was fulfilled by using five separate concentrations of PNEO (10, 20, 30, 40, and 50 %). The treated insects were allowed to dry before being transferred to experimental plastic dishes (21 cm × 12 cm × 13.5 cm) with pored covers for further observation. Insects were checked 24 h post-treatment, and mortality was recorded. Insects were considered dead when they failed to respond to gentle touch with a camel hairbrush or finger grasping. Two control groups of RPW adults were used; one group was treated with 50 % ethanol only, while the other remained untreated.
2.5.2 Evaluation of the feeding method of J. Curcas seeds oil on the mortality rate of RPW adults and antifeedant activity
For the bioassay, 175 RPW Adults were used. For each treatment, 25 adults were used (each treatment contained five replicates, and each replicate contained five adults). Adult insects were placed individually in experimental plastic dishes (9.5 cm × 9.5 cm × 4 cm) with pored covers without starving them. The adults were then fed separately from the sugarcane that had been immersed for 30 s in various concentrations of J. curcas oil (10 %, 20 %, 30 %, 40 %, and 50 %). Two groups of insects were used as controls; one was fed with sugarcane and immersed in 50 % ethanol only, and the other was left untreated. The mortality count was conducted after 24 h. Adults were considered dead when they failed to respond to gentle touch with a camel hairbrush or finger grasping. The unconsumed sugarcane in the dish was weighed on day three and day six after treatment, and the amount of food consumed was estimated to evaluate antifeedant activity. The % antifeedant index was calculated by applying the formula of Ben Jannet et al. (2000). where C = Consumed sugarcane in control, T = Consumed sugarcane in treatment.
2.6 Repellency test
Zhang et al.'s (2011) method was adopted with some modifications to assess the repellent activity of J. curcas against RPW adults. Whatman filter paper No.1 was divided into two halves; the first was treated with 3 ml of ethanol, and the other half was coated with 3 ml of PNEO at (10, 20, 30, 40, and 50 (v/v). The treated filter papers were allowed to dry for 15 min before being tested for repellency. Following solvent evaporation, the remaining residue on the filter paper was carefully spread into a uniform layer, transforming into a makeshift floor for a circular plastic box; the control was a circular white filter paper neatly divided in half. One half received ethanol, while the other remained untouched. For each treatment, 12 adults were used (each treatment contained three replicates, and each replicate contained four adults). Insect deployment (4 per box) commenced from central positions, followed by distribution monitoring. The distribution of the insects was observed every hour for 14 h and post 24 h. After this exposure period, the percentage repellency (PR%) was calculated according to Nerio et al.(2009) using the formula shown below: where Nc = number of insects found in the untreated zone. Nt = number of insects found in the treated area.
According to McDonald et al. (1970), To measure the effectiveness of different repellents, scientists devised a classification system with five distinct levels. Class 0, represented by a thin red line on a graph (think barely a blip), marks the baseline of minimal protection. As the lines thicken and climb (green for Class I, blue for Class II, and so on), they depict increasing repellency, reaching a plateau of near-impenetrability with the golden line of Class V. This system allows researchers to compare and categorize repellents based on their performance against RPW.
2.7 Data analysis
Laboratory toxicity results were analyzed using a standardized statistical program (LDP Line Program) following Finney's (1971) method, which guided the analysis of in vitro bioinsecticide toxicity data with specialized software facilitating curve generation and statistical parameter extraction (Bakr, 2005).
In each test, treatments were arranged in a completely randomized design. Each treatment was replicated five times and three times for the repellency test. The data on the larvicidal potency of PNEO were collected and then carefully arranged in a table showcasing the tested PNEO concentrations, the resulting mortalities, and the calculated Chi-square values. Like detectives sifting through clues, scientists used these numbers to extract key insights: the lethal concentrations, LC50 and LC90, revealing the doses where PNEO's power reaches its peak.
Windows version of GraphPad Prism (10.0.2, 2010, Boston, USA (https://www.graphpad.com) was used to analyze the mean of triplicate data. The data collected were subjected to a comparative statistical analysis using analysis of variance (ANOVA) to calculate the significant difference. Results with P ≤ 0.05 were considered statistically significant; (*, and #) represent the significant difference. Mean ± SEM was also calculated for the analysis of variance. We used the Tukeys' standardized range test to compare the variation between time intervals.
3 Results
3.1 Insecticidal efficacy of J. Curcas oil on RPW adults
The present study aimed to investigate the insecticidal effect of J. curcas seeds oil against RPW adults, and the results are shown below.
3.1.1 Evaluation of the topical application of J. Curcas seeds oil on the mortality rate of RPW adults
The results of the topical application method on RPW adults are shown in Table 2. The results of the topical application method on adults of RPW (Fig. 2) demonstrated a significant increase in mortality when 20 %, 30 %, 40 %, and 50 % of J. curcas oil concentrations were used when compared to 10 % concentration (P < 0.0001). Moreover, there was a significant increase in mortality when 50 % of J. curcas oil concentrations were compared to 20 % concentration (P < 0.05). However, there was no sense in mortality caused by PNEO at 30, 40, and 50 % when compared to each other. a: Each replicate contains five insects. b: Tabulated χ2 = 7.8. A χ2 value in the table greater than the one calculated at a significance level of 0.05 suggests that the results are homogeneous. c: Lethal Concentration (LC). d: Low Concentration Limit (LCL), Upper Concentration Limit (UCL).
Treatment Method
Concentration%
Mortality (%)a
Slope
χ2 b
LC 50 %
LC 90 % c
(LCL-UCL) d
(LCL-UCL)
Topical Application
10
8
5.53 ± 0.83
1.91
16.5(13.90–18.96)
28.14(24.17–35.15)
20
76
30
92
40
96
50
100
Feeding
10
32
4.41 ± 0.68
20.52
24.42
−31.30
−
20
32
30
100
40
100
50
100
3.1.2 Evaluation of the feeding method of J. Curcas seeds oil on the mortality rate of RPW adults
The PNEO concentration in the feeding method ranged from 10 % to 50 %. The results are shown in Table 2 and Figs. 1 and 3, with a mortality ratio of 32–100 % for the lowest and highest concentrations, respectively. In contrast, neither the control nor the ethanol groups experienced mortality. The LC50 and LC90 were determined to be 24.42 % and 31.30 % respectively (p < 0.05). Furthermore, the toxicity slope was 4.41 ± 0.68.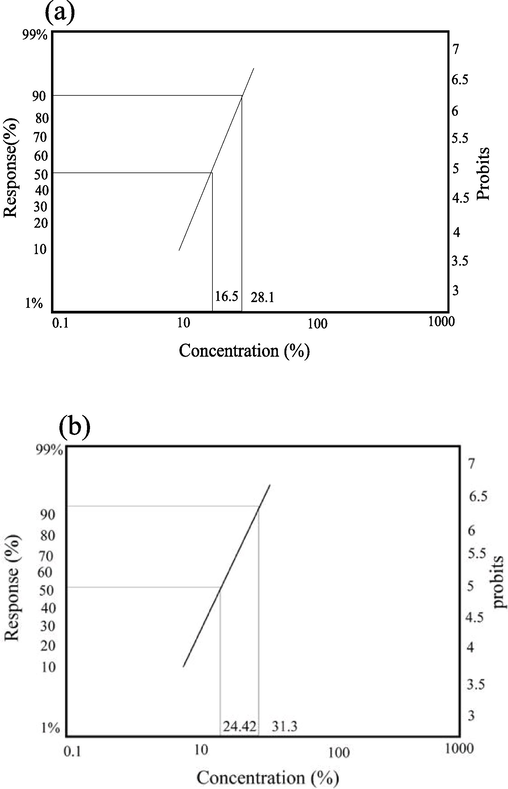
The regression line for J. curcas seeds oil against RPW adults using topical application (a) and feeding method (b). For the topical application (a), The LC50 was 16.50, and the LC90 was 28.14 at P < 0.05. The toxicity slope was 5.53 ± 0.83. For the feeding method (b), the LC50 was 24.42 and the LC90 was 31.30 at P < 0.05. The toxicity slope was 4.41 ± 0.68.
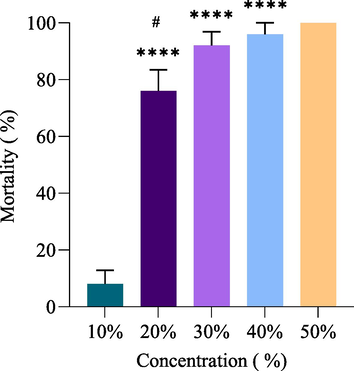
Effect of J. curcas by the topical application method on the mortality of RPW adults after 24 h. Data are presented as Mean ± SEM; **** significant versus 10 % of J. curcas oil concentration and # significant versus 50 % of J. curcas oil concentration. P-value ≤ 0.05 was recognized as statistically significant.
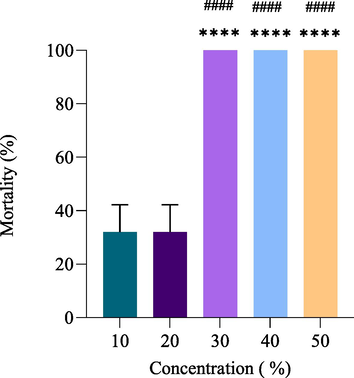
Effect of J. curcas seeds oil by feeding method on the mortality of RPW adults after 24 h. Data are presented as Mean±SEM; **** significant versus 10% of J. curcas oil concentration; #### significant versus 20% of J. curcas oil concentration. P-value ≤0.05 was recognized as statistically significant.
Results from the feeding method on adults of RPW (Fig. 3) demonstrated a significant increase in mortality when 30 %, 40 %, and 50 % of J. curcas oil concentrations were used compared to using 10 %. of J. curcas oil concentration (P < 0.0001). Compared to 10 % J. curcas oil concentration, 20 % of J. curcas oil concentrations showed no significant difference in mortality. Compared to 20 % of J. curcas oil concentration, 30 %, 40 %, and 50 % of PNEO showed a considerable increase in mortality (P < 0.0001). However, compared to each other, there is no sense in mortality between PNEO 30 %, 40 %, and 50 % dose levels.
3.1.3 Effect of J. Curcas seed oil on RPW adult's food consumption (antifeedant activity)
Fig. 4 and Table 3 show the effect of PNEO on adults's food consumption. When 30 %, 40 %, and 50 % of J. curcas seed oil were used, all the adult insects died after 24 h. On the third day, there was no significant decrease in food consumed by adults when 10 % and 20 % of J. curcas seed oil was used compared to controls. All insects died after 24 h when 30 %, 40 %, and 50 % of J. curcas seed oil were used. Data are presented as Mean ± SEM. P-value ≤ 0.05 was recognized as statistically significant. E: ethanol-treated control; C: untreated-control.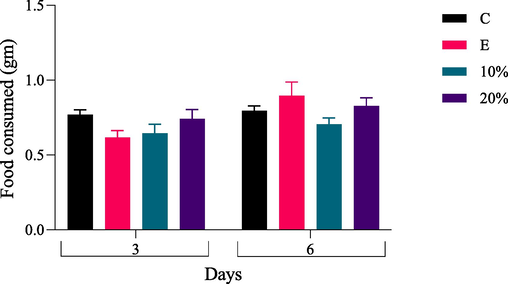
Impact of PNEO (10 and 20 %) levels on adult's food consumption on the third and sixth day of treatment. All insects died after 24 h when 30 %, 40 %, and 50 % of J. curcas seed oil were used. Data are presented as Mean ± SEM. P-value ≤ 0.05 was recognized as statistically significant; Each treatment contained five replicates, and each replicate contained five insects. E: ethanol-treated control; C: untreated-control; gm: gram.
Days
Groups
C
E
10 %
20 %
3rd Day
0.77 ± 0.03
0.62 ± 0.05
0.64 ± 0.06
0.74 ± 0.06
6th Day
0.80 ± 0.03
0.90 ± 0.09
0.71 ± 0.04
0.83 ± 0.05
Furthermore, on the sixth day, there was no significant decrease in food consumed by adults when 10 and 20 % of J. curcas seed oil were used compared to controls.
The results of the antifeedant efficacy of J. curcas seeds oil on the adults of RPW are shown in Table 4. The percentage of the antifeeding index of 10 and 20 % of J. curcas seeds oil concentrations were 8.74 and 1.78 %, respectively, whereas ethanol-treated control was 10.98 % on the third day. However, on the sixth day, the percentage of the antifeeding index of 10 %, and 20 % of J. curcas seeds oil concentrations were 6.04 % and −1.89 %, respectively, whereas ethanol-treated control was −5.86 %. There was a non-significant decrease in the antifeeding index on the 6th day of treatment compared to the 3rd day.
Treatment
3rd day
6th day
Untreated-control (C)
−
−
Ethanol-treated control (E)
10.98 %
−5.86 %
J. curcas oil 10 %
8.74 %
6.04 %
J. curcas oil 20 %
1.78 %
−1.89 %
3.2 Repellency test
The repellency of PNEO concentrations is shown in Table 5 and Fig. 5. The study revealed an increased repellent effect on RPW at higher concentrations. All concentrations assayed on RPW adults produced a repellent effect significantly higher than the controls (P-value ≤ 0.05). Compared to 10 % of J. curcas oil concentration, 20 %, 30 %, 40 %, and 50 % of J. curcas oil concentrations showed a significant increase in PR% (P = 0.0017, P < 0.0001, P < 0.0001, and P < 0.0001, respectively). There was a considerable increase in PR% when 40 % and 50 % of J. curcas oil concentrations were compared to 20 % of J. curcas oil concentration (P = 0.0043 and P = 0.0005, respectively). However, there was no significant difference in PR% when 30 % of J. curcas oil concentration was compared to 20 % of J. curcas oil concentration. In addition, there was no significant difference in PR% between 30 %, 40 %, and 50 % of J. curcas oil concentrations when compared to each other. The RPW showed the highest percentage repellency (70 %) at 50 % v/v, while the least repellent effect was observed at 10 % v/v, with only 33 % repellency after 24 h of exposure. Data are presented as Mean ± SEM; * significant versus the control group (ethanol (E)). P-value ≤ 0.05 was recognized as statistically significant; # significant versus 10 % of J. curcas oil concentration, and +significant versus 20 % of J. curcas oil concentration.
Concentration (% v/v)
PR%
Class
Control (ethanol)
16.67 ± 1.93
I
10 % J. curcas seed oil
33.33 ± 1.93**
II
20 % J.curcas seed oil
50.37 ± 1.61****, ##
III
30 % J.curcas seed oil
60 ± 1.93****, ####
III
40 % J.curcas seed oil
65.56 ± 1.11****, ####, ++
IV
50 % J.curcas seed oil
70 ± 3.85****, ####, +++
IV
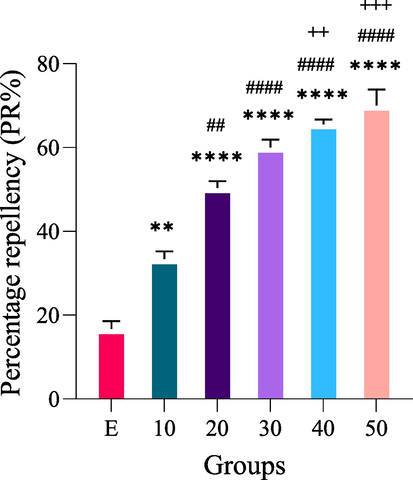
Percentage repellency (PR%) of J.curcas seed oil extract on RPW adults. Data are presented as Mean ± SEM; * significant versus the control group (ethanol (E)); # significant versus 10 % of J. curcas oil concentration; and +significant versus 20 % of J. curcas oil concentration. P-value ≤ 0.05 was recognized as statistically significant.
4 Discussion
The prolonged use of synthetic insecticides has raised serious health and environmental concerns. Consequently, plant insecticidal phytochemicals garner considerable scientific interest (Alghamdi, 2021). Thus, bioagents such as PNEO may effectively manage pests without harming the environment or human health (Valdez-Ramirez et al., 2023). Accordingly, this study investigates the insecticidal effect (contact and feeding methods), antifeedant potential, and repellent activity of PNEO against RPW adults. In general, the current results of the J. curcas seeds oil extract showed higher insecticidal potentiality against RPW adults by both methods. Furthermore, this investigation demonstrated a positive correlation between the PNEO concentrations and the % mortality of insects attributed to these concentrations.
There was a slight no significant decrease in the antifeedant index, which could be attributed to the biodegradability property of J. curcas seeds oil (Woma et al., 2019). The mortality results obtained through the feeding method were consistent with the antifeedant activity results. On the other hand, the lower concentrations did not significantly impact mortality through the feeding method compared to the higher concentrations used.
The current study's findings align with Valdez-Ramirez et al. (2023) systematic review, which summarizes the previous studies on the impact of J. curcas on insects in various crops. Based on the review of Valdez-Ramirez et al., 39 articles investigated the insect-static and insecticidal activity of various extracts of PNEO on different taxonomic orders. Insect pests that infest stored cereals, cabbage, and sorghum, including fruit flies and arid locusts, were effectively controlled with physic nut seeds and leaves' methanolic and aqueous extracts, seeds' extract of ether, and seed oil. A high mortality rate, control of the population, reduction in oviposition, diminishment in hatchability, and an increase in antifeedant effect were caused by the extracts. Previous studies have shown that using the appropriate solvent and application method (contact or food) is crucial to enhancing the bioactivity of botanical extracts.
Similar findings were observed by Al-Otaibi et al. (2020) in examining the susceptibility of RPW larvae to several chemical insecticides commonly employed in the Makkah region of Saudi Arabia. Feeding and dipping techniques were used to test the effectiveness of three pesticides. According to Al-Otaibi et al. (2020), feeding techniques mixed with diet were more effective than dipping. Previous investigations demonstrated that the efficacy of injecting insecticide into date palm trunks in the field, simulating the process of combining the pesticide with diet in a laboratory setting, was superior to sprinkling. It is possible that spraying is ineffective because the insects are hidden in the base of the frond palm, so it is difficult for an insecticide to reach these areas. In addition, injection treatment was more effective than spraying because sunlight photolyzed insecticide compounds. Consequently, incorporating the active ingredient into the plant body or the diet safeguards it against environmental influences and mitigates the rate of functional ingredient degradation (Al-Otaibi et al., 2020).
Moreover, the present study revealed that J. curcas seeds oil extract was also an efficient antifeedant against RPW larvae. This result agreed with several previous studies that were done on different insects.
Physic nut seed oil has been found to have insecticidal and antifeedant properties against a wide range of insects. In some cases, its effectiveness is comparable to synthetic insecticides (Bashir & El Shafie, 2013). Also, physic nut oil has been shown to exhibit insecticidal effects by contact with aphids (Aphis fabae) that infest broad beans (Vicia fabae). Likewise, physic nut oil has been shown to influence the pests infested cowpea (Vigna unguiculata (Habou et al., 2011).
Moreover, Habou et al. (2014) reported that J. curcas seed oil is effective against two bruchid beetle species, Callosobruchus maculatus Fab and Bruchidius atrolineatus Pic, which cause great damage to stored cowpea seeds (Vigna unguiculata). J. curcas oil concentrations ranging from 0.25 to 7.5 ml were mixed with 200 g of cowpea seeds before introducing 10 pairs of Callosobruchus maculatus or Bruchidius atrolineatus insects. In both species, J. curcas oil reduced adult survival. As a result, they concluded that the seed oil of J. curcas had a toxic effect on the adults of C.maculatus and B. atrolineatus. As the concentration increased, the level of toxicity also increased. When weaker concentrations were utilized, the females of both species laid fewer eggs. B. atrolineatus showed a higher emergence rate of adults than C. maculatus.
A previous study carried out two experiments on adult insects of Rhyzorpertha dominica (S.) (Coleoptera: Bostrychidae) and Sitophilus zeamais (M.) (Coleoptera: Curculionidae). The prepared water extract from PN seeds and pericarps at 10 % and 5 % resulted in 100 % and 75 % mortality of R. dominica and S. zeamais insects, respectively. In another study, PN seed oil prepared with various extraction procedures (roasting, crude extract, and cooking) was evaluated on the adult insect of corn weevils S. zeamais. Roasted seed oil was associated with the highest level of mortality (84.68 %). Furthermore, seed oil and leaf extract from J. curcas were evaluated on S. zeamais in a previous study. Using seed oil increased grain damage resistance, oviposition inhibited, adult hatching decreased, and mortality increased. In another study, an ingestion bioassay was conducted where seed powders from J. curcas were used to control adult insects of S. zeamais. Seed powder increased the mortality rate (Valdez-Ramirez et al., 2023).
In addition, aqueous extracts and powders of PN seeds were used to control Callosobruchus maculatus (C maculatus), which infested cowpea seeds by contact. During the exposure to the extract, the mortality was increased. Another study evaluated the impact of ethanolic extracts of PN seeds on cowpea seeds infested by adults of C. maculatus. Insects exposed to the highest extract concentration showed an increase in lipid peroxidation and suffered from oxidation, indicating the impairment of their essential organs (Valdez-Ramirez et al., 2023).
The impact of PN seeds' aqueous extract on adult P. sjostedti (Jac.) (Coleoptera: Chrysomeloidea) and Podagrica uniforma (Jac.) was also reported in a previous study. Ingestion bioassays of the extract reduced beetle populations (Valdez-Ramirez et al., 2023).
Results from previous studies conducted on insects in the same taxonomic order (Coleoptera) were consistent with the findings reported in this study. In this regard, several studies have shown that oils, extracts, or compounds from various parts of the J. curcas possessed insecticidal and deterrent properties against pest insects. Its seeds are particularly toxic to insects, and this toxicity is attributed to several components, including saponins, lectins, curcine, phytates, protease inhibitors, curcalonic acid, and phorbol esters (Valdez-Ramirez et al., 2023).
Previous investigation has noted that PN seeds' aqueous extracts contain many saponins that exhibit pronounced bitterness, insect repellency, and toxicity. The consumption of this substance resulted in stunting and reduced ration energy value. J. curcas compounds have been found to affect insects' chemoreceptors and prevent food intake (Diabaté et al., 2014).
In two studies, gas chromatography and mass spectrometry were used to determine the main compounds in the seeds of J. curcas. The primary fatty acids in the PN seeds were vaccenic, octadecenal, stearic, linoleic, oleic, palmitoleic, and palmitic acid (Babarinde et al., 2019; Figueroa-Brito et al., 2021). Based on some studies on Helicoverpa zea, these fatty acids caused mortality and inhibited neuronal cells by inducing apoptotic cell death (Ren et al., 2019). The linolenic and mono expoy-linolinc acid had a transient uncoupling effect on S. frugiperda (Sf-21) cells, decreasing respiration by elevating the oligomycin amount. In contrast, linoleic acid diols blocked the electron transport chain (Moran et al., 2001).
In two other studies, phytochemical screening of J. curcas seeds and leaves identified secondary metabolites, including phenols, saponins, flavonoids, tannins, steroids, alkaloids, and terpenoids (Jide-Ojo et al., 2013; Ukpai et al., 2017).
Although most J. curcas botanical extracts are not harmful to humans, seeds can be poisonous due to the phorbol esters up to 6 mg/g in toxic species grown in areas of Mexico, Asia, and Africa. However, some Mexican regions use nontoxic PN varieties that contain 0.27 mg/g phorbol esters for human and livestock consumption (Valdez-Ramirez et al., 2023).
Phorbol esters are the most toxic compound found in J. curcas. It can lead to severe toxicity, triggering a robust inflammatory reaction and chronic toxicity. It stimulates tumor development by binding and activating protein kinase C (PKC), which is responsible for causing cell differentiation and promoting growth regulation. Apoptosis or cellular proliferation results from this phenomenon. Although PKC receptors are found in all tissues, their abundance is most significant in the neuronal tissues of insects, where phorbol esters are inserted into the cell membrane. This enzyme is essential for signal transduction which controls cell growth and differentiation. Proliferation and differentiation of cells are manifested by inflammation when this enzyme is activated. Therefore, phorbol ester consumption would irritate mucous membranes and cause hemolysis (Diabaté et al., 2014; Muniz et al., 2020).
Devappa et al. (2012) evaluated the efficacy of fractions enriched in phorbol ester from J. curcas seed oil on Spodoptera frugiperda. Toxic effects and reduction in feed consumption were observed upon topical contact.
The seeds and leaves of PN had a toxic protein called curcin with toxic lectin activity and protease inhibitor properties. Curcin is a potent plant toxin that negatively impacts insect biology and results in the mortality of larvae and adults. The seeds of J. curcas contained more curcin than the leaves. It has been shown that curcin is a ribosome-inactivating protein that inhibits protein translation by inactivating ribosomal subunits in the liver. In addition, previous studies have shown that the aqueous extracts of J. curcas containing protease inhibitors were utilized to impede insect growth and development by reducing pests' digestibility of plant tissues. Additionally, inducing excessive losses of unprocessed fecal proteins (Diabaté et al., 2014).
According to the repellency test, the results of the current study revealed that J. curcas seed oil has insect-repellent properties, confirming previous studies on different insects. This study's results matched the results of Diabaté et al.(2014) study. Based on the findings of their study, the aqueous extract of J. curcas seeds had a repellent effect on P. xylostella larvae.
Similarly, Ubulom et al.(2021) found that J curcas seeds oil had repellent and insecticidal properties against American cockroaches. These results suggest that J. curcas seeds extract could be a potential alternative to the existing chemical insecticides used in crop protection, and the reason for this was the chemical compounds that were present in the oil.
By using botanical extracts from J. curcas, farmers can reduce the consumption of synthetic chemical insecticides while producing food in a sustainable manner and reducing the harmful impact of chemical insecticides on the environment. Thus, extracts of J. curcas can be deployed as a sustainable pest control method since they have fungicidal, nematicidal, molluscicidal, acaricidal, insecticidal, and antifeedant effects (Valdez-Ramirez et al., 2023).
5 Conclusion
J. curcas seed oil exhibits insecticidal and repellent properties against RPW adults. According to the results of the current study, both application methods (food or contact) were effective against RPW. Thus, the menace caused by this pest could be reduced using J. curcas seed oil. The results of the current study, along with other studies, showed that botanical extracts are an excellent alternative to synthetic chemical insecticides because they are easy to apply, eco-friendly, sustainable, and affordable. Therefore, oil extract from seeds of J curcas may be used to replace synthetic insecticides against RPW and can be incorporated into integrated and sustainable insect control practices.
5.1 Limitations
The study primarily focuses on the short-term efficacy of PNEO in controlling RPW adults through insecticidal, antifeedant, and repellent activities. However, the long-term effects of repeated or widespread use of PNEO on non-target organisms, ecological interactions, and ecosystem dynamics are not evaluated. While the study suggests that PNEO may offer an environmentally friendly alternative to synthetic insecticides, the potential ecotoxicological risks associated with its use are not thoroughly examined. This includes the impacts of PNEO residues on beneficial insects, soil organisms, aquatic ecosystems, and non-target plants. The study does not investigate the likelihood of RPW developing resistance to PNEO over time with repeated exposure.
5.2 Future research on the J. Curcas seed oil
As this study was conducted under laboratory conditions, it is recommended that field trials are conducted to evaluate the bioactivity of PNEO against RPW. Future research should include comprehensive environmental risk assessments that consider the long-term impacts of PNEO on both target and non-target organisms and ecosystem-level effects. Additionally, studies should evaluate strategies to mitigate potential risks associated with using PNEO, such as implementing integrated pest management approaches and monitoring signs of resistance development.
6 Authors' contributions
Conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing–review and editing the manuscript: Asmaa Alharbi; experiment design, methodology, collecting and preparing the materials, investigation, software and data analysis, writing the initial draft: Ahlam Alanazi.
7 Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Ethics approval
Not applicable in this paper.
CRediT authorship contribution statement
Asmaa Alharbi: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization. Ahlam Alanazi: Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Formal analysis, Data curation.
Acknowledgements
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (G:318-247-1443). The authors, therefore, gratefully acknowledge the DSR technical and financial support. The authors would like to thank Dr. Nashi Alqahtani, Dr. Hamadttu Elshafie, and Ibrahim Bou-Khowa from Date Palm Research Center of Excellence, King Faisal University, for their assistance and support.
Declaration of competing interest
The authors declare no conflict of interest or personal relationship that could have appeared to influence the work reported in this study. All authors have read and approved this submission and have given appropriate credit to everyone who participated in this work.
References
- Virulence of Beauveria bassiana Balsamo to red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Egypti. J. Biol. Pest Control.. 2021;31(1):77.
- [CrossRef] [Google Scholar]
- Date palm tree (Phoenix dactylifera L.): natural products and therapeutic options. Front. Plant Sci.. 2017;8(5):1-12.
- [CrossRef] [Google Scholar]
- Impact of the invasive plant species “Nicotiana glauca” toxins on the larvae of the invasive insect species “Rhynchophorus ferrugineus”: a damaging pest of date palm trees in Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(1):1154-1157.
- [CrossRef] [Google Scholar]
- The impact of the onion-garlic extracts to control date palm aphids in Saudi Arabia. J. Saudi Soc. Agric. Sci.. 2022;21(8):546-551.
- [CrossRef] [Google Scholar]
- Management of the red palm weevil Rhynchophorus ferrugineus (Olivier) using sustainable options in Saudi Arabia. Arab J. Plant Protect.. 2019;37(2):163-169.
- [CrossRef] [Google Scholar]
- Evaluation of fiprol, imidaprid and dueracide insecticides against larval stage of red palm weevil Rhynchophorus ferrugineus (Olivier) in Makkah Al-Mukarramah region. Biosci. Biotechnol. Res. Asia. 2020;17(2):319-327.
- [CrossRef] [Google Scholar]
- Chemical composition and toxicity of Jatropha curcas seed oil against Sitophilus zeamais Motschulsky as affected by pre-extraction treatment of seeds. Biocatal. Agric. Biotechnol.. 2019;21(7):101333
- [CrossRef] [Google Scholar]
- A new software for measuring leaf area, and area damaged by Tetranychus urticae Koch. J. Appl. Entomol.. 2005;129(3):173-175.
- [CrossRef] [Google Scholar]
- Insecticidal and Antifeedant Efficacy of Jatropha oil extract against the Desert Locust, Schistocerca gregaria (Forskal) (Orthoptera: Acrididae) Agric. Biol. J. N. Am.. 2013;4(3):260-267.
- [CrossRef] [Google Scholar]
- Toxicity, antifeedant and growth regulating potential of three plant extracts against the desert locust Schistocerca gregaria Forskal (Orthoptera: Acrididae) Am. J. Exp. Agric.. 2014;4(8):959-970.
- [CrossRef] [Google Scholar]
- Responses of Spodoptera littoralis larvae to Tunisian plant extracts and to neo-clerodane diterpenoids isolated from Ajuga pseudoiva leaves. Fitoterapia. 2000;71(2):105-112.
- [CrossRef] [Google Scholar]
- Potential of using phorbol esters as an insecticide against Spodoptera frugiperda. Ind. Crop. Prod.. 2012;38(1):50-53.
- [CrossRef] [Google Scholar]
- Toxicity, antifeedant and repellent effect of Azadirachta indica (A. Juss) and Jatropha carcus L. aqueous extracts against Plutella xylostella (Lepidoptera: Plutellidae) J. Basic Appl. Sci. Res.. 2014;4(11):51-60. www.textroad.com
- [Google Scholar]
- Chemical composition of Jatropha curcas1 seed extracts and its bioactivity against Copitarsia decolora2 under laboratory and greenhouse conditions. Southwestern Entomol.. 2021;46(1):103-114.
- [CrossRef] [Google Scholar]
- Insecticidal effect of Jatropha curcas L. seed oil on Callosobruchus maculatus Fab and Bruchidius atrolineatus Pic (Coleoptera: Bruchidae) on stored cowpea seeds (Vigna unguiculata L. Walp.) in Niger. Afr. J. Agric. Res.. 2014;9(32):2506-2510.
- [CrossRef] [Google Scholar]
- Habou, Z. A., Haougui, A., Mergeai, G., Haubruge, E., Toudou, A., Verheggen, F.J., 2011. Insecticidal effect of Jatropha curcas oil on the aphid Aphis fabae (Hemiptera: Aphididae) and on the Main Insect Pests Associated with Cowpeas (Vigna unguiculata) in Niger. Tropicultura. 29(4), 225–230. http://www.doaj.org/doaj?func=abstract&id=903445%5Cnhttp://workspace.bananahill.net/library/Crops/Jatropha Curcas/2011_Habou_ZA_et_al_Jatro_insecticide_cowpeas.pdf.
- Extracts of Jatropha curcas L. exhibit significant insecticidal and grain protectant effects against maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae) J. Stored Prod. Postharvest Res.. 2013;4(3):44-50.
- [CrossRef] [Google Scholar]
- Rearing management of red palm weevil, Rhynchophorus ferrugineus (Olivier,1790) (Coleoptera, Dryophthoridae), in artificial diet and effects on biology and adult biometry. Boletín Sanidad Vegetal Plagas.. 2006;32:631-641.
- [Google Scholar]
- McDonald, L. L., Guy, R.H., Speirs, R.D., 1970. Preliminary evaluation of news candidates materials as toxicants, repellents and attractants against stored product insects. Marketing Research Report. No. 882. Wanshington, U.S. Department of Agriculture . In Agriculture Research Service.183. https://ageconsearch.umn.edu/record/312345/files/mrr882.pdf.
- Ministry of Environment, Water, and Agriculture, 2023. Available at: https://mewa.gov.sa/ar/MediaCenter/News/Pages/News6742020.aspx?fbclid=IwAR392ojuv3hscGkt915lcbpZVCU_(Accessed: 1 may 2024).
- Defining mechanisms of toxicity for linoleic acid monoepoxides and diols in Sf-21 cells. Chem. Res. Toxicol.. 2001;14(4):431-437.
- [CrossRef] [Google Scholar]
- Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera) J. Stored Prod. Res.. 2009;45(3):212-214.
- [CrossRef] [Google Scholar]
- Growing jatropha (Jatropha curcas L.) as a potential. Inventions.. 2021;6(4):60.
- [CrossRef] [Google Scholar]
- AW1 neuronal cell cytotoxicity: the mode of action of insecticidal fatty acids. J. Agric. Food Chem.. 2019;67(43):12129-12136.
- [CrossRef] [Google Scholar]
- Repellency and insecticidal properties of seed oil of Jatropha curcas L. against American cockroach, Periplaneta americana L. J. Basic Appl. Zool.. 2021;82(1)
- [CrossRef] [Google Scholar]
- Ukpai, O. M., Ibediungha, B. N., Ehisianya, C.N., 2017. Potential of seed dusts of Jatropha curcas L., Thevetia peruviana (Pers.), and Piper guineense Schumach. Against the maize weevil, Sitophilus zeamais (Motschulsky, 1855) (Coleoptera: Curculionidae) in storage of corn grain. Polish J. Entomol. 86(3), 237–250. doi: 10.1515/pjen-2017-0014.
- A systematic review of the bioactivity of Jatropha curcas L. (Euphorbiaceae) extracts in the control of insect pests. Sustainability.. 2023;15(15):11637.
- [CrossRef] [Google Scholar]
- Nigeria Jatropha oil as suitable basestock for biolubricant production. J. Tribol.. 2019;23:97-112.
- [Google Scholar]
- The Toxicology and Biochemistry of Insecticides (first ed.). CRC Press; 2008. 10.1201/9781420059762
- Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem.. 2011;59(18):9910-9915.
- [CrossRef] [Google Scholar]







