Translate this page into:
Studies on phytochemical, antioxidant, antimicrobial analysis and separation of bioactive leads of leaf extract from the selected mangroves
⁎Corresponding author at: Vignan’s Foundation for Science, Technology & Research, Vadlamudi, 522213 Guntur, Andhra Pradesh, India. venki_biotech327@yahoo.com (T.C. Venkateswarulu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present study, phytochemical and antimicrobial analysis of selected mangrove species: Suaeda nudiflora, Lumnitzera racemosa, Ipomoea tuba and Avicennia alba was performed. The phenol found in all selected mangroves and tannins found in the species: Suaeda nudiflora, Ipomoea tuba and Avicennia alba and terpenoids present in Lumnitzera racemosa, Ipomoea tuba and Avicenia alba. The steroids and emodins were not found in all selected mangrove species. The antioxidant analysis proved that maximum inhibitory potential of leaf extract was obtained in Lumnitzera racemosa and minimum scavenging activity was found in Suaeda nudiflora. The leaf extract with different solvents was screened for antibacterial activity against pathogenic strains: Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsella pneumonia and Bacillus subtilis. The methanol extracts from Squeeda nudiflora and Ipomoea tuba was shown highest zone of inhibition compared to other solvent extracts. The order of antimicrobial activity expressed as inhibitory zones of methanol > acetone > hexane observed for pathogenic strains. Further, thin layer chromatography (TLC) studies revealed presence of bioactive compounds in the extracts of the mangrove species.
Keywords
Mangrove species
Phytochemical
Medicinal property
TLC separation
Rf values
1 Introduction
Mangroves plants are spread in the regions of inter tidal zones of tropical and sub tropical area mostly on the eastern coast of continents. Mangrove species contain a wide variety of chemical compounds that could be used as drugs for many epidemic diseases (Bobbarala et al., 2009; Behbahani et al., 2018). Mangrove forests near estuarine areas act as a barrier against cyclones and tsunami (Abeysinghe, 2010). Mangrove wetland serves as spawning and nursery grounds for many economically important estuarine fishes and migratory birds (Thomas et al., 2017; Haq and Wodeyar, 2002). Mangrove plants are used as the potential sources of biologically active chemicals which have commercial applications in the field of ethno- pharmaceutical sector (Nebula et al., 2013). The mangroves species contain neutraceuticals which are used as traditional food supplements and also widely used as folk medicines in some countries (Rasyid et al., 2016). Mangroves are biochemically unique and produce a wide range of natural products widely used for several human remedies (Nurdiani et al., 2012). The mangrove, Avicennia officinalis contain phytochemicals, anti-diabetic and free radical scavenging activities (Zhou et al., 2018). The leaf extracts of mangrove species namely Rhizophora mucronatz scvatxe and Sonneratia caseolaris have antimicrobial and antioxidant property (Zhao et al., 2011; Gawali and Jadhav, 2011). The methanol leaf extracts from Suaeda maritima have phytochemicals namely Saponins, terpenoids, tannins, alkaloids, steroids and are reported for antimicrobial and scavenging activity (Santhi and Sengottuvel, 2016). However, till now no study reported on antimicrobial and phytochemical studies of some mangrove species such as Suaeda nudiflora, Lumnitzera racemosa, Ipomoea tuba and Avicenia alba. Hence, the present study is aimed to study the antimicrobial, antioxidant and phytochemical analysis of selected mangrove species.
2 Materials and methods
2.1 Sample preparation and photochemical analysis
The mangrove species namely Suaeda nudiflora, Lumnitzera racemosa, Ipomoea tuba and Avicennia alba were collected from Nizampatnam sanctuary, Guntur, Andhra Pradesh, India. The mangrove plant species are washed with tap water and cut into small fragments and make dried under shade away from sun light. The dried leaves are making into powder and stored in polythene bags. Ten grams of powdered plant material was mixed with 100 ml of methanol and stirred for 72 h and then filtered. The solvent was removed by rotary vacuum evaporation. The extracts were used for phytochemical, Antimicrobial and antioxidants analysis (Sheel et al., 2014).
2.2 Antioxidant assay
The antioxidant potential of methanol leaf extracts of selected mangrove species was determined by standard 1, 1-diphenyl-2-picryl hydroxyl (DPPH) free radical activity method. The scavenging activity was calculated by using following formula and is expressed in percent (Reddy and Grace, 2016). where A = control and B = sample.
2.3 Antimicrobial analysis
The extracts from the mangrove species were prepared using different solvents namely: methanol, acetone and hexane. The quantity, 1 mg of leaf sample was mixed in 1 ml of solvent and then sample extracts were tested against pathogenic species such as Micrococcus luteus, Staphylococcus aureus, Pseudomonas aeuriginosa, Bacillus subtilis. Sterile cotton swab dipped into culture of pathogenic microbes and inoculated on the surface agar medium and then agar surface allowed for drying for 5 min. Then, the sterile filter paper dipped in solvent extract of mangrove species and antibiotic solution and sterile medium were kept on agar surface. All the plates were incubated for 24 to 48 h at 37 °C. The zone of inhibition was measured in millimeters (mm) and the experiment was performed with different concentrations of the leaf extract i.e., 25 µg, 50 µg, 100 µg and 200 µg and the antibiotic standard and control plates are maintained (Mukherjee et al., 2014).
2.4 Compound separation thin layer chromatography (TLC)
TLC was performed for alkaloids, tannins, phenols, emodins, terpenoids etc. The quantity, 50 gm of powdered plant samples of mangrove species separately extracted with methanol by subjecting it, to maceration at room temperature for overnight and then filtered with Whatmans filter paper. The filtrate consists of phytochemicals are separated by TLC. The TLC plates are prepared by mixing 25 gm of Silica Gel -G with 50 ml distilled water (1:2) for two minutes and then, the suspension was distributed over plate and plates were dried in hot air oven at 110 °C for 30 min and then stored in dry atmosphere further, which are used for spotting of sample. The samples were prepared by diluting crude extracts of methanol in all the four selected mangrove species separately and then, 1–10 µl samples was applied on TLC plate at 2 cm above its bottom with capillary tubes (Sonam et al., 2017; Biradar and Rachetti, 2013).
2.5 Statistical analysis
All experiments performed in triplicates and the results were expressed as mean ± standard deviation (SD) for antimicrobial and DPPH assays. Data were analyzed by ANOVA (p < 0.05) with data analysis system in MS office Excel 2007.
3 Results and discussion
3.1 Phytochemical analysis
The plants extract contain high medicinal values and these compounds can be used to design and develop pharmaceutical products. The leaf extract of mangrove species contain different phytochemicals and bioactive compounds namely alkaloids, terpenoids, steroids and terpenoids (Saranraj and Sujitha, 2015). The selected mangrove species were found to be positive for presence of phenols. The plant species Ipomoea tuba and Avicennia alba contain both tannins and alkaloids. Terpenoids was found in leaf extracts of Lumnitzera racemosa, Ipomoea tuba and Avicennia alba and are absent in leaf extracts of Squeeda nudiflora. Steroids and emodins are absent in selected mangrove species. The most common compounds are Tannins, phenols and terpenoids found in Ipomoea tuba and Avicennia alba (Table 1). In previous studies reported that the species: Avicenia marina and Avicennia officinalis are rich in phytochemicals like alkaloids, flavonoids, terpenoids and phenol. The Avicenia marina fruit extracts contain saponin and amino acid but these compounds were absent in Avicenia officinalis (Ramanathan et al., 2012). (+) Indicates presence of phytochemical & (−) Indicates absence of phytochemical
S.No
Name of the bioactive compound
Suaeda nudiflora
Lumnitzera racemosa
Ipomoea tuba
Avicennia alba
Tannins
+
_
_
+
Phenols
+
+
+
+
Steroids
_
_
_
_
Emodins
_
_
_
_
Terpenoids
_
+
+
+
Alkaloids
_
_
+
+
3.2 DPPH radical scavenging activity
DPPH (2, 2-diphenyl-hydrazyl -hydrate) is a stable free radical which accepts an electron to become a stable molecule. The methanolic leaf extracts of selected mangrove species showed good free radical scavenging activity. The scavenging activity increases with an increase in concentration of the extract. The DPPH turns yellow from purple indicate presence of antioxidants in the selected leaf extracts. The percent of scavenging activity of methanol extract of selected mangrove species was calculated and graph was plotted against concentration Vs percent of scavenging activity. The maximum scavenger activity, 95.62% was found in the leaf exact of Lumnitzera racemosa at concentration of 200 µg/ml and the minimum scavenging activity, 37.78% was found in the leaf extract of Suaeda nudiflora at concentration of 50 µg/ml. Ascorbic acid used as standard for the assay and the scavenging activity of each sample at different concentration was showed in the Fig. 1. The residual concentration of DPPH depends exclusively on the structure of the phenolic compounds. The accessibility of the radical centre of DPPH to each poly phenol could influence the order of the antioxidant power (Loganayaki et al., 2013). The mangroves has rich antioxidant system and enzymatic defense system of mangrove plants include different endogenous enzymes such as superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase and non-enzymatic antioxidants i.e., ascorbate, tocopherols and phenolic compounds serve as protectors against a wide variety of environmental stresses in plants (Thatoi et al., 2014).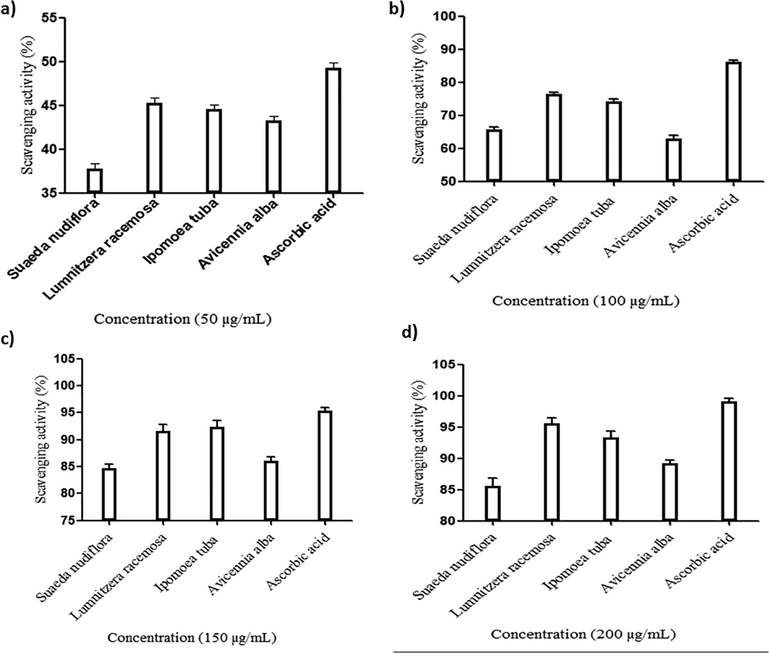
The antioxidant activity of mangrove species at different concentrations: (a) 50 µg/mL; (b). 100 µg/mL; (c). 150 µg/mL and (d). 200 µg/mL.
3.3 Antimicrobial analysis for leaf extracts of Suaeda nudiflora
Antimicrobial activity of leaf extracts of Suaeda nudiflora was performed against the pathogenic bacterial strains namely Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Klebsiella pneumonia with different concentrations. The methanol extracts of leaves of Sqseeda nudiflora showed highest inhibitory zone at the highest concentration (200 µg) against Staphylococcus aureus and against Micrococcus luteus and even at low concentration of 25 µg showed significant inhibitory zones against Staphylococcus aureus. The acetone extracts of leaf sample showed the antimicrobial effect against Staphylococcus aureus and Micrococcus luteus than other bacterial strains and leaf sample with hexane has not showed significant inhibitory zones (Table 2). In previous studies reported that leaf extract of Avicennia officinalis showed higher degree of inhibitory zones against other bacterial strains (Thatoi et al., 2016). The methanol and acetone extracts of Avicennia officinalis showed the positive results against bacterial strains (Sharief et al., 2014). The crude extract of Suaeda nudiflora with different solvents like hexane, ethyle acetate, methanol and chloroform showed higher degree of inhibitory zones against selected human pathogenic bacterial strains (Nayak et al., 2018).
Pathogenic strains
Zone of inhibition(mm)
Methanol (µg)
Acetone (µg)
Hexane (µg)
25
50
100
200
25
50
100
200
25
50
100
200
Control
–
–
–
–
–
–
–
–
–
–
–
–
M. luteus
4.76 ± 0.25
6.53 ± 0.47
7.46 ± 0.41
8.73 ± 0.25
2.33 ± 0.15
2.83 ± 0.15
3.73 ± 0.25
4.36 ± 0.15
0.4 ± 0.1
0.83 ± 0.15
1.76 ± 0.25
2.13 ± 0.15
S. aureus
7.03 ± 0.15
8.8 ± 0.2
10.03 ± 0.25
12.86 ± 0.15
0.03 ± 0.15
3 ± 0.1
4 ± 0.2
5.76 ± 0.25
1.5 ± 0.2
1.76 ± 0.25
2.76 ± 0.25
3.8 ± 0.2
P. aeroginosa
3.03 ± 0.15
6.76 ± 0.25
6.73 ± 0.25
9 ± 0.2
0.73 ± 0.25
0.5 ± 0.1
1.9 ± 0.1
3 ± 0.2
0.83 ± 0.15
1 ± 0.2
1.76 ± 0.25
2.33 ± 0.15
B. subtilis
2 ± 0.2
2.33 ± 0.15
2.53 ± 0.15
3.46 ± 0.15
1.96 ± 0.15
2.5 ± 0.2
3.5 ± 0.1
4.46 ± 0.15
2 ± 0.2
1.7 ± 0.3
2.5 ± 0.1
2.96 ± 0.15
K. pneumonia
1.96 ± 0.15
2 ± 0.1
3 ± 0.2
4.76 ± 0.25
1.33 ± 0.15
1.86 ± 0.15
1.76 ± 0.25
2.73 ± 0.25
0.53 ± 0.15
1.93 ± 0.20
2 ± 0.2
2.5 ± 0.2
A. niger
1.8 ± 0.2
3.36 ± 0.15
4.46 ± 0.15
4.96 ± 0.25
1.36 ± 0.15
1.76 ± 0.25
1.96 ± 0.25
2.5 ± 0.2
1.1 ± 0.36
1.86 ± 0.15
2.5 ± 0.2
3.06 ± 0.20
Rhizopus
1.7 ± 0.2
2.46 ± 0.15
2.73 ± 0.20
3.13 ± 0.15
2 ± 0.2
2.66 ± 0.75
3.3 ± 0.2
4 ± 0.2
1.1 ± 0.36
2.36 ± 0.15
3.03 ± 0.25
4.75 ± 0.25
3.4 Antimicrobial analysis for leaf extracts of Lumnitzera racemosa
The leaf extracts of Lumnitzera racemosa showed high inhibitory zone with highest concentration (200 µg) against Staphylococcus aureus and significant inhibitory effect against Bacillus subtilis. Acetone extracts of leaf sample represents the inhibitory zones against Pseudomonas aeruginosa than other bacterial strains. The leaf sample with hexane showed minimal antimicrobial effective incomparision with other solvent extracts (Table 3). Earlier, the studies from Saad et al., (2011) reported that the methanolic leaf extracts of Lumnitzera littorea showed the potent inhibitory effect against the pathogenic bacterial strains. But, till now no report found on antibacterial activity of Lumnitzera racemosa leaf extracts with methanol, acetone and hexane.
Pathogenic organisms
Solvent extract
Methanol (µg)
Acetone (µg)
Hexane (µg)
25
50
100
200
25
50
100
200
25
50
100
200
Control
–
–
–
–
–
–
–
–
–
–
–
–
M. luteus
2.16 ± 0.15
3.33 ± 0.15
4.13 ± 0.15
5.8 ± 0.15
1.36 ± 0.15
1.83 ± 0.15
1.83 ± 0.15
2.83 ± 0.15
1.16 ± 0.15
0.9 ± 0.1
2.33 ± 0.15
2.36 ± 0.15
S. aureus
4.13 ± 0.15
6.33 ± 0.15
7.8 ± 0.2
8.83 ± 0.15
3.36 ± 0.15
5.8 ± 0.2
5.8 ± 0.2
7.3 ± 0.2
1.83 ± 0.15
2.3 ± 0.2
3.33 ± 0.15
3.4 ± 0.1
P. aeroginosa
3.16 ± 0.15
3.8 ± 0.2
4.36 ± 0.15
5.8 ± 0.2
2.2 ± 0.2
3.86 ± 0.15
3.86 ± 0.15
5.33 ± 0.15
1.13 ± 0.15
1.86 ± 0.15
2.13 ± 0.15
2.8 ± 0.2
B. subtilis
2.8 ± 0.2
3.8 ± 0.2
4.3 ± 0.2
5.8 ± 0.2
2.16 ± 0.15
4.1 ± 0.1
4.1 ± 0.1
5.33 ± 0.15
1.1 ± 0.1
1.8 ± 0.2
2.13 ± 0.15
2.8 ± 0.2
K. pneumonia
1.16 ± 0.15
2.3 ± 0.2
2.86 ± 0.15
4.8 ± 0.2
2.13 ± 0.15
2.83 ± 0.15
2.83 ± 0.15
3.8 ± 0.2
1.16 ± 0.15
1.3 ± 0.2
2.3 ± 0.2
4.13 ± 0.15
A. niger
0.7 ± 0.2
1.16 ± 0.15
2.3 ± 0.2
3.3 ± 0.2
0.66 ± 0.15
2.16 ± 0.20
2.16 ± 0.20
2.3 ± 0.2
1.36 ± 0.15
1.83 ± 0.15
1.8 ± 0.2
2.53 ± 0.15
Rhizopus
0.63 ± 0.15
1.33 ± 0.15
1.83 ± 0.15
2.8 ± 0.2
0.66 ± 0.15
2.13 ± 0.15
2.13 ± 0.15
2.3 ± 0.2
1.33 ± 0.15
1.8 ± 0.2
2.86 ± 0.15
3.3 ± 0.2
3.5 Antimicrobial analysis for leaf extracts of Ipomoea tuba
The methanol leaf extract of Ipomoea tuba produced highest zone of inhibition against Micrococcus luteus and Pseudomonas aeruginosa and least inhibitory effect was found against Klebsiella pneumonia. The findings of study also proved superior results against Staphylococcus aureus with different concentrations of 25 µg, 50 µg, 100 µg, 200 µg. The acetone leaf extracts showed the highest inhibitory effect against Micrococcus luteus with concentration of 200 µg and least inhibitory effect was found against M. luteus at the concentration (25 µg). Hexane leaf extracts showed minimal effect against effective inhibitory zones against Staphylococcus aureus and no effect against S. aureus and K. pneumonia and the no effect was found on M. luteus, P.aeroginosa and B. subtilis. Methanol extracts showed higher degree of inhibitory zones against pathogenic strains than the leaf extracts of Acetone and hexane (Table 4). The previous studies reported that leaf extracts of Rhizophora mangle showed higher degree of inhibitory zones against selected human pathogenic bacterial strains namely Staphylococcus aureus ,Pseudomonas aeruginosa and Bacillus subtilis (Cruz et al., 2015). The mangrove species have wide ranges of phytochemicals and bioactive compounds which were used for pharmacological activities like antimicrobial treatment. Leaf extracts of mangrove plant namely Ceriopsde candra was reported for presence of many bioactive compounds which have antibacterial properties (Ravikumar et al., 2010).
Zone of inhibition (mm)
Pathogenic organisms
Solvent extract
Methanol (µg)
Acetone (µg)
Hexane (µg)
25
50
100
200
25
50
100
200
25
50
100
200
Control
–
–
–
–
–
–
–
–
–
–
–
–
M. luteus
4.72 ± 0.04
6.03 ± 0.15
8.83 ± 0.15
10.86 ± 0.15
1.10 ± 0.02
3.3 ± 0.2
5 ± 0.1
5.73 ± 0.25
1.0 ± 0.03
2.83 ± 0.15
3.3 ± 0.2
4.36 ± 0.15
S. aureus
4.83 ± 0.15
7.86 ± 0.15
8.83 ± 0.15
10.33 ± 0.15
2.83 ± 0.15
3.36 ± 0.15
4.83 ± 0.15
5.83 ± 0.15
1.86 ± 0.15
2.83 ± 0.15
3.3 ± 0.2
4.96 ± 0.25
P. aeroginosa
3.3 ± 0.2
5.83 ± 0.15
8.83 ± 0.15
11.03 ± 0.15
1.83 ± 0.15
2.4 ± 0.1
3.86 ± 0.15
4.8 ± 0.2
1.33 ± 0.15
1.83 ± 0.15
1.88 ± 0.15
2.8 ± 0.2
B. subtilis
3.36 ± 0.15
3.83 ± 0.15
5.86 ± 0.15
9.83 ± 0.15
1.86 ± 0,15
2.83 ± 0.15
4.8 ± 0.2
5.46 ± 0.15
1.33 ± 0.15
1.86 ± 0.15
2.3 ± 0.2
3.83 ± 0.15
K. pneumonia
2.42 ± 0.20
1.86 ± 0.15
3.8 ± 0.2
4.76 ± 0.20
1.36 ± 0.15
1.86 ± 0.15
2.83 ± 0.15
4.83 ± 0.15
2.4 ± 0.1
2.83 ± 0.15
3.33 ± 0.15
4.36 ± 0.15
A. niger
0.86 ± 0.15
1.86 ± 0.15
3.03 ± 0.15
3.33 ± 0.15
0.83 ± 0.15
0.86 ± 0.15
1.83 ± 0.15
2.8 ± 0.2
1.3 ± 0.2
1.86 ± 0.15
3.36 ± 0.15
4.86 ± 0.15
Rhizopus
1.33 ± 0.15
2.4 ± 0.1
2.83 ± 0.15
3.330.15
1.83 ± 0.15
2.8 ± 0.15
3.96 ± 0.15
4.3 ± 0.2
0.83 ± 0.15
1.33 ± 0.15
1.4 ± 0.2
2.3 ± 0.2
3.6 Antimicrobial analysis for leaf extracts of Avicennia alba
The methanol extracts from the leaves of Avicennia alba showed significant inhibitory zones against Micrococcus luteus, Pseudomonas aeruginosa, Bacillus subtilis with concentrations 25 µg, 50 µg, 100 µg and 200 µg and showed highest inhibitory zones on Bacillus subtilis. The acetone extracts of leaf sample showed inhibitory zones against Staphylococcus aureus and least on P.aeroginosa. The hexane extracts of leaf showed significant inhibitory zones against Staphylococcus aureus and Bacillus subtilis. The solvent leaf extracts of Avicennia albahas not shown the antimicrobial effect on the A. niger (Table 5). The studies from Haq et al., (2014) reported that the ethanolic leaf extract of mangrove, Sonneratia alba showed the antibacterial activity against pathogenic strains: Staphylococcus aureus, Bacillus cereus, Escherichia coli I and Pseudomonas aeruginosa. The methanol leaf extract of marine mangrove plant Avicennia marina showed the highest antimicrobial activity against pseudomonas aeruginosa (Thamizharasan and Anbusaravanan, 2016). Compared to other Avicennia species the leaf extract of Avicennia alba is potential because the significant inhibitory effect was found on different pathogenic strains.
Zone of inhibition(mm)
Pathogenic strains
Solvent extract
Methanol (µg)
Acetone (µg)
Hexane (µg)
25
50
100
200
25
50
100
200
25
50
100
200
Control
–
–
–
–
–
–
–
–
–
–
–
–
M. luteus
3.86 ± 0.15
5.83 ± 0.15
5.83 ± 0.20
7.3 ± 0.2
2.86 ± 0.15
3.3 ± 0.2
4.8 ± 0.2
5.8 ± 0.2
1.86 ± 0.15
2.3 ± 0.2
2.86 ± 0.15
3.36 ± 0.15
S. aureus
4.33 ± 0.15
5.76 ± 0.20
7.83 ± 0.15
9.33 ± 0.15
3.3 ± 0.2
3.5 ± 0.43
5.86 ± 0.15
6.83 ± 0.15
1.33 ± 0.15
1.86 ± 0.15
3.83 ± 0.15
4.3 ± 0.2
P. aeroginosa
3.8 ± 0.2
5.86 ± 0.15
6.83 ± 0.15
7.3 ± 0.2
1.86 ± 0.15
3.33 ± 0.15
4.3 ± 0.2
5.83 ± 0.15
1.86 ± 0.15
2.3 ± 0.2
2.36 ± 0.15
3.36 ± 0.15
B. subtilis
4.83 ± 0.15
0.86 ± 0.15
6.8 ± 0.2
8.83 ± 0.15
2.13 ± 0.75
5.03 ± 0.15
5.3 ± 0.2
6.83 ± 0.15
2.36 ± 0.15
2.86 ± 0.15
3.8 ± 0.2
4.76 ± 0.20
K. pneumonia
1.03 ± 0.15
1.3 ± 0.2
1.83 ± 1.03
5.33 ± 0.15
1.86 ± 0.15
2.33 ± 0.15
3.3 ± 0.2
3.83 ± 0.15
1.33 ± 0.15
1.36 ± 0.15
2.76 ± 0.15
3.33 ± 0.15
A. niger
1.83 ± 0.15
2.4 ± 0.1
3.36 ± 0.15
3.8 ± 0.2
1 ± 0.1
1.83 ± 0.15
2.33 ± 0.15
2.83 ± 0.15
0.5 ± 0.1
1.33 ± 0.15
1.86 ± 0.15
2.83 ± 0.15
Rhizopus
2.36 ± 0.15
2.83 ± 0.15
3.76 ± 0.15
5.86 ± 0.15
1.83 ± 0.15
2.36 ± 0.15
2.8 ± 0.2
4.86 ± 0.15
0.83 ± 0.15
2.33 ± 0.15
2.83 ± 0.05
3.86 ± 0.15
3.7 Compound separation by TLC
The colour spots represent presence of bioactive compounds and pale yellow spots on TCL plates was indicative for antioxidants from mangrove species. The Rf and hRf values calculated for all separated bands (Table 6). The bands pattern on TLC sheet is indicated the presence of similar type of phytochemicals. The major bands developed from selected mangrove species have similar band pattern (Fig. 2). The yellow color spots on TLC sheet represent the presence of bioactive compounds and antioxidants. Hanna et al. in (2008) reported that the yellow colour spots indicate presence of carotenoids and other phenolic compounds. The phenolic compounds from plants are known to be good anti-oxidants.
Number of similar bands
S. nudiflora
L. racemosa
I. tuba
A. alba
Rf
hRf
Rf
hRf
Rf
hRf
Rf
hRf
01
0.38 ± 0.005
39.33 ± 0.57
0.35 ± 0.005
36 ± 1
0.36 ± 0.01
35.33 ± 0.57
0.43 ± 0.005
43.66 ± 0.57
02
0.43 ± 0.007
43.33 ± 0.37
0.38 ± 0.005
38.33 ± 1
0.42 ± 0.01
42 ± 0.03
0.64 ± 0.01
64 ± 1
03
0.64 ± 0.005
64.33 ± 0.41
0.7 ± 0.01
70 ± 0.03
0.48 ± 0.01
48 ± 1
0.72 ± 0.01
72 ± 0.24
04
0.7 ± 0.01
70 ± 1
0.83 ± 0.005
83.66 ± 1
0.7 ± 0.01
70 ± 1
0.86 ± 0.01
86 ± 0.02
05
0.86 ± 0.005
86.33 ± 0.57
0.86 ± 0.01
86 ± 0.04
0.85 ± 0.57
85.33 ± 0.57
0.89 ± 0.01
89 ± 1
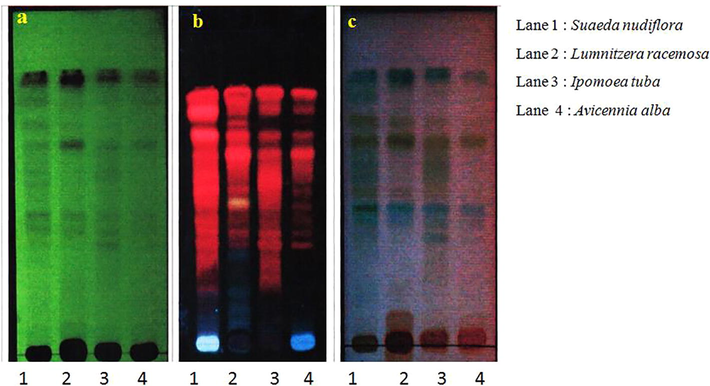
The compound in methanol leaf extract of mangrove species separated on TLC sheet: (a). Detection of bands at 254 nm, (b). Detection of bands at 366 nm & (c). Detection of bands after spray with Anisaldehyde sulphuric acid reagent (ANS).
4 Conclusion
The leaf extracts of mangroves species selected for the study reveals the presence of phytochemicals and among the four samples, potent antioxidant activity is found in the leaf extract of Lumnitzera racemosa. The methanolic leaf extract from the selected mangroves showed highest antimicrobial activity in comparison with other solvent extracts and the highest the zone of inhibition found on Staphylococcus aureus from the leaf extract of Suaeda nudiflora. The TLC study revealed the presence of the bioactive molecules with scavenging activity. Hence, the study proved that the mangrove extracts used for development of novel bioactive molecules for therapeutic applications and further studies in loop for selective isolation and identification of bioactive molecules of mangrove plants.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
Authors acknowledge the facilities supported by DST-FIST and VFSTR.
References
- Antibacterial activity of some medicinal mangroves against antibiotic resistant pathogenic bacteria. Indian J. Pharm. Sci.. 2010;72:167-172.
- [Google Scholar]
- Phytochemical analysis and antibacterial activities extracts of mangrove leaf against the growth of some pathogenic bacteria. Microb. Pathog.. 2018;114:225-232.
- [Google Scholar]
- Extraction of some secondary metabolites & thin layer chromatography from different parts of Centellaasiatica L (URB) Am. J. Life Sci.. 2013;1:243-247.
- [Google Scholar]
- Antimicrobial potentialities of mangrove plant Avicennia marina. J. Pharm. Res.. 2009;2:1019-1021.
- [Google Scholar]
- Evaluation of Mangrove (Rhizophora mangle L.) products as coloring, antimicrobial and antioxidant agents. Int. J. Phytocosmetics Nat. Ingredients. 2015;2:1-7.
- [Google Scholar]
- Antioxidant activity and antioxidant phytochemical analysis of mangrove species Sonneratia alba and Bruguiera cylindrica. Asian J. Microbiol. Biotechnol. Environ. Exp. Sci.. 2011;13:257-261.
- [Google Scholar]
- Evaluation of marine algae Ulvalactuna Las a source of natural preservative ingredient. Am.-Eur. J. Agric. Environ. Sci.. 2008;3:434-444.
- [Google Scholar]
- Antioxidant and antibacterial activities of different extracts and fractions of a mangrove plant Sonneratia alba. Int. J. Agric. Biol.. 2014;16:706-714.
- [Google Scholar]
- An ecological study of habitat of mangrove forest of Bangladesh. J. Human Ecol.. 2002;13:225-230.
- [Google Scholar]
- Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol.. 2013;50:687-695.
- [Google Scholar]
- Ecosystem service valuations of mangrove ecosystems to inform decision making and future valuation exercises. PloS one. 2014;9:e107706
- [Google Scholar]
- Phytochemical, antioxidant and antimicrobial screening of Suaeda maritima L (Dumort) against human pathogens and multiple drug resistant bacteria. Indian J. Pharmaceutical Sci.. 2018;80:26-35.
- [Google Scholar]
- Metabolites and bioactivities of Rhizophoraceae mangroves. Nat. Products Bioprospecting. 2013;3:207-232.
- [Google Scholar]
- Phytochemical screening and antibacterial activity of methanol extract of mangrove plant (Rhizophora mucronata) from porong river estuary. J. Basic Sci. Technol.. 2012;1:27-29.
- [Google Scholar]
- Phytochemical characterization and antimicrobial efficiency of mangrove plants Avicennia marina and Avicennia officinalis. Int. J. Pharmaceutical Biol. Archive. 2012;3:348-351.
- [Google Scholar]
- Impact of human interventions on mangrove ecosystem in spatial perspective. In IOP Conference Series: earth and environmental science. IOP publishing. 2016;47:012-041.
- [Google Scholar]
- Antibacterial potential of chosen mangrove plants against isolated urinary tract infections bacterial pathogens. Int. J. Med. Med. Sci.. 2010;2:94-99.
- [Google Scholar]
- In vitro evaluation of antioxidant activity of methanolic extracts of selected mangrove plants. Med. Aromat. plants.. 2016;5:2167-10412.
- [Google Scholar]
- Antimicrobial activity of mangrove plant (Lumnitzera littorea) Asian Pac. J. Trop. Biomed.. 2011;4:523-525.
- [Google Scholar]
- Qualitative and quantitative phytochemical analysis of Moringa concanensis Nimmo. Int. J. Curr. Microbiol. App. Sci.. 2016;5:633-640.
- [Google Scholar]
- Mangrove medicinal plants: a review. Am.–Eurasian J. Toxicol. Sci.. 2015;7:146-156.
- [Google Scholar]
- Quantification of phytochemicals and antibacterial activity of fruit extract of Avicennia officinalis. Asian. J. Pharm. Clin. Res.. 2014;7:127-130.
- [Google Scholar]
- Preliminary phytochemical screening of methanolic extract of Clerodendronin fortunatum. IOSR.J.Appl. Chem.. 2014;7:10-13.
- [Google Scholar]
- Phytochemical screening and TLC profiling of various extracts of Reinwardtia indica. Int. J. Pharmacognosy Phytochem. Res. 2017:523-527.
- [Google Scholar]
- Antibacterial potential of mangrove plant Avicennia marina against a clinical pathogen. Int. J. Zool. Stud.. 2016;1:14-16.
- [Google Scholar]
- The genus Avicennia, a pioneer group of dominant mangrove plant species with potential medicinal values: a review. Front. Life Sci.. 2016;9:267-291.
- [Google Scholar]
- Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta physiologiae plantarum.. 2014;36:561-579.
- [Google Scholar]
- Distribution and drivers of global mangrove forest change, 1996–2010. PloS one. 2017;12:e0179302
- [Google Scholar]
- The diversity and anti-microbial activity of Endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr. Microbiol.. 2011;62:182-190.
- [Google Scholar]
- Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South china sea. PloS one. 2018;13:e0197359
- [Google Scholar]







