Translate this page into:
Study on the role of flavonoids derived extract from seed residues of hippophae rhamnoides on high-fat diet induced obese mice
⁎Corresponding author. lishuangmdj8458@163.com (Shuang Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The objective of the paper was to explore the active mechanism underlying the role of flavonoids, which were derived from seed residues of hippophae rhamnoides (Flavonoids from the Seeds of H. rhamnoides L., FSH), in obesity. A total of 50 SPF healthy male C57BL/6 mice were selected and divided into 5 groups (with 10 mice in each group), i.e., the normal control group, the high fat diet control group, the 100 mg/kg FSH group, the 300 mg/kg FSH group, and the rosiglitazone group. The basic biochemical indicators of the mice were detected, and the liver and white adipose tissue samples were collected, stained and analyzed via Western Blot to study the effects of flavonoids on obesity. In the experiment, rosiglitazone was used as a traditional weight-inhibiting drug and its efficacy was compared with that in the FSH groups. It was found that compared with that in the high-fat diet control group, the weight gain of obese mice administered with FSH was slowed, along with reduced levels of serum total cholesterol, plasma glucose, and liver fat accumulation. Meanwhile, it was also discovered that FSH could reduce the expression of PPARγ in liver and white adipose tissues, thus helping to reduce fat accumulation. It was proved that the flavonoids derived from seed residues of hippophae rhamnoides exerted an inhibitory effect on obesity to protect the liver.
Keywords
Obesity
Flavonoids from seed residues of hippophae rhamnoides
PPARγ protein
C57BL/6 mice
1 Introduction
Obesity has gradually become a global issue. The total number of over-weight people has reached 1.4 billion worldwide, of which 500 million are obese, and this number is expected to reach 1 billion by 2020 (Bamford et al., 2016). Obesity is closely related to the occurrence of various disease, including hypertension, hyperlipidemia, cardiovascular diseases, and tumors (Mosleh and Almalik, 2016). Morbid obesity, especially central obesity, often leads to insulin resistance, one of the major factors causing metabolic syndrome (Girousse et al., 2018). Metabolic syndrome is associated with dyslipidemia, hypertension, type 2 diabetes and cardiovascular diseases (Grundy, 2016). Obesity is correlated with lifestyle and exposure to different xenobiotics, especially endocrine disruptors chemicals (Komada et al., 2018; Gardner et al., 2018). It is a chronic metabolic disease with complicated pathology and is featured by its long-term imbalance of energy. If energy intake exceeds energy consumption for a long time, excessive energy will gradually accumulate as triglycerides (TG) in white adipose tissues and lead to obesity (Bamford et al., 2016). Of all the clinical treatments for obesity, drugs such as orlistat are often used to effectively inhibit the gain of body weight (Heck et al., 2000); however, as a drug for diabetes mellitus, research on rosiglitazone is rarely reported (Kocarnik et al., 2017).
In the last year’s many natural extracts, nutraceuticals, prebiotics or probiotics have been tried in obesity management (Ríos-Hoyo and Gutiérrez-Salmeán, 2016; Choque Delgado et al., 2018; Lai et al., 2016).
Hippophae rhamnoides L., commonly called Seabuckthorn, is a fruit plant in the family of Elaeagnaceae, native to the Asian parts of Mongolia, China, and Europe. Berries of Seabuckthorn were used in ancient Tibetian, Chinese, Russian and Mongolian traditional medicine for the treatment of different diseases (Fontané et al., 2018). As the main components extracted from seabuckthorn seed residues, Flavonoids are the main components extracted from seed residues of hippophae rhamnoides (Flavonoids from the Seeds of H. rhamnoides L., FSH) and consist of various biologically active substances, such as quercetin, kaempferol, isorhamnetin, and myricetin, with the ability to regulate the lipid metabolism (Attri et al., 2018). This study aimed to evaluate the effects of FSH on obesity and lipid metabolism-related metabolic disorders. It is discussed the effects of chronic systematic inflammation on obesity. A murine model of high fat diet-induced obesity was used in order to clarify the molecular mechanism underlying the role of FSH and its Flavone glycosides in the regulation of lipid metabolic disorders. This paper aimed to provide a theoretical basis for the research and development of clear-functioned and clear-targeted hippophae rhamnoides (seabuckthorn) extracts.
2 Materials and methods
2.1 Extraction and purity determination of FSH
The seabuckthorn used in this study was collected from Lvliang City, Shanxi Province, China. The samples needed for the experiment were identified by the Botany Teaching and Research Group of Mudanjiang Medical University. The seabuckthorn seeds were collected and dried. After removing the impurities from the seabuckthorn seeds, they were pulverized with a pulverizer, sieved through a 50-mesh sieve, dried in an oven at 60 °C, and stored in a sealed bag after being dried. The processed seabuckthorn seeds were added into petroleum ether (boiling range 60–90 °C) at a material-liquid ratio (g/mL) of 1:8. Then, the mixture was heated for reflux at 70 °C for 2 h. After being sieved, the filtrate was discarded, and the filter residue was dissolved in petroleum ether to obtain the skimmed seabuckthorn seeds. Then, 10 g of skimmed seabuckthorn seeds were put into a 500 mL round-bottom three-neck flask. Next, the seabuckthorn seeds were added with 80 mL of 70% ethanol and heated for reflux at 80 °C for 2 h in the water bath. The filtrate was obtained through suction filtration. The filter residue was repeatedly extracted twice. The filtrates obtained from the above 3 extraction processes were put together, and the ethanol was removed by a rotary evaporator at 55 °C. After being concentrated until there were no alcoholic smells, the concentrate was poured into a 50 mL centrifuge tube, dissolved in hot water, and centrifuged at 10,000 rpm for 10 min to obtain the pretreatment solution. Then, the pretreatment solution was adsorbed on a DI01 macro-porous resin adsorption column for 3 h. Afterward, the solution was washed with ultra-pure water to remove the water-soluble impurities until the reducing sugar reaction was negative. The eluate was obtained by eluting with 30% (V/W) ethanol, freeze-dried in a vacuum freeze drier, and weighed with a precision electronic balance to obtain the weight of crude-extracted flavonoids. The extraction process was shown in Fig. 1.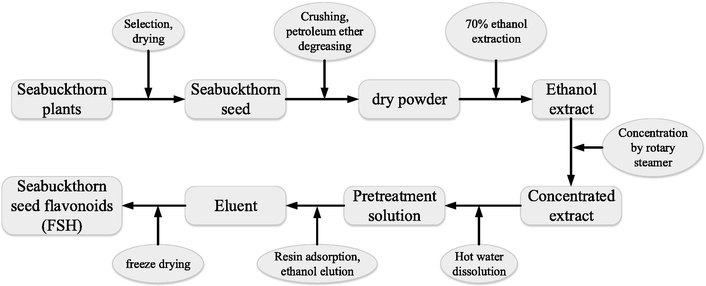
Schematic diagram of the extraction process of FSH.
In terms of purity determination, a precise electronic balance was used to weigh 25 mg of each crude FSH extract, dissolved in an appropriate amount of 60% ethanol solution, transferred to a 50 mL volumetric flask, washed three times, and made up to the dial to obtain the FSH crude-extracted solution with a concentration of 0.5 mg/mL. Then, 3.0 mL of the FSH crude-extracted solution was accurately absorbed. The absorbance at 500 nm was measured by using a UV–visible spectrophotometer. The results were calculated by being brought into the standard curve to obtain the total flavonoid content in the FSH crude extracts.
2.2 Animals
A total of 50 SPF healthy male C57BL/6 mice, weighing 21–25 g and aging 6–8 weeks, were purchased and housed in the Animal Experimental Center of Mudanjiang Medical University. The animals were kept in the environment with a controlled temperature between 20 and 24 °C and controlled humidity between 40 and 50%. All the animals had free access to food and water during the experiment. The animal experiment was approved by the Ethical Committee of Mudanjiang Medical University.
The mice were randomly divided into 5 groups (10 animals in each group) as follows:
Group A: the normal control (NC) group (n = 10) – the animals received normal diet;
Group B: the high-fat diet (HFD) control group – the animals received a high-fat diet (the ratio of fat energy supply was 45%, Shanghai Slaccas Experimental Animal Corporation, China);
Group C: the FSH100 group – the animals received a high-fat diet and after 50 days it started the daily intragastric administration of 100 mg/kg body weight of FSH (Suzhou Fulu Biotechnology, China) for 60 days;
Group D: the FSH300 group – the animal received a high-fat diet and after 50 days it started the daily intragastric administration of 300 mg/kg body weight of FSH for 60 days;
Group E: the rosiglitazone group (the Ros2 group), mice in which were given a high-fat diet and after 50 days it started the daily intragastric administration of 2 mg/kg of rosiglitazone for 60 days.
After 50 days of high-fat diets, the average weight of mice in groups B, C, D, and E was 20% higher than that of the normal control group, indicating that the animal model of obesity was successfully established. Subsequently, the intragastric administration of various drugs was started. The mice were administered with 0.2% Carboxymethylcellulose Sodium (CMC-Na) by gavage for 60 consecutive days, and the volume of administration should be ≤200 μL to prevent excessive stimulation of the mice. The mice in the control group received the same volume of saline solution intragastric as the mice in the experimental groups. After the successful model establishment, the mice were marked and then treated for 60 days with intragastric administration of various compounds. The entire experiment lasted for 117 days. Food and water intake, weights, and weight variations of all mice were recorded every 3 days.
2.3 Preparation of mouse blood and tissue samples
After 60 days of treatment by intragastric administration, the blood samples of all mice were collected from the ophthalmic arteries after 12 h of fasting to isolate the serum. Then, the mice were euthanized via cervical dislocation and the liver and adipose tissues were collected. The tissue samples were divided in 2, one part was fixed in pre-cooled 4% paraformaldehyde and the other part was quick to freeze with liquid nitrogen and stored at −80 °C.
The tissue samples were weighed and phosphate-buffered saline (PBS) (National Vaccine & Serum Institute, China) was added in a ratio of 1:9 (w/v) to uniformly triturate the tissues. The samples were centrifuged at 4 °C and 2500 rpm for 15 min, the supernatant was collected and stored at −80 ͦC till further analysis.
Each liver tissue section of 1.0 × 1.0 cm in size was lysed in 1 mL of pre-cooled radioimmunoprecipitation assay (RIPA) (Proteintech Corporation, USA) lysate, homogenized, and centrifuged at 4 °C and 300 rpm for 15 min to collect the supernatant for Western Blot (WB). In addition, each liver tissue section of 0.2 × 0.2 cm in size was lysed in 1 mL of pre-cooled Trizol (Takara Corporation, Japan) solution, triturated into homogenate, and centrifuged at 4 °C and 12000 rpm for 15 min to collect the supernatant for Ribonucleic Acid (RNA) extraction. All tubes and materials used in the reaction must be RNase free to prevent contamination.
2.4 Detection of biochemical markers
The triglyceride detection reagent kit (Shanghai Kexin Biotechnology Research Institute, China) and the ultra-violet visible spectrophotometer (Shanghai Yaxin Biotechnology, China) spectrum method were utilized to detect the triglyceride (TG) concentrations of mice; the total cholesterol detection reagent kit (Shanghai Kexin Biotechnology Research Institute, China) and the ultra-violet visible spectrophotometer (Shanghai Yaxin Biotechnology, China) spectrum method were utilized to detect the total cholesterol (TC) levels of mice; the high density lipoprotein-cholesterol reagent (Zhejiang Dong’ou Diagnostic Products, China) and the automatic biochemical analyzer (Shanghai Huxi Analysis Instrument Factory, China) were utilized to detect the high density lipoprotein-cholesterol (HDL-C) of mice.
2.5 Mouse tissue sectioning and staining
Collected tissues were fixed by xylene (Shanghai Beyotime Biotechnology, China), rinsed overnight with running water to remove paraformaldehyde (Shanghai Beyotime Biotechnology, China) residual, successively soaked in 50%, 70%, 85%, 95%, and 100% ethanol (1 h each) for dehydration, incubated for 30 min in a 1:1 solution of absolute ethanol and xylene, incubated for 35 min each in xylene I and xylene II, embedded in a 65 °C paraffin solution I for 8–12 h, soaked for another 2.5 h in a fresh paraffin solution, and then placed in a 4 °C refrigerator until they were completely solidified. Afterward, the tissues were sliced, flattened, stained with Hematoxylin-Eosin (HE) and mounted with neutral gum. The sections were observation and imaging under an optic microscope (Bio-Rad Corporation, USA).
2.6 Fluorescence Real-time quantitative polymerase chain reaction (q-PCR)
The extracted RNA was violently reacted with 200 μL of chloroform for 15 s and centrifuged at 12000 rpm for 15 min to collect the supernatant, which was then mixed with an equal volume of 100% isopropanol, oscillated for 10 min and centrifuged again at 12000 rpm for 15 min. The pellet was suspended with 1 mL of pre-cooled 75% ethanol and then centrifuged at 4 °C and 8500 rpm for 5 min. After the supernatant was discarded and the ethanol residue was evaporated, the pellet was suspended with diethyl pyrocarbonate (DEPC) (Amresco Corporation, USA) water and placed on the ice to prevent degradation. Then, the RNA concentration was measured by a micro-spectrophotometer, while an OD260/280 ratio of 1.8–2.0 indicated that the quality of RNA was satisfactory. Then, reverse transcription was performed using a reaction system of 20 μL containing 4 μL of 5 × PrimerScript Buffer (Shanghai Watson Biotechnologies, China) (for Real-time q-PCR), 1 μL of oligo dT primer (Amresco Corporation, USA), 1 μL of Random 6 mers (Amresco Corporation, USA), and 1μg of RNA. The reaction was performed at 37 °C for 15 min. Afterward, 20 μL of obtained cDNA was diluted to 100 μL with RNase Free dH2O (Shanghai Watson Biotechnologies, China). The q-PCR reaction was carried out in accordance with the relevant instructions in a 25 μL reaction system containing 12.5 μL of 2xSYBRMix, 1 μL of ForwardPrimer5uM, and 1 μL of ReversePrimer5uM. The reaction steps: pre-denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and extension/annealing at 60 °C for 1 min.
2.7 Detection of PPARγ expression by WB
The extracted protein samples were quantified using a BCA protein quantification kit (Nanjing Jiancheng Technology Corporation, China). Then, the samples were bathed at 100 °C for 1 min and 25 μL of each sample were resolved by SDS-PAGE electrophoresis using a 12% separation gel and a 5% stacking gel. The conditions of electrophoresis: 70 V, 30 min; 120 V, 1 h. After the electrophoresis was finished, the gel containing the target proteins was cut off in accordance with the instructions of the manufacturer. Then, the proteins were transferred to nitrocellulose membranes using “wet-transfer” and an 80 V transmembrane voltage. After the transfer, the membranes were washed with Tris-HCl Buffer Solution Tween (TBST) (Shanghai Watson Biotechnologies, China) and blocked with 5% skim milk powder at room temperature for 1 h, washed with TBST, incubated overnight at 4 °C with primary antibodies (PR rabbit monoclonal antibody, 1:800) (Shanghai Ybio, China), washed with TBST again, incubated for 1 h at room temperature with secondary antibodies (goat anti-rabbit IgG, 1:4000) (Nanjing Sunshine Biotechnology, China), washed with TBST again, and scanned with an Infrared Imaging System (LI-COR, USA). The contrast, saturation, and brightness of images were adjusted as needed.
2.8 Statistics analysis
Data collected in the experiments were expressed as mean number ± standard deviation (Mean ± SD). All data were analyzed by SPSS 17.0 statistics software (IBM, USA). Paired comparisons between two different groups were analyzed by Analysis of Variance (ANOVA) or Student’s t-test, while the comparisons among multiple groups were performed with Duncan or Dunnett's T3 test. P < 0.05 indicated a significant difference while P < 0.01 indicated extremely significant difference.
3 Results
3.1 Effects of FSH on weight, food intake, and abdominal adipose tissues of obese mice
As shown in Fig. 2, compared with the normal control group, the FSH100 group and the FSH300 group showed a significant increase in body weights at the beginning of treatment, which was statistically different (P < 0.01); compared with the HFD control group, weigh gains in the FSH100 group and the FSH300 group were slowed down after being treated with FSH. At the same time, the positive control rosiglitazone could also reduce the weight gains of obese mice. The weight variations in the HFD control group were the most obvious, and the difference was statistically significant (P < 0.01); in other groups, no significant differences in the body weight variations were found. No significant difference in food intake between the HFD control group and the FSH group was found. In addition, the food intake of the HFD group was slightly lower than that of the normal control group, while the food intake of the rosiglitazone group was slightly higher than that of the HFD control group, and the differences were statistically significant (P < 0.05).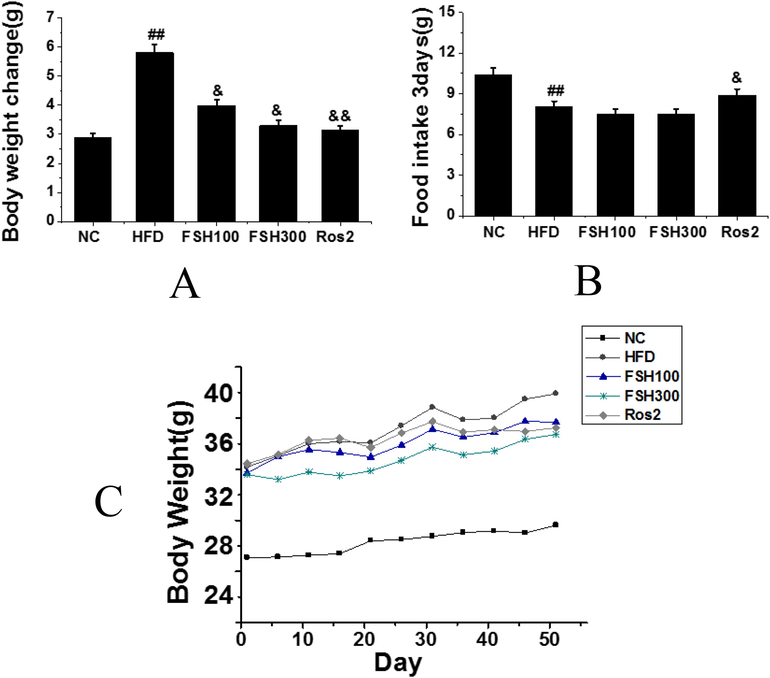
A: Records of weight variations of mice at every 3 days; B: Food intake of mice at every 3 days; C: Mouse weight variation during the treatment period (Compared with the control group, ## P < 0.01 indicated obviously statistical significance; compared with the HFD group, & P < 0.05 indicated statistical significance, && P < 0.01 indicated obviously statistical significance).
3.2 Biochemical markers
As shown in Fig. 3, the results obtained through the measurement and statistical analysis of blood glucose, TG, TC, HDL-C, liver TG content, serum HDL-C, and TC ratio. Serum triglyceride (TG) levels in the HFD control group, FSH100 group, and FSH300 group were significantly increased compared with the normal control group and the rosiglitazone group, and the differences had obviously statistical significance (P < 0.01) (Fig. 3A). However, the serum TG in the FSH300 group was significantly decreased compared with that of the HFD group, while the serum TG in the rosiglitazone group was slightly decreased compared to the normal control group. Serum level of total cholesterol (TC) in the HFD group was significantly increased compared to the normal control group, while no significant difference was observed among the different test groups, and the differences had obviously statistical significance (P < 0.01), indicating that the FSH and rosiglitazone had no significant effects on the regulation of TC (Fig. 3B). The ratio of high-density lipoprotein cholesterol (HDL-C) to TC in the serum of the normal control group was significantly higher than that in all other groups, and the differences had obviously statistical significance (P < 0.01) (Fig. 3C). The glucose level in the HFD control group significantly increased compared to the normal control group, and the differences were statistically significant (P < 0.05) (Fig. 3D). The glucose level in the 3 groups treated with FSH and rosiglitazone were significantly decreased compared to the HFD control group, with the highest decrease in FSH300 group. The liver triglyceride level in the HFD control group significantly increased compared to the normal control group, and the differences were statistically significant (P < 0.05) (Fig. 3E). Compared with the HFD control group, the liver triglyceride level both FSH100 and FSH300 groups decreased in a dose-dependent manner. The liver triglyceride level in the rosiglitazone group was about 4 times more than the level observed in the normal control group.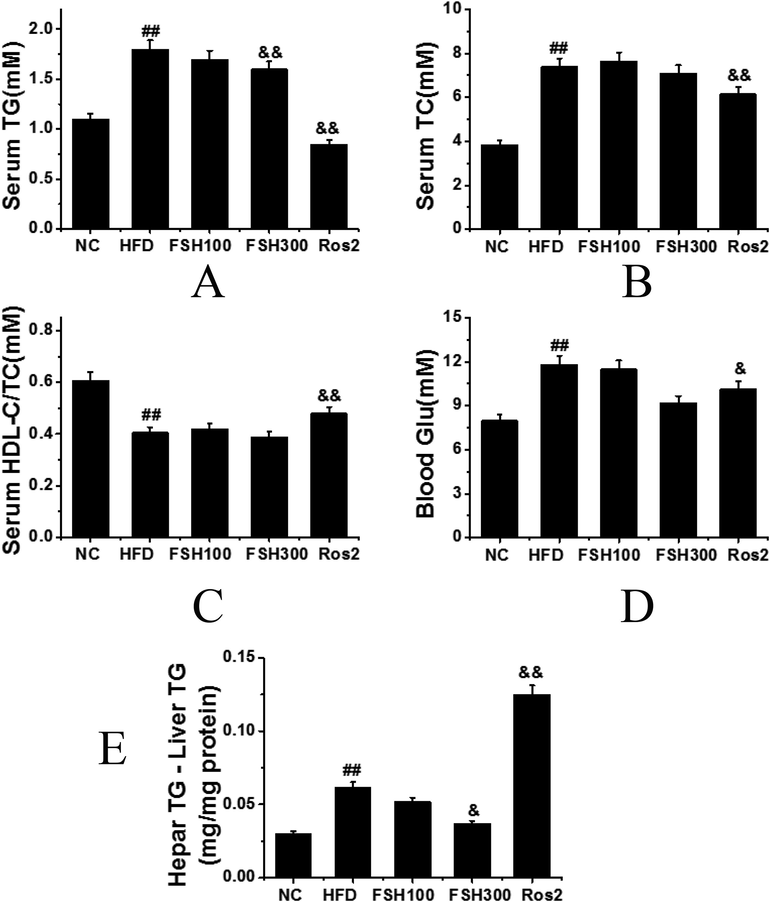
Biochemical markers in the groups: A: Serum triglyceride; B: Serum total cholesterol; C: The ratio of high-density lipoprotein cholesterol (HDL-C) to TC (HDL-C/TC) in the serum; D: Blood glucose level; E: Hepar TG – Liver TG level; (Compared with the control group, ## P < 0.01 indicated obviously statistical significance; compared with the HFD group, & P < 0.05 indicated statistical significance, && P < 0.01 indicated obviously statistical significance).
3.3 Histopathological evaluation of liver and epididymal adipose tissues
Sections of mouse liver and epididymal adipose tissues in each group were stained, as shown in Fig. 4. The adipocytes in the HFD control group were significantly enlarged compared with those in the normal control group, and the differences had obviously statistical significance (P < 0.01). The adipocytes in the FSH100 and FSH300 groups were reduced compared with those in the HFD control group, and the differences were statistically significant (P < 0.05). Through HE staining of liver tissues, it was found that the fat content in the liver tissues increased with the filling of a large number of adipocytes and infiltration of fat tissues. Meanwhile, the number of adipocytes was reduced in the liver tissues of the FSH100 and FSH300 groups, and the differences were statistically significant (P < 0.05), which also showed significantly alleviated fat infiltration caused by the high-fat diet.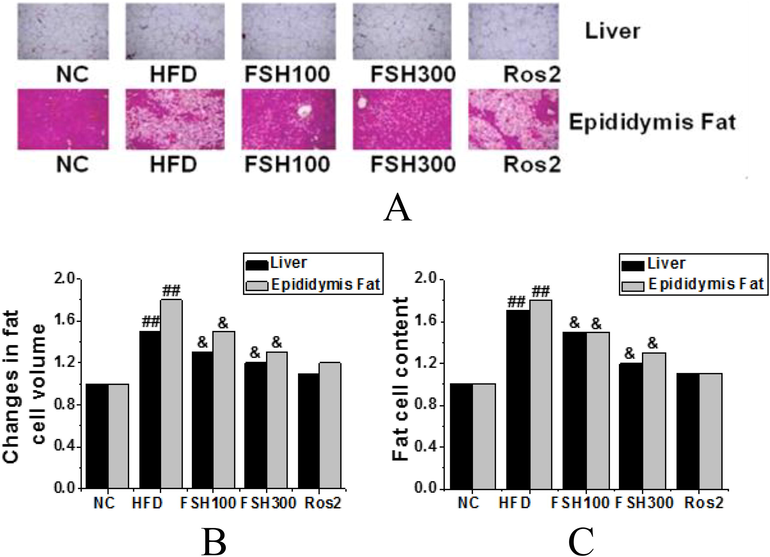
The analysis of staining results of mouse liver sections and mouse epididymal adipose tissues stain in the groups (A: Liver and epididymal adipose tissue H&E staining (x200); B: The histogram of volume variances of adipocytes in the groups; C: The histogram of quantity variances of adipocytes in the groups (Compared with the control group, ## P < 0.01 indicated obviously statistical significance; compared with the HFD group, & P < 0.05 indicated statistical significance, && P < 0.01 indicated obviously statistical significance)).
3.4 Effects of FSH on PPARγ expressions in livers and white adipose tissues of obese mice
As shown in Fig. 5, the expression levels of PPARγ in the livers and white adipose tissues of mice were analyzed. It was found that the expressions PPARγ in the HFD group increased significantly compared with the normal control group, and the differences were statistically significant (P < 0.01). After FSH administration, the expressions of PPARγ in livers and white adipose tissues of mice decreased significantly compared with the HFD group, and the differences were statistically significant (P < 0.01). Therefore, it could be speculated that the level of FSH could affect the influences and regulation mechanisms of PPARγ and related lipolytic cell signaling pathways.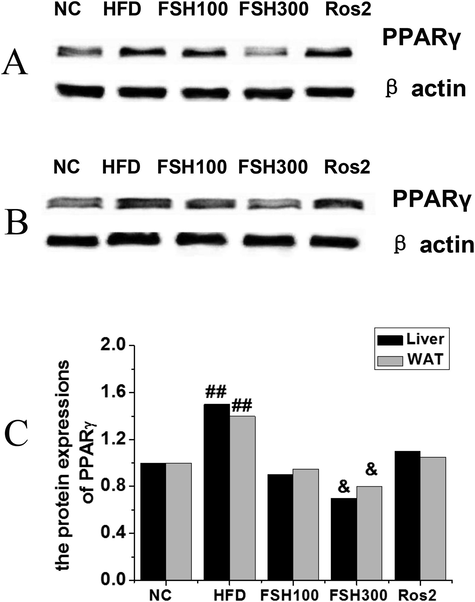
Effects of FSH on PPARγ expression in liver and white adipose tissues of mice (A: Electrophoretogram of the effects on liver tissues; B: Electrophoretogram of the effects on white adipose tissues; C: Analysis of the changes in PPARγ contents in livers and white adipose tissues (Compared with the control group, ## P < 0.01 indicated obviously statistical significance; compared with the HFD group, & P < 0.05 indicated statistical significance)).
4 Discussion
Obesity often leads to a declined quality of life and reduced life expectancy. Studies have shown that obesity, especially central obesity, often leads to insulin resistance, a leading cause of metabolic syndrome (Bamford et al., 2016), which in turn can cause dyslipidemia, hypertension, type 2 diabetes and cardiovascular diseases (Girousse et al., 2018; Grundy, 2016). In this study, through the research on the body weight variations and food intake of mice, compared with the normal control group, the FSH100 group and the FSH300 group showed a significant increase in body weights at the beginning of treatment, which was statistically different (P < 0.01); compared with the HFD control group, weigh gains in the FSH100 group and the FSH300 group were slowed down after being treated with FSH. In addition, the food intake of the HFD group was slightly lower than that of the normal control group, while the food intake of the rosiglitazone group was slightly higher than that of the HFD control group, and the differences were statistically significant (P < 0.05). The analysis of biochemical indicators found that the liver TC levels in the FSH100 group and the FSH300 group decreased in a dose-dependent manner compared with the HFD control group. The level of hepatic TC in the rosiglitazone group was about 4 times than that of the normal control group.
PPARγ could express the genes that were involved in adipocyte differentiation. Although both PPARγ and C/EBPα could promote the expression of adipocyte-associated adipogenic genes, some scholars believed that PPARγ was more decisive. If the PPARγ gene was silenced, C/EBPα could not take on the role of promoting the differentiation of pre-adipocytes, and its expression level would be much lower than normal (Broekema et al., 2019). In this study, HE staining analysis showed that the adipocytes in the HFC group were significantly increased in volume and number compared with the normal control group, while the adipocytes in the livers of the FSH100 group and the FSH300 group were significantly decreased, which could significantly alleviate the fat infiltration of liver tissues caused by high-fat diet; in the analysis of PPARγ expressions, it was found that the level of FSH could affect the influences and regulation mechanisms of PPARγ and related lipolytic cell signaling pathways, which was consistent with previous studies.
In conclusion, FSH is proved to be a safe drug that can effectively inhibit weight gains and alleviate the symptoms of obesity, and it appears to have no side effects on the research content, which provides the inhibition of symptoms of obesity with experimental reference and basis. However, certain deficiencies were found in the experimental process; for example, the sample capacity was relatively small. Therefore, the sample capacity would be further increased in the subsequent research to deeply explore the mechanism of obesity symptoms thereby making the experimental results more valuable.
Acknowledgement
This work was supported by Mudanjiang Science and Technology Planning Project (Z2018s043); The Scientific research projects of basic scientific research in colleges and universities operating expenses of Heilongjiang Province in 2018, China (Grant No. 2018-KYYWFMY-0014)”.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Colonic fermentation of polyphenolics from Sea buckthorn (Hippophae rhamnoides) berries: Assessment of effects on microbial diversity by Principal Component Analysis. Food Res. Int.. 2018;105:324-332.
- [Google Scholar]
- Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals. Vet J.. 2016;214:14-20.
- [Google Scholar]
- Natural helix 9 mutants of PPARγ differently affect its transcriptional activity. Mol. Metab.. 2019;20:115-127.
- [Google Scholar]
- Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res. Int.. 2018;113:183-188.
- [Google Scholar]
- Influence of the microbiota and probiotics in obesity. Clin. Investig Arterioscler.. 2018;30(6):271-279.
- [Google Scholar]
- Surplus fat rapidly increases fat oxidation and insulin resistance in lipodystrophic mice. Mol. Metab.. 2018;13:24-29.
- [Google Scholar]
- Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA. 2018;319(7):667-679.
- [Google Scholar]
- Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy. 2000;20(3):270-279.
- [Google Scholar]
- Weight change after initiation of oral hypoglycemic monotherapy for diabetes predicts 5-year mortality: an observational study. Diabetes Res. Clin. Pract.. 2017;123:181-191.
- [Google Scholar]
- Pancreatic fat content assessed by 1 H magnetic resonance spectroscopy is correlated with insulin resistance, but not with insulin secretion, in Japanese individuals with normal glucose tolerance. J. Diabetes Investig.. 2018;9(3):505-511.
- [Google Scholar]
- Multi-strain probiotics inhibit cardiac myopathies and autophagy to prevent heart injury in high-fat diet-fed rats. Int. J. Med. Sci.. 2016;13(4):277-285.
- [Google Scholar]
- Illness perception and adherence to healthy behaviour in Jordanian coronary heart disease patients. Eur. J. Cardiovasc. Nurs.. 2016;15(4):223-230.
- [Google Scholar]
- New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep.. 2016;5(2):262-270.
- [Google Scholar]







