Translate this page into:
Structural, electrical and optical properties of Zn1−xCuxO (x = 0.00–0.09) nanoparticles
⁎Corresponding authors at: Department of Mathematics, Huzhou University, Huzhou 313000, China (Y.-m. Chu). Department of Basic Sciences, University of Engineering and Technology, Peshawar 25000, Pakistan (H. Ahmad). Department of Physics and Astronomy, College of Science, King Saud University, Riyadh 11451, Saudi Arabia (M. Atif). muhatif@ksu.edu.sa (M. Atif), chuyuming2005@126.com (Yu-ming Chu), hijaz555@gmail.com (Hijaz Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Pure and copper doped zinc oxide nanoparticles (Zn1−xCuxO) of different concentrations (x = 0.0, 0.03, 0.06 and 0.09 wt%) were synthesized by using co precipitation method. During the next phase, effect of Cu concentration on structural, electrical and optical properties of ZnO-NPs were investigated with the multiple techniques like XRD, UV–visible spectroscopy, FTIR, and two probe methods. The XRD analysis confirmed hexagonal wurtzite crystal structure having crystallite sizes ranging between 18.3 and 24.2 nm. Rotational and vibration mode of different functional groups (OH, CH, C = O and ZnO) were calculated using FTIR spectra. Moreover, the UV visible spectroscopy identified a band gap (3.10–2.30 eV) of pure and Cu doped ZnO-NPs. Finally, electrical properties like conductivity was increased and whereas, the resistivity decreased with increasing Cu dopant concentrations. Overall, addition of Cu as doping agent improved the structural, optical and electrical properties ZnO-NPs and could enable multiple further potential applications like solar cell, energy storage and other optoelectronic devices.
Keywords
Zinc oxide NPs
Co-precipitation
Hexagonal wurtzite crystal structure
Conductivity
Resistivity
Solar cell
Energy storage
1 Introduction
Synthesis of II–VI semiconductor nanomaterials is of prime focus owing to their excellent electrical, optical and thermal properties. Potential applications include gas sensors, piezoelectric transducer and light – emitting diode, various optoelectronic devices, biomedical applications, plant science, and wastewater treatment (Mazitova et al., 2019; Lima et al., 2014; Caglar et al., 2015). Zinc oxide is one such potential semiconductor material with a tunable band gap; it can be doped with higher electronegative elements such as F, Cu, In, Al, and Co to modify its electrical and optical properties (Xiong et al., 2008). Moreover, ZnO is also relatively inexpensive and non-toxic nanomaterial. It has extraordinary electron mobility, which is also preferable for high-temperature applications because not easily oxidized due to the considerable band gap maximum excitation binding energy (Ryu et al., 2000; Pan et al., 2008). Furthermore, compared to TiO2 and SiO2; nZnO has been preferably used in killing harmful microorganisms, protecting ultraviolet radiation, and treating cancer sensing devices for clinical applications (Zhang et al., 2008).
Presently, the primary focus of researchers is to enhance the electro-optical properties by using the various cation dopant agents (Al+3, Mn+2, Ni+2, Co+2 and Cu+2) on ZnO lattice. Addition of dopant agents alters the morphology and related characters. In this context, the use of Cu+2 ions could improve physical properties of nZnO due to its higher ionization energy as well as the excellent electron and hole recombination (lilarassi and Chandrasekaran, 2010).
Presently indium tin oxide (ITO), an expensive material is used as a transparent conductive oxide (TCO) in various electrical and optical applications and Cu-doped nZnO could be a feasible/ ideal substitute material. We therefore synthesized a relatively low-cost material with properties comparable to ITO. It is pertinent to mention here that, dopants of different transition elements are used to alter ZnO physical and chemical properties (Senthilkumar et al., 2008). Recent reports also showed that elements with suitable ionic radii are preferable as a dopant compared to that having a larger radius. So, the ionic radius of Cu (87 pm) is smaller than the ionic radius of Zn (88 pm), so it can be a good substitute which does not produce stress in the lattice of ZnO nanomaterials (Munir et al., 2020). Numerous other studies reported use of inner-transition element dopant ZnO-NPs by co-precipitation technique (Pal and Giri, 2010), chemical vapour deposition (Shanmugam and Jeyaperumal, 2018), laser ablation (Thakur et al., 2014), precipitation method (Haq et al., 2018), combustion method (Vijayaprasath et al., 2016) and sol–gel process (Arshad et al., 2015). Among these methods, co-precipitation technique offers low cost and eco-friendly synthesis of nanomaterials (Moulahi and Sediri, 2014; Mittal et al., 2014; Munir et al., 2020) during which the stoichiometry and homogeneity of the particles can be controlled simply by controlling the reagents. The present study is related to synthesis of Cu doped ZnO-NPs through co-precipitation method and subsequent changes in electrical and optical properties were studied and compared with pure ZnO-NPs.
2 Experimental procedures
2.1 Synthesis of zinc oxide nanoparticles
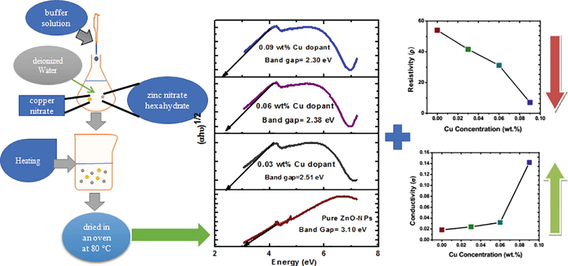
To synthesize ZnO-NPs via co-precipitation method, zinc nitrate hexahydrate (0.1 M) was dissolved in 250 mL deionized water with continuous stirring on a magnetic stirrer for 2 h. A buffer solution was also prepared (using NaOH-Na2CO3) and added drop by drop in a zinc nitrate hexahydrate solution which yielded white glutinous precipitate of ZnO material. The resultant white precipitate was filtered, washed with deionized water, dried in an oven for two hours at 80 °C. Finally, the ZnO powder was annealed in a furnace for three hours at 600 °C and ground in mortar and pestle. After that using the direct addition method, the Cu-doped nZnO were prepared by adding 0.03 wt% copper nitrate in 0.1 M zinc nitrate hexahydrate solution with continued stirring on a magnetic stirrer for two hours at room temperature. The same procedure was repeated as described earlier.
3 Results and discussion
3.1 Characterization techniques
Prepared Cu dopant ZnO-NPs were characterized by using various techniques including XRD, FTIR, UV–VIS and two probe method. The XRD analysis of Cu dopant ZnO-NPs was evaluated by using a PanalyticalX’Pert-Pro apparatus at 30 mA, 40 kV. Moreover, FTIR (Spectrum 2, Perkin Elmer) was engaged to examine rotational and vibrational mode attached on the surface of nanomaterials. After that the absorbance spectra was studied by UV–visible spectroscopy (double beam spectrometer, Lambda 25, Perkin Elmer) LP74 Processor Module. Finally, parameter DC resistivity has been determined by using two probe IV measurement techniques.
3.2 Structural analysis
The X-ray diffraction technique was used to study the crystal structure and lattice parameter of pure and Cu doped ZnO-NPs. Pure and copper doped ZnO-NPs showed no secondary peak in response to increasing Cu concentrations (Fig. 1). The hexagonal wurtzite crystal structure resulted in the most prominent peak (1 0 1) and Miller indices of different diffracted peaks at 31.76°, 34.45°, 36.35°, 47.62°, and 56.6° corresponding to (1 0 0), (0 0 2), (1 0 1), (1 0 2) and (1 1 0) were calculated. All the peaks and Miller indices compared with standard JCPDS card (36–451) and Cu ions substituted to the position of Zn ions because the ionic radius of Cu2+ (87 pm) closed to Zn2+ (88 pm) (Sajjad et al., 2018; Okeke et al., 2020). Finally, the crystallite size of pure and Cu dopant ZnO-NPs by using the Debye-Scherer Eq. (1) was calculated. It showed that as the Cu concentration increased, then crystallite size decreased Table 1.
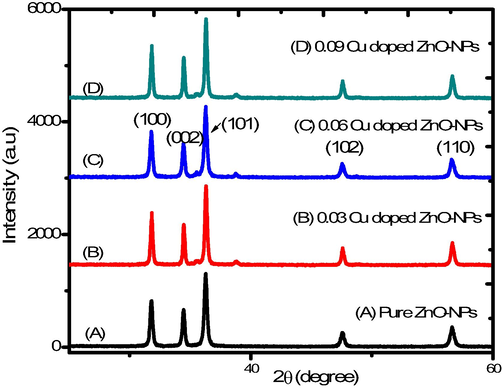
XRD spectrum of Cu doped ZnO-NPs.
Sample Name
Lattice Constant (Å) a = b c
Crystallite size (1 0 1) nm
Pure ZnO-NPs
3.25
5.20
24.21
0.03 Cu doped ZnO-NPs
3.25
5.20
20.61
0.06 Cu doped ZnO-NPs
3.25
5.20
19.23
0.09 Cu doped ZnO-NPs
3.24
5.20
18.30
D = crystalline size,
λ = wavelength of x-ray
β = Full width half maxima
θ = Bragg angle.
3.3 Fourier transforms infrared radiation (FTIR) analysis
The broad absorption spectrum in between 3511 cm−1 to 1578 cm−1 was evident (Fig. 2) which indicated the O–H stretching mode (Munir et al., 2019). Furthermore, another sharp peak of 1611 cm−1 indicated the H-O–H vibration mode, assigned to the small amount of H2O in the ZnO-nanocrystals (Osali et al., 2019). After that, the FTIR spectrum contains several absorption bands from 402 to 3511 cm−1. The band at 3511 cm−1 wave number presented O–H (hydroxyl) band. The band near about 2366 cm−1 wave number represented the C–H group. At 1400–1600 cm−1 due to C = O stretching mode, a vibrational band exists. At the level of 460 cm−1, the peak represented the Zn-O stretching group. The peaks from 518.04 cm−1 to 809.9 cm−1 represented the presence of Cu atom in the lattice of ZnO.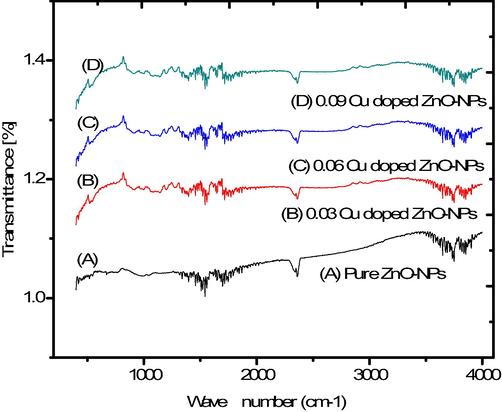
FTIR spectrum of pure and copper doped ZnO-NPs.
3.4 Electrical properties
Current-Voltage (I−V) response of pure and Cu dopant ZnO-NPs was observed at room temperature using two-probe I–V technique. It is reported that a linear behavior can be seen between current and voltage curve of pure and doped sample as expected because of Ohm’s law. Reversing the voltage gives same trend as for positive voltage. Slope of this I–V curve gives conductance for that sample. Inverse of conductance gives the value of resistance (R). Electrical conductivity is calculated by using the following relation:
Here L and A is the thickness and area of palette. For our experiment, L is 2.826 mm and A is 41.894 mm2 because same die is used and samples are pressed under same pressure. Inverse of conductivity gives resistivity (ρ). Calculate the value for conductivity and resistivity of prepared sample for various concentration of Cu (0.00, 0.03, 0.06, 0.09 wt%). Furthermore, increase in copper concentration as dopant material improved its conductivity and decreased resistivity (Fig. 3). A possible explanation of these effects is the successful substitution of Zn ions by Cu, which contributed to better electrical properties of Cu doped ZnO NPs as compared to pure ZnO-NPs (Bylsma et al., 1986).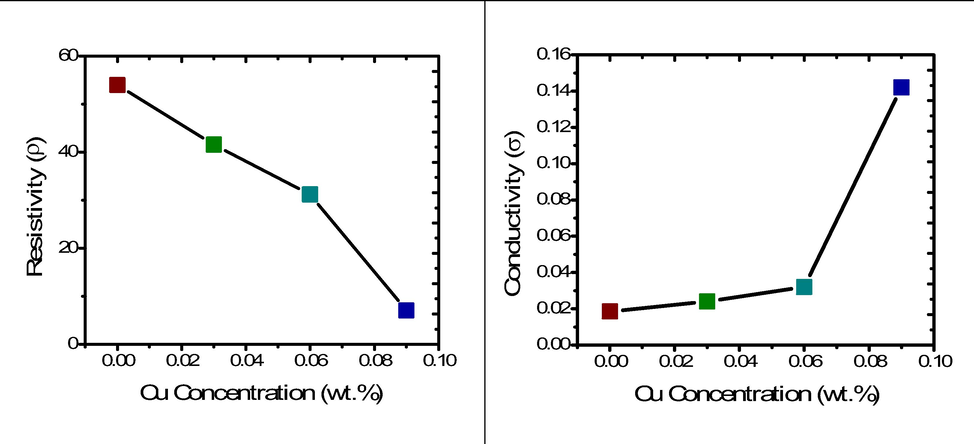
Resistivity and Conductivity of Cu doped ZnO-NPs.
3.5 UV–visible spectroscopy analysis
The UV–VIS absorbance showed that peak of the absorbance appearing at a wavelength of around 325.5 to 383 nm [25]. The maximum absorption recorded for pure ZnO-NPs sample where as the absorption decreased in the visible region for the doped samples (Fig. 4). Moreover, the Tauc, Davis and Mott relation was used to calculate the band gap of pure and Cu doped ZnO-NPs. it can be express as
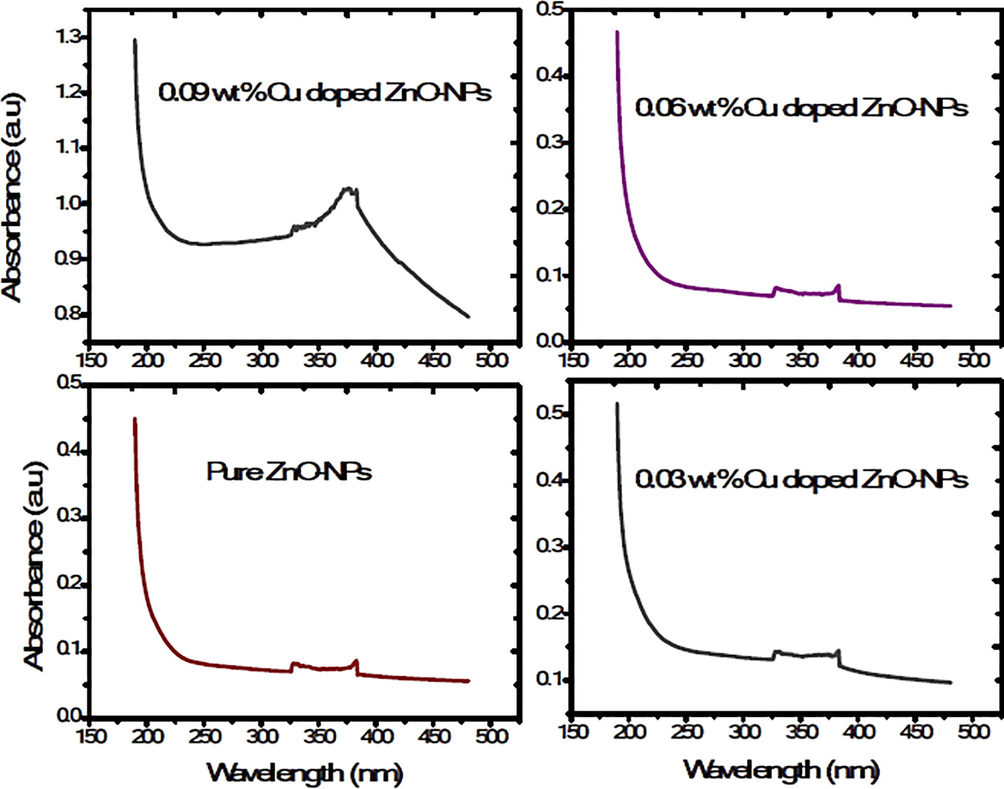
Absorbance spectrum of UV–Visible spectroscopy.
Here α = absorption coefficient, ν = light frequency, A = proportionality constant and Eg = band gap. The numerical value of band gap of pure ZnO-NPs approximately equal to 3.10 eV and it was also reported that the band gap decrease by increasing the dopant concentration (Munir et al., 2019). The (Fig. 5) shows that the band gap decreases (3.10–2.30 eV) as the copper concentration increases in pure ZnO-NPs. The Cu dopant ZnO-NPs not any structural change because both are d block element in a periodic table. The band gap decreased due to strong mixing the p-d sub shell of Cu and O. Furthermore, band gap shrinking depends upon the electron interaction and it also depends upon the merging the impurity into conduction band. Finally, the red shift in Cu doped ZnO nanoparticles was explained Bylsma by using second-order perturbation theory [26]. After that experimentally, confirm the red shift by substituting copper ion in lattice of pure ZnO-NPs.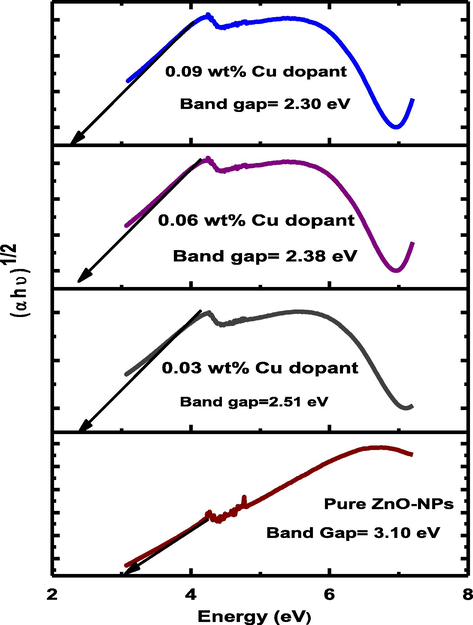
Band gap of pure and Cu doped ZnO-NPs.
4 Conclusions
Cu doped ZnO-NPs were synthesized by using Co-precipitation method. Hexagonal crystals structure and crystallite size (18.3 ∼ 24.21 nm) of pure and copper dopant ZnO-NPs were achieved. The absorption of the synthesized particles decreased with increasing Cu concentrations and strong absorption in between 325.5 and 383 nm. In addition, the electrical resistivity of doped ZnO NPs decreased whereas conductivity increased indicating successful substitution of Cu+ or Cu2+ ions in place of Zn2+. As a way forward and due to prominent conductive nature of the synthesized material, it could be suitable alternative material for multiple applications like solar cell and energy storage.
Acknowledgement
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURG-1442-293.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Band gap engineering and enhanced photoluminescence of Mg doped ZnO nanoparticles synthesized by wet chemical route. J. Luminescence. 2015;161:275-280.
- [CrossRef] [Google Scholar]
- Dependence of energy gap on x and T in Zn 1–x Mn x Se: The role of exchange interaction. Phys. Rev. B. 1986;33(12):8207.
- [Google Scholar]
- Effect of channel thickness on the field effect mobility of ZnO-TFT fabricated by sol gel process. J. Alloys Compounds. 2015;621:189-193.
- [CrossRef] [Google Scholar]
- Enhanced room temperature ferromagnetism in Cr-doped ZnO nanoparticles prepared by auto-combustion method. J. Semicond.. 2018;39(4):043001.
- [CrossRef] [Google Scholar]
- Structural, optical and magnetic characterization of Cu-doped ZnO nanoparticles synthesized using solid state reaction method. J. Mater. Sci.: Mater. Electr.. 2010;21(11):1168-1173.
- [Google Scholar]
- Co-doped ZnO nanoparticles synthesized by an adapted sol–gel method: effects on the structural, optical, photocatalytic and antibacterial properties. J. Sol-Gel Sci. Technol.. 2014;72(2):301-309.
- [CrossRef] [Google Scholar]
- Synthesis and properties of zinc oxide nanoparticles: Advances and prospects. Ref. J. Chem.. 2019;9(2):127-152.
- [CrossRef] [Google Scholar]
- UV–Visible light induced photocatalytic studies of Cu doped ZnO nanoparticles prepared by co-precipitation method. Solar Energy. 2014;110:386-397.
- [CrossRef] [Google Scholar]
- ZnO nanoswords and nanopills: Hydrothermal synthesis, characterization and optical properties. Ceram. Int.. 2014;40(1):943-950.
- [CrossRef] [Google Scholar]
- Impact of silver dopant on structural, optical and electrical properties of ZnO nanoparticles. J. Ovonic Res.. 2019;15(3):173-179.
- [Google Scholar]
- Influence of IP-injected ZnO-nanoparticles in Catla catla fish: hematological and serological profile. Naunyn-Schmiedeberg's Arch. Pharmacol.. 2020;393(12):2453-2461.
- [CrossRef] [Google Scholar]
- Impact of Ni dopant on optical and magnetic properties of ZnO nanoparticles for biomedical applications. J. Ovonic Res.. 2020;16(3):165-171.
- [Google Scholar]
- Impact of Cu doping on ZnO nanoparticles phyto-chemically synthesized for improved antibacterial and photocatalytic activities. J. Nanoparticle Res.. 2020;22(9):1-18.
- [Google Scholar]
- Structural and electro-optical properties of electrospun Cu-Doped ZnO thin films. Solid State Sci.. 2019;98:106038.
- [CrossRef] [Google Scholar]
- High temperature ferromagnetism and optical properties of Co doped ZnO nanoparticles. J. Appl. Phys.. 2010;108(8):084322.
- [CrossRef] [Google Scholar]
- Mater. Sci. Eng.. 2008;R62:1.
- Optical and structural properties of ZnO films deposited on GaAs by pulsed laser deposition. J. Appl. Phys.. 2000;88(1):201-204.
- [CrossRef] [Google Scholar]
- Structural and optical properties of pure and copper doped zinc oxide nanoparticles. Results Phys.. 2018;9:1301-1309.
- [CrossRef] [Google Scholar]
- Biocalorimetric and respirometric studies on biological treatment of tannery saline wastewater. Appl. Microbiol. Biotechnol.. 2008;78(2):249-255.
- [CrossRef] [Google Scholar]
- Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl. Surface Sci.. 2018;449:617-630.
- [CrossRef] [Google Scholar]
- Structural and optical properties of copper doped ZnO nanoparticles and thin films. Adv. Appl. Sci. Res.. 2014;5(4):18-24.
- [Google Scholar]
- Optical and magnetic studies on Gd doped ZnO nanoparticles synthesized by co-precipitation method. J. Luminescence. 2016;178:375-383.
- [CrossRef] [Google Scholar]
- J. Am. Chem. Soc.. 2008;130:7522.
- Cryst. Growth Des.. 2008;8:2609.







