Translate this page into:
Structural and vibrational characterization of anhydrous and dihydrated species of trehalose based on the FTIR and FTRaman spectra and DFT calculations
⁎Corresponding author. Fax: +54 381 4248169. sbrandan@fbqf.unt.edu.ar (Silvia Antonia Brandán)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The dihydrated trehalose form was characterized using the FTIR and Raman spectra. The dihydrated species present the higher solvation energy value in aqueous solution. The AIM study predicted an intra-molecular H bond with the glycosidic linkage oxygen. High gap values for the trehalose species could explain their nonreducing properties. The complete vibrational assignments for anhydrous and dihydrated forms were reported.
Abstract
In this study, three anhydrous species of trehalose and their dihydrated form were studied using the Fourier Transform Infrared (FTIR) and Raman spectroscopy combined with theoretical calculations derived from the theory of the functional of the density (DFT). Here, the structural and vibrational properties were predicted using the hybrid B3LYP/6-31G∗ method. The complete vibrational assignments were performed using the scaled mechanical force fields (SQMFF) methodology and their internal normal coordinates. The natural bond orbital (NBO) and atoms in molecules (AIM) calculations predicted high stabilities for the dihydrated species in gas and aqueous solution phases. The little variation observed in the dipole moment for the dihydrated species in solution could be related to a small perturbation of water hydration shell and short hydrogen bonds, as revealed by NBO and AIM studies. The lower volume expansion, low solvation energy and the higher nucleophilicity index observed for trehalose species in solution, in relation to maltose and sucrose could probably explain that different water molecules are around of trehalose/water mixtures generating higher “rigidity” in trehalose, as was experimentally reported in the literature. On the other hand, the low f(νO–H)H2O force constant value for trehalose, as compared with maltose and lactose could justify the very fragile character from the trehalose/water system to the temperature and concentration changes, as was experimentally observed from viscosity and Raman scattering studies.
Keywords
Trehalose
Molecular structure
Vibrational spectra
DFT calculations
Force field
1 Introduction
The structural and vibrational properties of some important disaccharides were recently reported by our investigation group (Brizuela et al., 2012a,b, 2014; Márquez et al., 2015a,b; Iramain et al., 2016). In this work, those properties for anhydrous and dihydrated species derived from trehalose were also studied because they are important due to the variety of their uses and applications (Schebor et al., 2010; Shibata and Nagashima, 2016; Yano et al., 2015; Kawashima and Goto, 2011; Connolly et al., 2010; Magazù et al., 2010a,b; Calabrò and Magazù, 2012; Siddhanta et al., 2015; Takahashi et al., 2006; Govindarajan et al., 2006; Belton and Gil, 1994) and, also, because its sugar is a nonreducing disaccharide of glucose that modifies the physical properties of membrane phospholipids in the dry state and, as a consequence, the notable stability of membranes in anhydrobiotic organisms can be explained, as was reported by Crowe and Crowe (1984). There are many studies related to the use of the vibrational spectroscopy on trehalose species, for instance, recent studies on the bioprotective effect of trehalose on human hemoglobin (Connolly et al., 2010; Calabrò and Magazù, 2012), on the interaction of trehalose with Hen egg white lysozyme (Belton and Gil, 1994) and, also on the low-frequency vibrations below 200 cm−1 of crystalline αα-trehalose dihydrate (Takahashi et al., 2006) using far-infrared spectroscopy but, so far, the infrared and Raman spectra of trehalose were not completely assigned. From a structural point of view, there are three known trehalose species anhydrous, one of them, the αα-trehalose isomer (α-D-glucopyranosyl α-D-glucopyranoside) is found in natural form while the other two αβ-trehalose and ββ-trehalose isomers were synthesized. On the other hand, the crystal structure of trehalose dihydrate in the solid phase was already reported in an independent form by Taga et al. (1972) and Brown et al. (1972). Hence, we have studied the structural and vibrational properties of those three trehalose anhydrous species and their αα-dihydrated form which were compared first among them and, later with the properties reported for similar sugars (Brizuela et al., 2012a,b, 2014; Márquez et al., 2015a,b; Iramain et al., 2016). Hence, the aim of this work is to perform a combined study using theoretical calculations based on the density functional theory (DFT) and the experimental infrared and Raman spectra for the αα-trehalose dihydrated in the solid state. With this purpose, the structures of those four trehalose species were optimized using the B3LYP/6-31G∗ level (Becke, 1993; Lee et al., 1988) in gas and aqueous solution phases and, later the atomic charges, molecular electrostatic potentials, stabilization energies, bond orders, frequencies and topological properties were calculated at the same level of theory. On the other hand, the force fields for those structures in both media were also calculated using the scaled quantum mechanical force field (SQMFF) methodology (Rauhut and Pulay, 1995a,b) accomplished with the corresponding normal internal coordinates in order to perform the vibrational analyses using the Molvib program (Sundius, 2002). In addition, the reactivities and behaviors of those four trehalose species in the different media were also predicted using the corresponding frontier orbitals and some known descriptors reported in the literature (Parr and Pearson, 1983; Brédas, 2014; Márquez and Brandán, 2014; Cataldo et al., 2014; Romani and Brandán, 2015a; Romani et al., 2015b; Márquez et al., 2015a,b). Here, the structural and vibrational properties for the trehalose species in aqueous solution were evaluated and analyzed in accordance with those publications reported for trehalose by different authors (Pagnotta et al., 2008; Varga et al., 2008; Magazù et al., 2007; Olsson et al., 2016; Magazù et al., 1998, 1999, 2010a,b, 2011; Ballone et al., 2000; Branca et al., 1999, 2001, 2002; Bonanno et al., 1998).
2 Experimental methods
A commercial sample of anhydrous αα-trehalose dihydrate in pure solid state was used to prepare KBr pellets. The infrared spectrum was recorded on a Fourier Transform Infrared (FT-IR) Perkin Elmer spectrophotometer in the wavenumbers ranging from 4000 to 400 cm−1 provided with a Globar source and DGTS detector. The Raman spectra of the compound in solid state was recorded between 4000 and 10 cm−1 with a Bruker RF100/S spectrometer equipped with a Nd:YAG laser (excitation line of 1064 nm, 800 mW of laser power) and a Ge detector cooled at liquid nitrogen temperature. The IR and Raman spectra were recorded with 200 scans and a resolution of 1 cm−1.
3 Computational details
The initial structures of those four species of trehalose were modeled with the GaussView program (Nielsen and Holder, 2008) and, then, they were optimized in the gas phase and in aqueous solution using the hybrid B3LYP/6-31G∗ method and the Gaussian 09 program (Frisch et al., 2009). In this study, two different αα-dihydrated species were considered and, also, the dimeric species corresponding to the most stable dihydrated species in accordance to the experimental structures reported (Taga et al., 1972; Brown et al., 1972). The calculations for all the species in aqueous solution were performed using the self consistent reaction field (SCRF) method together with the polarized continuum (PCM) and solvation (SD) models (Tomasi and Persico, 1994; Miertus et al., 1998; Marenich et al., 2009). Both models were used to compute the solvation energies while the changes in the volume of these species in solution in relation to the values in gas phase were estimated with the Moldraw program (Ugliengo, 1998). The three anhydrous species can be seen in Fig. 1 while the αα-dihydrated species is shown in Fig. 2. The dimeric structure for the most stable αα-dihydrated structure is presented in Fig. 3. On the other hand, in Fig. S1 the detailed theoretical structures of αα-, αβ- and ββ-trehalose anhydrous species showing the identification of the R1 and R2 glucopyranose rings are presented. Here, two atomic charges types were studied, the Merz–Kollman (MK) and the natural population atomic (NPA) which were computed with the natural bond orbital (NBO) and Gaussian programs (Besler et al., 1990; Glendening et al., 1996). On the other hand, the molecular electrostatic potential values for all the species were calculated using the MK charges while the bond order and the stabilization energy values for all those species were obtained from the NBO calculations at the same level of theory. The inter-molecular interactions were also analyzed by means the topological properties which were calculated using the AIM2000 program (Biegler-Köning et al., 2001). In addition, the reactivities of the anhydrous and dihydrated species in the different media were predicted using the corresponding frontier orbitals (Parr and Pearson, 1983; Brédas, 2014) while the chemical potential (μ), electronegativity (χ), global hardness (η), global softness (S) and global electrophilicity index (ω) descriptors were employed in order to predict their behaviors at the same level of theory (Márquez and Brandán, 2014; Cataldo et al., 2014; Romani and Brandán, 2015a; Romani et al., 2015b; Márquez et al., 2015a,b). Here, it is necessary to clarify that the equations corresponding to these descriptors were presented in the Supporting material because they are widely known in the literature. On the other hand, the internal coordinates used in the determination of the force fields for the anhydrous and dihydrated species were those similar to the ones reported for other sugars (Brizuela et al., 2012a; 2014) and, for this reason, they were not presented here. The force fields obtained for all the species with the SQMFF procedure (Rauhut and Pulay, 1995a,b) and the Molvib program (Sundius, 2002) in Cartesian coordinates were later transformed to internal coordinates. Here, we have used the potential energy distribution components (PED) ⩾ 10% in order to perform the complete vibrational assignments of those anhydrous and dihydrated species while the assignments for the dimeric species were performed with the aid of the GaussView program (Nielsen and Holder, 2008).
Theoretical anhydrous structures of αα-, αβ and ββ-trehalose indicating the atoms labeling for the O atoms corresponding to the glycosidic and glucopyranose rings.
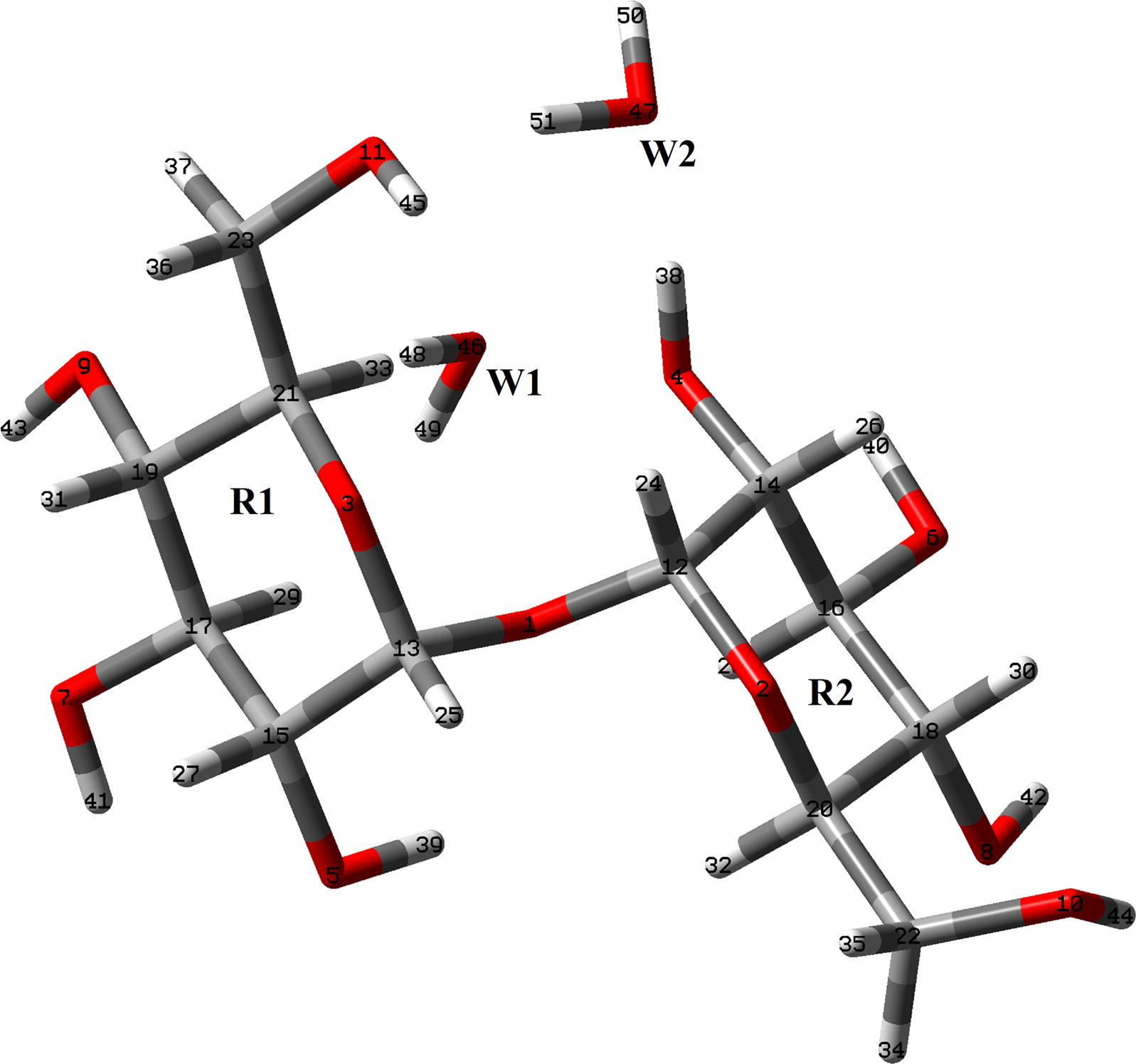
Theoretical structure of αα-dihydrated trehalose species together with the atoms labeling. The water molecules are indicated by W letters.
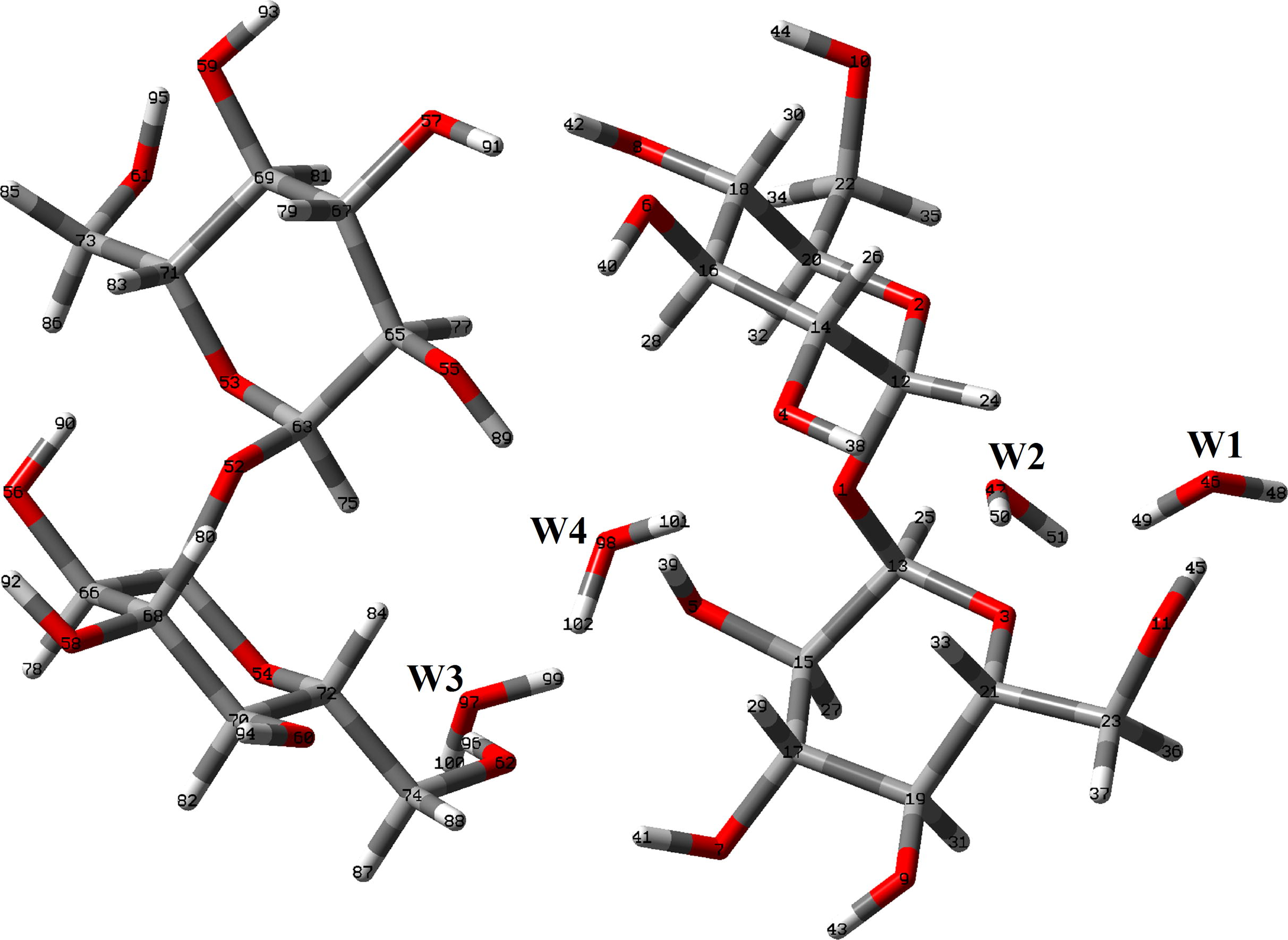
Theoretical dimeric structure of αα-dihydrated trehalose species together with the atoms labeling.
4 Results and discussion
4.1 Structural analysis
Table 1 shows the calculated total and relative energies, dipole moments and populations for all the studied trehalose species in gas and aqueous solution phases. Here, we have considered two different structures for the αα-trehalose anhydrous and dihydrated species, named C1 and C2 in order to find the most stable species, as observed in Table 1. The results clearly show that the αβ anhydrous and the C1αα- dihydrated species are the most stables and, hence, the higher populations are found for the αβ- anhydrous species in gas phase with a value of 60% while in solution the value decreases up to 35.33%, then, the population of the ββ species increases drastically in this medium from 0.16% in the gas phase up to 46.30%. In relation to the dipole moment values, in gas phase the three anhydrous species show similar values while their values notably change in solution observing a particular decrease in the value for the ββ species. This notable reduction in the dipole moment value for the anhydrous ββ species could probably be related to their major volume expansion in solution and to their higher solvation energy value, as will see later. The dipole moment values for the two dihydrated species also increase in solution presenting the most stable species the higher value in gas phase but the lower value in solution, in relation to the C2 species. Here, the few variations observed in the dipole moments for the dihydrated species in solution could be related to a small perturbation of water hydration shell and short hydrogen bonds between trehalose oxygens and water hydrogens, as reported by Pagnotta et al. (2008). The αα dimeric species present an energy value of −2901.5588 Hartrees in gas phase with a dipole moment value of 6.05 D. This way, this dimeric species show a higher stability as compared to two units of αα dihydrated species, it is 2 × −1450.7569 = −2901.5138 Hartrees.
B3LYP/6-31G*
GAS
PCM
Species
E (hartree)
μ (D)
ΔE kJ/mol
Population %
E (hartree)
μ (D)
ΔE kJ/mol
Population %
Anhydrous
C1αα
−1297.8870
2.80
1.05
39.33
−1297.9415
4.36
0.52
18.37
αβ
−1297.8874
2.84
0.00
60.51
−1297.9417
3.35
0.00
35.33
ββ
−1297.8818
2.72
14.69
0.16
−1297.9412
0.81
1.31
46.30
C2αα
−1297.8733
3.25
36.98
0.00
−1297.9372
3.70
11.80
0.00
Dihydrated
C1αα
−1450.7569
3.70
0.00
100
−1450.8214
4.54
0.00
100
C2αα
−1450.7376
3.44
50.63
0.00
−1450.8096
6.05
30.95
0.00
A comparison of the calculated geometrical parameters for the anhydrous and dihydrated species of trehalose in gas phase with those corresponding experimental ones for the dihydrated species (Taga et al., 1972 and Brown et al., 1972) by means of the root-mean-square deviation (RMSD) values can be seen in Table 2. The RMSD values show a very good agreement for the bond lengths and angles of all the species and, especially, in the dihedral angles of the dihydrated species with a difference only of 7.4° while for the anhydrous species the RMSD values vary between 65.5 and 38.1°, as expected because the compared experimental species is also dihydrated. Note that the higher differences among the structures are observed in the dihedral C18–C20–C22–O10 angles where only the dihydrated species has a value close to the experimental one, as explained before. In general, the optimized parameters are underestimated by the B3LYP/6-31G∗ calculations, in reference to the experimental values and, only some calculated distances are approximately similar in all the species such as those C–C bonds belonging to the ring R1. Probably, this observation can be explained because the α-D-glucopyranosyl R1 ring remains practically constant in the αα, αβ and ββ species including the dihydrated one while the other α-D-glucopyranoside ring R2 change in the αβ and ββ species. For this reason, both αα anhydrous and dihydrated species have practically similar bond lengths values with exception of the C13–O1, C20–C22, C21–C23, C14–O4 and C18–O8 bonds which show slightly variations in the values, as indicated in Table 2. Bold letter: RMSD values.
B3LYP/6-31G*a
Parameter
Anhydrous
Dihydrated
α-α-trehalose
α-β-trehalose
β-β-trehalose
α-α-trehalose
Expb α-α-trehalose
Bond lengths (Å)
C12–O1
1.431
1.434
1.437
1.431
1.417 (5)
C13–O1
1.433
1.431
1.427
1.406
1.421 (5)
C12–O2
1.405
1.401
1.400
1.408
1.423 (5)
C20–O2
1.446
1.446
1.446
1.442
1.437 (5)
C13–O3
1.399
1.401
1.408
1.425
1.407 (5)
C21–O3
1.442
1.442
1.449
1.455
1.424 (6)
C14–C12
1.534
1.530
1.530
1.542
1.514 (6)
C14–C16
1.530
1.531
1.531
1.526
1.523 (6)
C16–C18
1.525
1.532
1.533
1.518
1.526 (6)
C18–C20
1.529
1.531
1.529
1.529
1.533 (6)
C13–C15
1.534
1.534
1.534
1.534
1.531 (6)
C15–C17
1.530
1.529
1.534
1.531
1.513 (6)
C17–C19
1.526
1.525
1.539
1.525
1.514 (6)
C19–C21
1.532
1.533
1.535
1.532
1.526 (6)
C20–C22
1.524
1.526
1.526
1.536
1.528 (6)
C21–C23
1.516
1.516
1.517
1.525
1.519 (6)
C14–O4
1.422
1.423
1.423
1.410
1.429 (6)
C15–O5
1.422
1.422
1.422
1.421
1.425 (5)
C16–O6
1.420
1.422
1.422
1.421
1.410 (6)
C17–O7
1.420
1.420
1.410
1.422
1.435 (5)
C18–O8
1.418
1.418
1.417
1.425
1.412 (5)
C19–O9
1.418
1.419
1.420
1.418
1.411 (6)
C22–O10
1.416
1.418
1.418
1.417
1.425 (5)
C23–O11
1.422
1.421
1.420
1.423
1.429 (6)
RMSD
0.011
0.011
0.013
0.014
Dihedral angles (°)
C12–O1–C13
114.4
114.2
114.3
115.5
115.7 (3)
C12–O2–C20
116.2
116.5
116.3
115.0
114.1 (3)
C13–O3–C21–
115.8
115.9
114.7
114.9
114.3 (3)
C12–C14–C16
110.6
110.9
111.0
110.0
109.5 (4)
C14–C16–C18
110.9
110.3
110.4
109.3
108.6 (4)
C16–C18–C20
109.2
109.6
109.5
109.5
113.1 (4)
C18–C20–O2
109.5
112.3
112.0
108.8
111.6 (4)
O2–C12–O1
112.5
112.7
112.5
111.6
111.9 (1)
O1–C13–O3
112.5
112.6
112.3
112.4
111.3 (4)
C20–C22–O10
111.2
111.1
111.0
114.2
112.9 (4)
O1–C12–C14
106.1
106.3
106.1
107.5
106.4 (1)
O1–C13–C15
106.3
106.2
106.6
106.3
105.9 (4)
C13–C15–C17
110.4
110.4
111.3
110.2
110.3 (4)
C15–C17–C19
111.0
111.0
110.3
111.3
112.2 (4)
C17–C19– C21
108.8
108.9
107.4
109.1
110.7 (4)
C19–C21–O3
109.2
109.3
108.0
107.9
112.6 (4)
C21–C23–O11
107.6
107.6
107.5
113.9
112.1 (4)
RMSD
2.1
2.0
2.3
1.8
Dihedral angles (°)
C13–O1–C12–O2
68.0
70.9
65.4
79.2
75.0
C12–O1–C13–O3
62.8
65.3
64.5
68.8
61.7
O2–C12–C14–C16
50.8
53.5
52.7
51.4
62.7
C12–C14–C16–C18
−52.4
−54.5
−53.6
−53.5
−56.8
C16–C18–C20–O2
−56.4
−51.9
−52.8
−59.5
−47.9
C18–C20–O2–C12
58.7
54.0
55.1
59.0
53.9
C18–C20–C22–O10
177.9
−177.7
−178.6
33.2
47.4
O3–C13–C15–C17
50.5
50.6
48.9
52.2
55.5
C13–C15–C17–C19
−51.8
−51.9
−51.0
−52.5
−51.6
C17–C19–C21–O3
−57.6
−57.1
−61.9
−58.2
−50.2
C19–C21–O3–C13
60.1
59.6
63.7
62.4
60.0
C19–C21–C23–O11
−169.8
−170.1
−170.7
−170.6
−167.7
O1–C13–C15–O5
52.0
51.8
51.1
53.8
O2–C20–C22–O10
57.8
59.0
58.4
–87.5
O3–C21–C23–O11
69.5
69.1
69.9
69.9
O1–C12–C14–O4
52.4
55.1
54.6
48.8
C16–C14–C12–O1
−72.0
−69.5
−70.2
−71.8
O1–C12–O2–C20
63.4
65.4
65.0
65.4
RMSD
38.1
65.1
65.5
7.4
4.2 Volume variations and solvation energies
The molecular volume variations (Ugliengo, 1998) observed in the anhydrous and dihydrated species in solution, in reference to the values in the gas phase and, the calculated solvation energies at the B3LYP/6-31G∗ calculations level using the polarized continuum (PCM) and solvation (SD) models (Tomasi and Persico, 1994; Miertus et al., 1998; Marenich et al., 2009) are summarized in Table 3. The results for all the trehalose species show expansions of volume in aqueous solution where the value increases according to the following order: αβ-trehalose (1.4 Å3) < αα-trehalose (2.1 Å3) < ββ-trehalose (2.8 Å3). This variation observed could suggest that the position of the OH group also has influence on the volume variation, thus when the position is ββ- the variation is higher. This way, the reduction in their dipole moment value could justify that observation. On the other hand, the volume expansions theoretically observed for trehalose, maltose and sucrose could probably explain that different water molecules are around disaccharide–water mixtures which generate a particular higher ‘‘rigidity’’ in trehalose due to their lower volume variation, as was experimentally observed by Varga et al. (2008). A similar relation is observed in the calculated solvation energy values, as indicated in Table 3. Thus, the species with higher volume variation, it is the ββ-trehalose form, present the higher solvation energy value, probably because only for this species in solution the dipole moment values significantly decrease their value from 2.72 D in gas phase to 0.81 D in solution while for the remain species the values increase in this medium. When these values are compared with the values corresponding to the two maltose anhydrous species we observed a contrary relation because the species with higher volume variation present the lower solvation energy value. On the other hand, when the solvation energies for the trehalose species are compared with the other sugars, we observed that the β anhydrous and monohydrated species have higher values than the corresponding α ones where, in particular, the values of the hydrated sugar species decreasing according to the following order: Sucrose dihydrated > β-maltose > Anhydrous sucrose > Anhydrous β-Lactose > Sucrose pentahydrated > Anhydrous α-Lactose > α-α-trehalose dihydrated > α-Lactose Monohydrated > Anhydrous maltose while the three anhydrous species of trehalose have the lowest solvation energy values, as observed in Table 3. These low solvation energies values observed for trehalose in relation to sucrose and maltose probably can explain in part why a trehalose/water mixture shows a larger structural resistance to temperature changes and a higher ‘‘rigidity’’ in comparison with maltose/H2O and sucrose/H2O mixtures, as mentioned by Varga et al. (2008). This property of trehalose could also be related to the most intense disaccharide–water interaction observed for trehalose by Magazù et al. (2007). ΔGc = ΔGuncorrected# − ΔGTotalnon electrostatic.
Molar Volume (Å3)
Trehalose Anhydrousa
Species
GAS
PCM/SMD
#ΔV = VAS–VG (Å3)
α-α-trehalose
327.1
329.3
2.1
α-β-trehalose
322.4
323.8
1.4
β-β-trehalose
320.9
323.7
2.8
Trehalose dihydrateda
α-α-trehalose
357.8
359.7
1.9
Solvation energies (kJ/mol)
Trehalose Anhydrousa
Species
ΔGu#
ΔGne
ΔGc
α-α-trehalose
−142.95
25.87
−168.82
α-β-trehalose
−142.28
25.66
−167.88
β-β-trehalose
−155.81
25.29
−181.10
Trehalose dihydrateda
α-α-trehalose
−169.18
29.97
−199.15
Maltose Anhydrousb
α-maltose
322.1
325.6
3.5
β-maltose
322.7
325.1
2.4
Maltose Monohydratedb
α-maltose
343.6
348.7
5.1
β-maltose
342.9
344.7
1.8
Solvation energies (kJ/mol)
Species
ΔGu#
ΔGne
ΔGc
Maltose Anhydrousb
α-maltose
−151.87
25.29
−177.16
β-maltose
−158.43
23.99
−182.42
Maltose Monohydratedb
α-maltose
−157.64
31.43
−189.07
β-maltose
−185.97
24.79
−210.76
Lactose Anhydrousc
α-Lactose
−174.95
26.33
−201.28
β-Lactose
−179.67
26.17
−205.84
Lactose Monohydratedc
α-Lactose
−165.25
30.18
−195.43
Sucrosed
Anhydrous
−182.00
28.38
−210.38
Sucrose.(H2O)2
−198.56
29.30
−227.86
Sucrose.(H2O)5
−160.00
41.97
−201.97
4.3 Charges, molecular electrostatic potentials (MEP) and bond orders (BO) studies
For the four trehalose forms, two charge’s types, the atomic MK and NPA charges (Besler et al., 1990; Glendening et al., 1996), were considered using the B3LYP/6-31G∗Method, as was mentioned in section computational details. These charges for those species in gas phase are summarized in Table S1 while the values in solution are given in Table S2. The exhaustive inspection of the values show that the NPA charges have in general higher values than the MK charges in both media and besides, these charges on the C22 and C23 atoms in the four species have negative signs, as observed in Tables S1 and S2. Note that the MK charge on the C19 atom belonging to the R1 ring of the dihydrated species present negative sign in gas phase and positive in solution while on the other ones are observed positive sings. On the other hand, these charges in solution are observed with negative signs on the C12 and C13 atoms in the dihydrated species while in gas phase on both atoms are observed positive signs. Note that for the dihydrated species in gas phase we observed positive signs in both charges on all the H atoms corresponding to the water molecules. On the contrary, in solution the NPA charges on the H atoms of the dihydrated species are observed with negative signs while the MK exhibits in this media positive signs. These variations in the charges observed for the species in solution could clearly explain the hydration process with water molecules of the solvent. Besides, these changes in the charges possibly will explain the most intense disaccharide–water interaction observed for trehalose in solution by Magazù et al. (2007) that moreover will justify their higher bioprotective effectiveness.
The MEPs were analyzed on all the atoms of the four species in both media and the corresponding values are presented in Table S3. Analyzing the results, we observed that in both media the most negative values are located on the O10 atoms of all the species while the less negative values for the anhydrous species are observed on the O1 atoms and for the dihydrated species on the O3 atom. Thus, these results for the anhydrous species could indicate that the inter-ring C13-O1–C12 angle is independent of the medium employed. Besides, this result probably is also related to that observed experimentally by Taga et al. (1972) for the dihydrated species because neither the ring oxygens nor the glycosidic linkage oxygen accept hydrogen bonds. Later, as it is expected, the lowest negative MEP values for all the trehalose species are observed on the H atoms where the anhydrous species show the highest values on the H34 atoms while the lower values are observed on the H38 and H39 atoms of these species probably because the distances between these atoms are close to the corresponding O1 atoms (2.248–2.241 Å) and, for these reasons, they are the more labile atoms. Analyzing, the values for the dihydrated species, the higher values are also observed on the H34 atoms while the lowest values on the H atoms of the water molecules. The different reaction sites can be obviously observed by the different colorations when the MEP mapped surfaces for the α-α-anhydrous and dihydrated species are compared in Fig. S2. Thus, the blue colors show the electrophilic sites observed on the H atoms while the nucleophilic sites are clearly observed in red color on all the O atoms. Here, it is interesting to note that in the dihydrated species a strong blue color are observed on the H atoms of the water molecules in agreement to the low MEP values, as observed in Table S3.
In relation to the bond order values, Table S4 show these values expressed as Wiberg indexes, for the four trehalose species. The results clearly show that for the four species the higher BO values are observed in the O atoms that belong to the R1 and R2 rings and to the inter-ring O atoms, these are the O1, O2 and O3 atoms, which have values between 2,030 and 1,978 while their values clearly decrease in solution. Here, newly we evidenced that experimental results found by Taga et al. (1972) for the dihydrated species. On the contrary, in general remaining O atoms of all the species belonging to different OH groups in the two rings are observed slight increase in the BO values in solution. These higher values in solution probably indicate that these H atoms linked to those O atoms are less labile. On the contrary, regarding the BO values for the C atoms, the C16 and C17 atoms in αα-trehalose and the C17 atoms in αβ and ββ-trehalose and the C14 and C19 atoms in the dihydrated species present the higher values in gas phase but, some of these values change few in solution. Probably, due to the different positions of the OH groups linked to the C atoms some values for these atoms are different in the four species. Other very important result is observed in the H atoms of the four species because when those atoms are linked to the C atoms have higher values than the other ones, as expected due to that the H atoms bonded to the OH groups are most labile and, as a consequence they have the lowest values. An expected result is observed in the dihydrated species, because the O and H atoms belonging to the water molecules present the lowest BO values but, they increase notably in solution due to the hydration of these atoms with water molecules. These results can explain that the water mobility within the first hydration shell is ca. 25% smaller than that of pure water, as observed by Bonanno et al. (1998) from molecular dynamics simulation studies for the water interaction with αα-trehalose.
4.4 Stability study
The stabilities of all the trehalose’s species in gas and aqueous solution phases were studied using NBO (Glendening et al., 1996) and AIM (Biegler-Köning et al., 2001) calculations. Thus, the donor–acceptor interaction energies obtained from the second order perturbation calculations in those media are presented in Table S5. Here, the main donor–acceptor interaction energies for the anhydrous species in both media are observed from the lone pairs of the O atoms to the antibonding O–C orbitals (LP(2)O1 → σ*O–C) while for the dihydrated species besides these are also observed those from the lone pairs of the O atoms to the antibonding O–H orbitals (LP(2)O1 → σ*O–H), as can be seen in Table S5. Thus, the contributions to the total energy show clearly that the dihydrated species are the most stable in both media than the anhydrous species. The presence of a additional H bonds interaction in solution in the dihydrated species will explain perhaps the role of trehalose in the stabilization of proteins because, as recently was mentioned by Olsson et al. (2016), the protein adsorbs water and thereby reduces the water content in the trehalose–water system.
The stabilities of all the trehalose’s species were also studied by means of the topological properties with the AIM2000 program (Biegler-Köning et al., 2001). Accordingly, the calculated charge electron density, (ρ) and the Laplacian values, ∇2ρ(r) in the bond (BCPs) and ring critical points (RCPs) for the R1 and R2 rings of the three anhydrous and dihydrated species in both media are given in Tables S6 and S7, respectively. Analyzing first the three anhydrous species we observed from Table S6 notable differences between the values in gas phase and those in solution. For instance, the αβ- and ββ- species in gas phase present the two expected RCPs due to the R1 and R2 rings and the H bonds O6–H42 interactions while this latter interaction it is not observed in the αα- species. On the contrary, in solution the αα- and αβ- species have the two RCPs and a new H–H interaction while in the ββ- species there is not observed the latter interaction. This observation can be justified by the large distance between the O6 and H42 atoms of 3.519 Å. On the other hand, the distance between the H33 and H38 atoms of 2.615 Å justifies that in the αα species in gas phase there is not observed interaction between both atoms. For the dihydrated trehalose species a very different situation is found when their topological properties are analyzed in gas and in aqueous solution phases. Thus, Table S7 shows their calculated properties using the B3LYP/6-31G∗ Method in both media. First, in both media are observed the two RCPs but in gas phase are observed six O–H interactions while in solution are observed only five because the O8–H44 interaction in solution present a distance of 3.139 Å. These results clearly show the high stability of the dihydrated species in both media and, besides, suggest the presence of this species in the solid state, in complete agreement with the NBO results. Here, another very important result is the formation of the H bond O3–H49 interactions in both media observed only for the dihydrated species because this O3 atom belong to the glucopyranose R1 ring. This result is different from that observed experimentally by Taga et al. (1972) for the dihydrated species where neither the ring oxygens nor the glycosidic linkage oxygen accept hydrogen bonds. The AIM study explains the ability of trehalose to form H bonds and, this way, to stabilize the membranes, as reported by Crowe and Crowe (1984).
The high stability of the dihydrated trehalose species predicted using NBO and AIM studies, as compared with the anhydrous species due to the H bonds formation could support: (i) the small perturbation of water hydration shell and short hydrogen bonds between trehalose oxygens and water hydrogens (Pagnotta et al., 2008), (ii) the water dynamics in the presence of bioprotectant systems which is affected by all the disaccharides and particularly by trehalose (Varga et al., 2008; Magazù et al., 2007), (iv) that trehalose is shown to affect the swelling properties of the polymer with temperature, stabilizing its conformation (Magazù et al., 1998), (v) the presence of the disaccharides inhibits the protein dynamical transition (Magazù et al., 2011) and, (vi) only the H bonds intra-molecular, as mentioned by Ballone et al. (2000), because the DFT results for the isolated molecule is a simplified scheme and provides a fairly good description of ground-state geometries. On the contrary, the cohesion of the crystal is mainly due to the formation of several intermolecular hydrogen bonds which involve every hydroxyl group in the system.
4.5 Frontier orbitals and descriptors study
Trehalose is a sugar that acts on the stability of membranes in anhydrobiotic organisms and this effect is only observed in this carbohydrate by Crowe and Crowe (1984), for this reason, it is very important to predict their reactivities and behaviors in gas and in aqueous solution in order to understand their mechanism of bio-protecting. Thus, their reactivities in those media were predicted using the frontier orbitals (Parr and Pearson, 1983; Brédas, 2014; Márquez and Brandán, 2014; Cataldo et al., 2014; Romani and Brandán, 2015a; Romani et al., 2015b), whose values are presented in Table S8, while the chemical potential (μ), electronegativity (χ), global hardness (η), global softness (S), global electrophilicity index (ω) and mucleophilic index (E) descriptors were employed (Márquez and Brandán, 2014; Cataldo et al., 2014; Romani and Brandán, 2015a; Romani et al., 2015b; Márquez et al., 2015a,b) to predict their behaviors in the two media and, they are summarized in Table S9. These properties calculated for the trehalose species were then compared with those reported for maltose and lactose (Iramain et al., 2016). Here, the high gap values observed for the anhydrous and dihydrated species of trehalose could probably explain that it is a no reducing disaccharide of glucose while the similar lower gap values for maltose and lactose probably justify that these carbohydrates are reducing sugars. Note that the reactivity in all the sugars increases in aqueous solution. In particular, if trehalose is less reactive in water in a protein–trehalose–water system the protein adsorbs water decreasing their content from the trehalose–water system, as reported by Olsson et al. (2016). Moreover, the major intensity in the trehalose–water interaction and its higher bioprotective effectiveness, as mentioned by Magazù et al., 2007 could also be justified by the high gap value and low reactivity. On the other hand, the high gap values observed for the anhydrous and dihydrated species of trehalose suggest a reduced reactivity that could support that this species acts as a nonreducing disaccharide of glucose while the similar lower gap values for maltose and lactose probably justify that these carbohydrates are reducing sugars. Here, it is necessary to remember that in a reducing disaccharide of glucose (maltose) one of the two monosacharides has one free hemiacetal unit that generates a reducing aldehyde group while the other one is occupied with the glycosidic bond which cannot act as reducing agent. On the contrary, trehalose is a nonreducing disaccharide of glucose because there is not a free hemiacetal unit but there is an acetal linkage between their anomeric centers, giving a structure without one free hemiacetal unit to act as reducing agent.
In relation to the descriptors, it is necessary to clarify that as their equations are widely known these were not included in the main manuscript but in Table S9. Regarding exhaustively the descriptors, we observed first that all the values observed in gas phase decrease in solution and, then, that the less reactive species (>gap) are the trehalose’s species which have > global hardness (η) and <global softness while the anhydrous species also present > nucleophilic and electrophilic indexes. Analyzing the nucleophilic and electrophilic indexes for the hydrated disaccharides in solution, we observed that the presence of two water molecules in trehalose, in relation to one in maltose and lactose, increase the nucleophilicity thus, the observed tendency is: trehalose > maltose > lactose while the trend change with the electrophilicity to: maltose > trehalose > lactose. These results are in agreement with those MEP values predicted for the nucleophilic sites on all the O atoms including all OH groups and, with the results suggested by Ballone et al. (2000), because the cohesion of the crystal is mainly due to the formation of several intermolecular hydrogen bonds which involve every hydroxyl group in the system. The nucleophilicity index observed especially in the hydrated species could probably be related with that important property to stabilize membranes due to their ability to form H bonds and, hence, to replace water around the head group of a phospholipid, according to the results observed by Crowe and Crowe (1984). In addition, that nucleophilicity index will explain why trehalose inhibits the protein dynamical transition during the addition of disaccharides to hydrated lysozyme and why the of hydrogen bond connectivity has influence on transport properties for the trehalose–water system, as revealed by Magazù et al. (2011) and 1998, respectively.
4.6 Vibrational analysis
All the trehalose species were optimized using B3LYP/6-31G∗ calculations with C1 symmetry where the three anhydrous species have 129 normal vibration modes while the dihydrated species present 147 normal vibration modes and, where all these modes show activity in the infrared and Raman spectra. The experimental infrared and Raman spectra of dihydrated trehalose in the solid phase can be seen in Fig. 4 while in Fig. 5 is presented a comparison of the IR spectrum of the αα-dihydrated species with the corresponding predicted for this species and their dimer in gas phase at the B3LYP/6-31G∗ level of theory. On the other hand, Fig. S3 shows the comparisons among the experimental IR of dihydrated trehalose in the solid phase with the corresponding predicted for the three anhydrous species in gas phase using that level of theory. The comparisons among the experimental IR of dihydrated trehalose in the solid phase in the 4000–2500 cm−1 region with the predicted for this species and their corresponding dimer in gas phase at the same level of theory are given in Fig. S4 while in Fig. S5 are presented these same comparisons but in the 2000–0 cm−1 region. These two comparisons clearly evidence the presence of more than one molecule in the solid state due to the increase in the numbers and intensities of the bands in both spectra for this species in relation to the observed in the dihydrated species. A similar situation is observed when the Raman spectra are compared in those two regions, as can be seen in Fig. S6 and S7. In the 4000–2500 cm−1 region the presence of a dimer is also observed in the form of the bands with higher intensities (Fig. S6, green spectrum) while the quantities and forms of the bands in the 2000–0 cm−1 region could support the presence of the dimer, as those two most intense bands are observed in the Raman spectra at approximately 1500 cm−1. In Table 4 is summarized the observed and calculated wavenumbers and assignments for the three anhydrous and dihydrated species in gas phase using SQM/B3LYP/6-31G∗ calculations while for the assignments of the dimer the GaussView program (Nielsen and Holder, 2008) was used. We observe from Table 4 that the assignments are clearly different for the αα-, αβ- and ββ-trehalose species indicating, this way, the influence of the position of the OH groups on the bands observed in both spectra. Here, the SQM force fields were calculated with the Molvib program (Sundius, 2002) using scale factors valid for B3LYP/6-31G∗ calculations (Rauhut and Pulay, 1995a,b). These results can be obtained at the request of the authors. On the other hand, the vibrational assignments for the anhydrous and dihydrated species were performed taking into account those assignments reported for other sugars (Brizuela et al., 2012a, 2014; Márquez et al., 2015a,b; Iramain et al., 2016), those reported by Takahashi et al. (2006) for the low-frequency vibrations of the dihydrated species and, in accordance with ours DFT calculations obtained here. It is necessary to clarify that Ballone et al. (2000) have studied structural properties and harmonic vibrational modes of trehalose in the gas phase and in the monohydrate crystal in order to analyze the local conformation and the relative strength of intra- and intermolecular hydrogen bonds, and the effects of the crystal environment on the molecular geometry and vibrational frequencies. Their results provide a fairly good description of ground-state geometries, while vibrational properties are only in qualitative agreement with those computed by the density functional method. Abbreviations: ν, stretching; β, deformation in the plane; γ, deformation out of plane; wag, wagging; τ, torsion; βR, deformation ring τR, torsion ring; ρ, rocking; τw, twisting; δ, deformation; a, antisymmetric; s, symmetric; (1), glucopyranose Ring1; (2), glucopyranose Ring2. aThis work, bFrom scaled quantum mechanics force field Bold letter, values taken from Refc (Takahashi et al., 2006).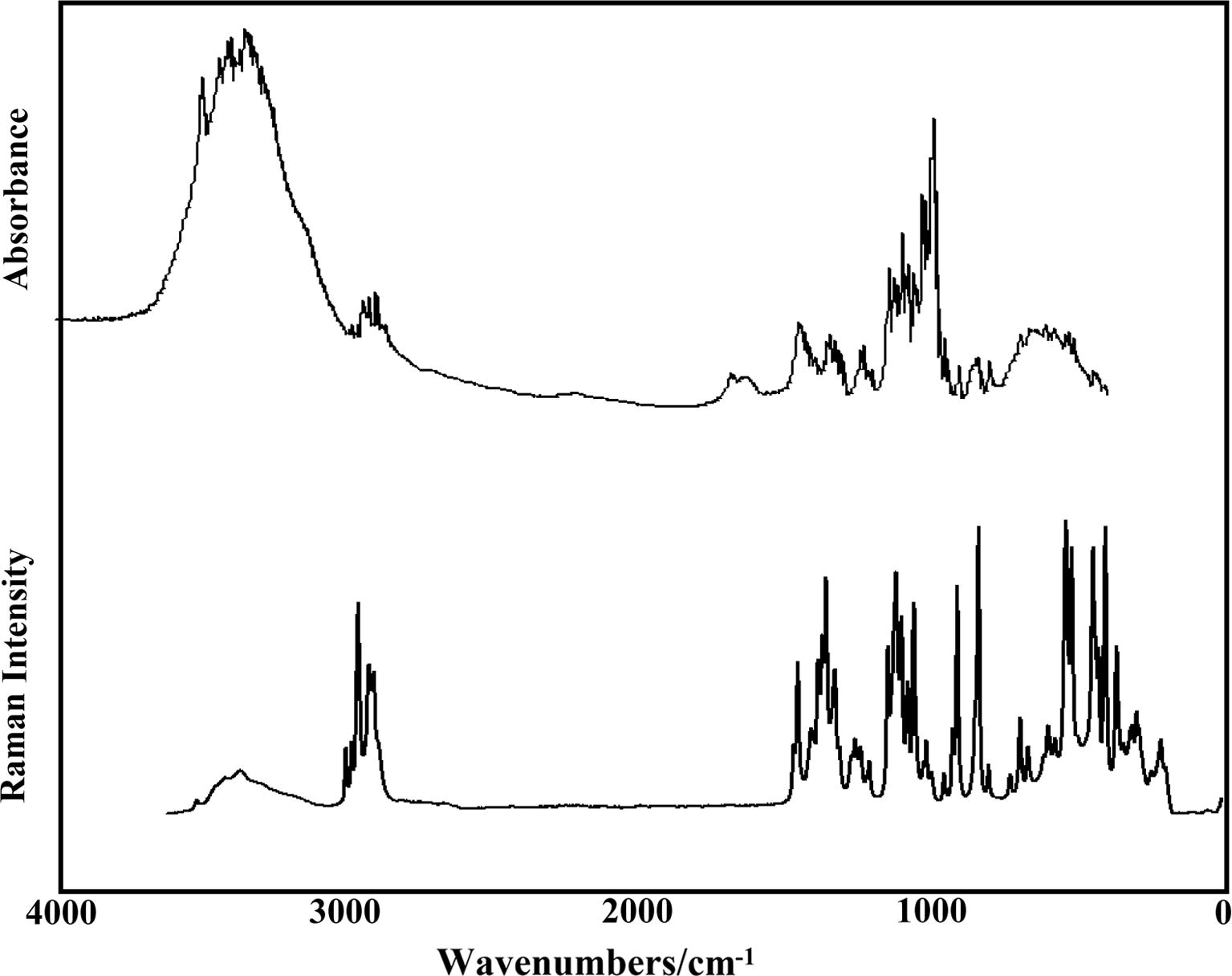
Experimental FTIR spectrum of αα-dihydrated in the solid phase in the 4000–400 cm−1 region compared with their corresponding FT-Raman spectrum in the 4000–10 cm−1 region.
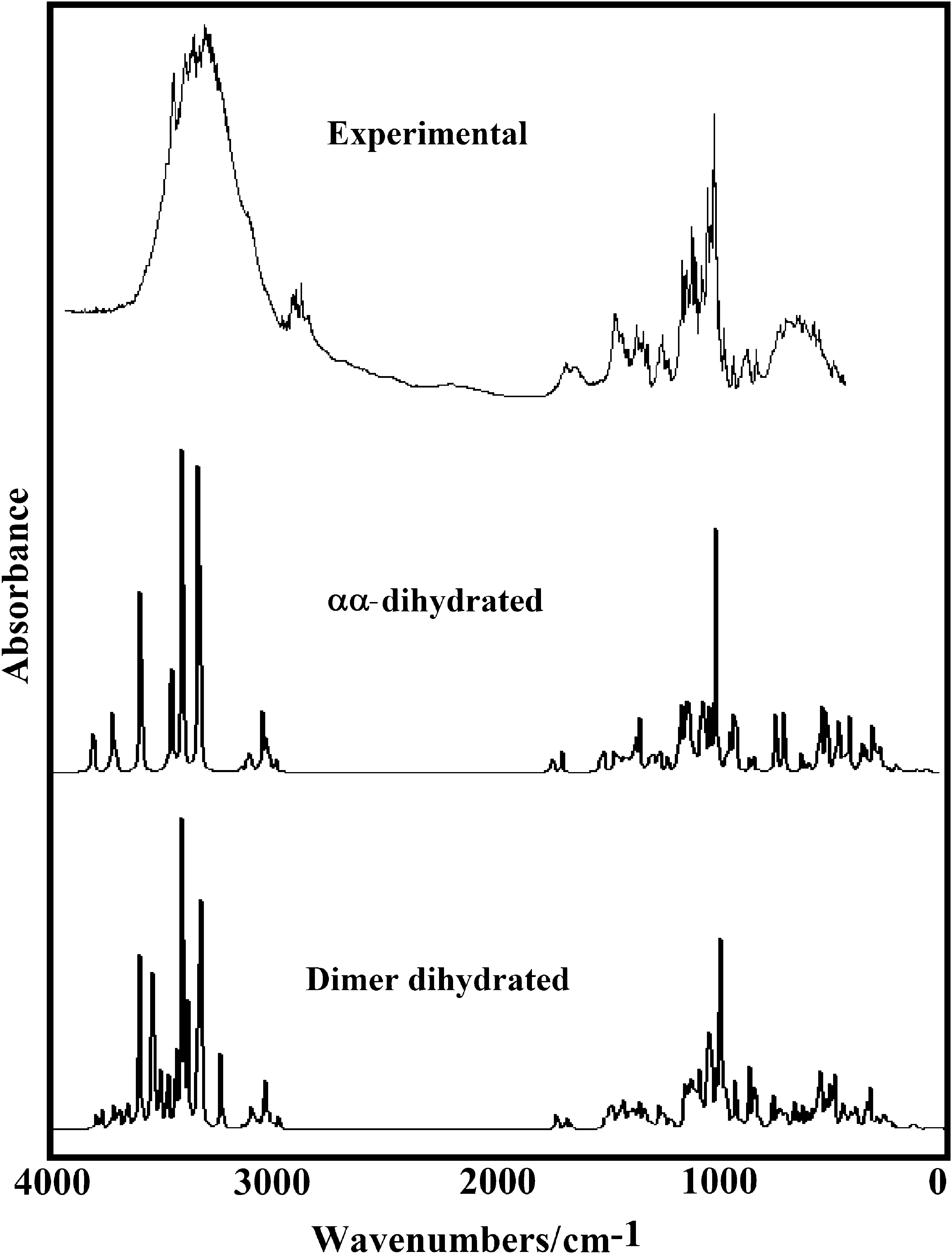
Experimental FTIR spectra of αα-dihydrated in the solid phase compared with the corresponding predicted for this species and their dimer in gas phase at the B3LYP/6-31G* level of theory.
Experimentala
Refc
Anhydrousa
Dihydrateda
IR
Raman
IR
αα-Trehalose
αβ-Trehalose
ββ-Trehalose
αα-Trehalose
Dimer
Solid
Solid
Solid
SQMb
Assig
SQMb
Assig
SQMb
Assig
SQMb
Assig
Calc.
Assig
3500 w
3504 w
3606
ν(O11–H45)
3606
ν(O11–H45)
3609
ν(O11–H45)
3651
νaH2O(W1)
3798
νaH2O
3580
ν(O10–H44)
3582
ν(O10–H44)
3585
ν(O10–H44)
3647
νaH2O(W2)
3604
νsH2O
3574
ν(O8–H42)
3570
ν(O9–H43)
3565
ν(O4–H38)
3569
ν(O7–H41)
3608
ν(O–H)
3570
ν(O9–H43)
3565
ν(O7–H41)
3562
ν(O5–H39)
3569
ν(O8–H42)
3604
νsH2O
3565
ν(O7–H41)
3561
ν(O4–H38)
3561
ν(O9–H43)
3568
ν(O9–H43)
3547
ν(O–H)
3565
ν(O6–H40)
3558
ν(O5–H39)
3554
ν(O7–H41)
3565
ν(O10–H44)
3536
ν(O–H)
3562
ν(O4–H38)
3548
ν(O6–H40)
3550
ν(O6–H40)
3557
ν(O6–H40)
3511
νaH2O
3474 sh
3559
ν(O5–H39)
3540
ν(O8–H42)
3542
ν(O8–H42)
3552
ν(O5–H39)
3479
ν(O–H)
3444 w
3445
νsH2O(W1)
3442
νaH2O
3374 s
3388
νaH2O
3357 s
3350 w,br
3347
ν(O–H)
3335 s
3312
νsH2O(W2)
3321 w
3323 sh
3265
ν(O11–H45)
3269 sh
3280 sh
3193
ν(O4–H38)
3242
ν(O–H)
3153 sh
3137 sh
3049
νsCH2(C23)
2995 w
2997 m
2993
3011
νsCH2(C22)
2996
ν(C12–H24)
2997
ν(C12–H24)
3047
νsCH2(C23)
2995 w
2997 m
2993
2988
νsCH2(C22)
2990
νsCH2(C22)
3008
νsCH2(C23)
3032
νaCH2(C23)
2995 w
2997 m
2993
2995
ν(C12–H24)
2988
ν(C14–H26)
2988
ν(C14–H26)
2989
νsCH2(C22)
3030
νaCH2(C23)
2988 sh
2984
ν(C13–H25)
2983
ν(C13–H25)
2985
ν(C13–H25)
2980
ν(C12–H24)
2984
ν(C–H)
2973 w
2973 m
2973
2969
ν(C14–H26)
2970
ν(C15–H27)
2972
ν(C15–H27)
2982
ν(C–H)
2957
2967
ν(C15–H27)
2968
ν(C15–H27)
2961
ν(C19–H31)
2962
ν(C21–H33)
2951 w
2953 m
2950
2942
νsCH2(C23)
2940
ν(C21–H33)
2942
νaCH2(C23)
2958
ν(C13–H25)
2941 sh
2943
2934
ν(C20–H32)
2930
νaCH2(C22)
2932
νaCH2(C22)
2934 w
2934
2930
ν(C21–H33)
2929
νsCH2(C23)
2928
ν(C21–H33)
2920
ν(C18–H30)
2908 w
2916 m
2908
2914
ν(C20–H32)
2914
ν(C20–H32)
2911
νaCH2(C22)
2900 m
2897
2903
νaCH2(C23)
2881 w
2886 sh
2880
2885
νaCH2(C23)
2883
νaCH2(C23)
2881
νsCH2(C23)
2898
ν(C14–H26)
2881 w
2886 sh
2877
νaCH2(C22)
2896
ν(C20–H32)
2861 sh
2859 sh
2873
ν(C19–H31)
2873
ν(C16–H28)
2873
ν(C16–H28)
2889
ν(C17–H29)
2861 sh
2859 sh
2869
ν(C18–H30)
2872
ν(C19–H31)
2880
ν(C16–H28)
2852 sh
2859
ν(C17–H29)
2859
ν(C17–H29)
2859
ν(C18–H30)
2859
ν(C19–H31)
2837 sh
2857
ν(C16–H28)
2858
ν(C18–H30)
2821
ν(C17–H29)
1687 w
1684 vw
1689
1660
δH2O(W2)
1697
δH2O
1639 w,br
1613 vw
1623
δH2O(W1)
1675
δH2O
1469 w
1481
δCH2 (C23)
1481
δCH2 (C23)
1473
δCH2 (C23)
1475
τO4–H38
τO47–H381516
δCH2
1460
1462
δCH2(C22)
1461
δCH2(C22)
1462
δCH2(C22)
1465
τO4–H38
τO47–H381494
δ(O–H)
1457 m
1452
δCH2(C22)
1455
ρC–H
1451 m
1452 s
1453
wagCH2(C23)
ν(C21–C23)1454
ν(C21–C23)
1457
wagCH2(C23)
1451
δCH2 (C23)
δ(O11–H45)1453
wagCH2
1429 w
1431
wagCH2(C22)
1428
wagCH2(C22)
ν(C20–C22)1433
ρC15–H27
1434
ρC18–H30
ρC16–H28
δ(O6–H40)1434
ρC–H
1429 w
1425
ρC14–H26
ρC15–H271423
ρC15–H27
wagCH2(C22)1429
wagCH2(C22)
1427
ρ’C21–H33
1428
ρC–H
1429 w
1423
δ(O9–H43)
ρC19–H311422
δ(O9–H43)
ρ’C19–H311422
ρC14–H26
1426
ρ’C18–H30
1426
ρC–H
1429 w
1420
ρC17–H29
1419
ρC17–H29
1424
ρC17–H29
ρC15–H271422
wagCH2
1417 sh
1417
wagCH2(C22)
ρC16–H281417
δ(O8–H42)
1419
δ(O8–H42)
1418
δ(O5–H39)
1419
wagCH2
1417 sh
1415
ρC14–H26
ρC16–H281414
ρC15–H27
ρC17–H291415
ρC19–H31
1417
ρC–H
1410 sh
1410 m
1411
ρC15–H27
1408
ρC19–H31
ρ’C16–H281410
wagCH2(C23)
1413
ρC–H
1405 sh
1406
δ(O4–H38)
ρC14–H261404
δ(O7–H41)
1405
wagCH2(C22)
δ(O10–H44)1409
ρC–H
1400 w
1397
ρC17–H29
1398
ρ’C12–H24
ρC13–H251398
ρC–H
1392 sh
1394
ρ’C12–H24
ρC13–H251392
ρ’C12–H24
1392
ρ’C12–H24
ρC13–H251389
τCO4–H38
τO47–H381388
ρC–H
1385 w
1384 s
1386
ρ’C12–H24
ρC16–H281384
ρ’C12–H24
1384
τCO4–H38
τO47–H381387
ρC–H
1385 w
1384 s
1383
ρ’C12–H24
1378
ρC20–H32
ρC13–H251383
ρC20–H32
1381
τCO4–H38
τO47–H381380
ρC–H
1373 sh
1374 sh
1375
δ(O10–H44)
1376
ρC16–H28
δ(O10–H44)1379
δ(O10–H44)
1374
τCO4–H38
τO47–H381375
ρC–H
1371 w
1370 s
1371
ρ’C13–H25
1372
δ(O10–H44)
ρC18–H301376
ρC16–H28
1371
ρC–H
1363 sh
1365
1367
ρ’C13–H25
1368
ρ’C13–H25
1362
ρ’C13–H25
ρC21–H331366
ρC–H
1356 m
1357 vs
1360
ρC12–H24
1361
ρ’C21–H33
1357
ρC12–H24
1359
ρC–H
1349 sh
1350 sh
1355
ρC13–H25
1356
ρC21–H33
ρ’C21–H33
1355
ρC–H
1348 sh
1354
ρC21–H33
1353
ρC21–H33
1347
ρC18–H30
1348
ρC21–H33
ρC13–H251349
ρC–H
1342 vw
1343
ρC18–H30
1345
ρC20–H32
ρC18–H301344
ρC21–H33
1345
ρC14–H26
ρ’C14–H261348
ρC–H
1336 sh
1333
1335
ρC19–H31
1337
ρC19–H31
1338
ρC19–H31
1333
ρC–H
1333 w
1330 sh
1331
ρC13–H25
1332
ρC12–H24
1338
ρC20–H32
1324
ρC–H
1328 s
1323
ρC20–H32
1337
ρC12–H24
1330
τCO4–H38
τO47–H381319
ρC–H
1328 s
1318
ρ’C21–H33
δ(O7–H41)1316
ρ’C21–H33
1305
ρ’C14–H26
1317
ρ’C21–H33
1317
δ(O–H)
1311 w
1314 w
1312
1308
ρ’C20–H32
δ(O8–H42)1303
ρ’C14–H26
1297
ρ’C15–H27
ρ’C19–H311314
ρ’C20–H32
δ(O8–H42)1307
δ(O–H)
1294 sh
1297
ρ’C15–H27
1291
ρ’C15–H27
ρ’C20–H321293
ρ’C20–H32
1287
ρ’C15–H27
1286
δ(O–H)
1275 sh
1280
ρ’C14–H26
1279
ρ’C14–H26
ρ’C20–H321278
δ(O11–H45)
1271
τCO4–H38
τO47–H381275
δ(O–H)
1268 sh
1272 w
1265
δ(O11–H45)
ρCH2(C23)1263
δ(O11–H45)
wagCH2(C23)1273
δ(O11–H45)
δ(O7–H41)1259
ρCH2(C23)
1266
ρCH2
1258 w,br
1258 m
1255
δ(O9–H43)
1254
δ(O8–H42)
1255
δ(O4–H38)
1255
δ(O7–H41)
1258
ρCH2
1241 w
1241 m
1249
δ(O6–H40)
δ(O4–H38)1251
δ(O7–H41)
1248
ρCH2(C23)
1237
ρC–H
1241 w
1241 m
1237
ρCH2(C23)
δ(O11–H45)1239
ρCH2(C23)
1239
ρ’C18–H30
δ(O6–H40)
1234 sh
1235
ρ’C18–H30
1237
ρ’C18–H30
δ(O6–H40)1238
δ(O9–H43)
1237
ρ’C16–H28
1230 sh
1231
ρ’C19–H31
1230
ρC–H
1222 sh
1225
δ(O5–H39)
1224
δ(O5–H39)
1219
δ(O11–H45)
1223
ρCH2(C22)
1225
δ(O–H)
1212 w
1219 sh
1211
ρCH2(C22)
δ(O10–H44)1212
ρCH2(C22)
1221
δ(O–H)
1209 m
1210
ρCH2(C22)
1199
ρ’C16–H28
1209 m
1194
ρ’C16–H28
1194
ρ’C17–H29
δ(O5–H39)1192
ρ’C17–H29
1188 vw
1187
ρ’C16–H28
1186
τCO4–H38
τO47–H38
1175 sh
1182
ρ’C17–H29
ρ’C19–H311180
ρ’C17–H29
1171
βR1(A61)
1150 m
1149 m
1167
βR1(A61)
1131 m
1134 sh
1132
ν(C13–O3)
ν(C13–C15)1131
ν(C13–C15)
1132
ν(C17–O7)
1135
ν(C14–O4)
βR1(A61)1135
βR1(A62)
1128 sh
1128
ν(C14–C16)
1128
ν(C15–C17)
ν(C12–C14)1126
ν(C17–O7)
1129
βR1(A62)
1132
βR1(A62)
1118 vs
1123
ν(C16–O6)
1120
ν(C18–O8)
1122
ν(C13–C15)
1124
ν(C15–C17)
1127
βR1(A62)
1118 vs
1118
ν(C15–C17)
1115
ν(C17–O7)
1115
ν(C18–O8)
ν(C20–C22)1117
τCO4–H38
τO47–H381119
βR1(A62)
1100 m
1101 s
1112
ν(C17–O7)
1110
ν(C14–C16)
1109
ν(C16–O6)
1106
ν(C12–O2)
1110
ν(C–C)
1100 m
1101 s
1110
ν(C12–O2)
1107
ν(C13–O3)
1102
ν(C15–O5)
1101
τO4–H38
δ(O1C13O3)1103
ν(C–O)
1098 sh
1100
ν(C18–O8)
ν(C16–O6)1101
ν(C16–O6)
1096
τR1 (A61)
1098
τR1 (A61)
1094 sh
1093
ν(C19–O9)
1092
ν(C19–O9)
1090
ν(C19–O9)
ν(C17–O7)1093
ν(C–O)
1084 m
1086 sh
1088
ν(C18–O8)
1089
ν(C19–O9)
1087
τCO4–H38
τO47–H381080
ν(C–O)
1079 s
1080
ν(C19–C21)
1081
ν(C17–O7)
ν(C19–C21)
1081
ν(C18–C20)
1073
ν(C–O)
1079 s
1071
ν(C14–O4)
1069
ν(C21–C23)
τwCH2(C23)
δ(C23C21O3)1074
ν(C19–C21)
ν(C21–C23)1071
ν(C–O)
1062 m
1059 vs
1067
ν(C15–O5)
1067
ν(C15–O5)
1064
ν(C14–O4)
ν(C16–O6)
ν(C12–O2)
1068
ν(C–O)
1062 m
1059 vs
1063
ν(C18–C20)
ν(C20–C22)1062
ν(C14–O4)
ν(C12–O2)1057
ν(C18–C20)
1063
τR1 (A62)
1063
ν(C–O)
1055 sh
1056
ν(C22–O10)
ν(C18–C20)1052
ν(C15–O5)
1057
τR1 (A62)
1052 sh
1049
ν(C22–O10)
1047
ν(C22–O10)
1046
ν(C22–O10)
1052
τwCH2
1048 sh
1041
ν(C22–O10)
1040
ν(C22–O10)
ν(C23–O11)1042
ν(C23–O11)
1041
ν(C12–O2)
1047
τwCH2
1032 s
1036
ν(C23–O11)
ν(C22–O10)
1032 s
1032
ν(C23–O11)
1032
ρC15–H27
1031
ν(C13–O3)
1031
ν(C–O)
1026 w
1025
ν(C19–C21)
1029
ν(C23–O11)
ν(C19–C21)
1021
ν(C15–O5)
1028
ν(C–O)
1016 m
1016 w
1009
ν(C12–O1)
1014
ν(C12–O1)
1015
ν(C–O)
998 vs
999 w
998
1004
τwCH2(C23)
1005
ν(C21–O3)
1000
ν(C13–O3)
999
ν(C13–O3)
1009
τwCH2
998 vs
998
993
ν(C20–O2)
τwCH2(C23)998
ν(C–O)
998 vs
998
988
ν(C20–O2)
987
ρC15–H27
987
ν(C21–C23)
991
ν(C20–O2)
ν(C18–O8) ν(C16–C18)987
τO–H
980 sh
975
ν(C16–C18)
978
τO–H
965 sh
973
ν(C17–C19)
974
ν(C17–C19)
973
ν(C16–C18)
974
ν(C17–C19)
960
ν(C–C)
957 w
955 w
956
966
ν(C16–C18)
967
ν(C13–O1)
955
ν(C–C)
948 sh
949
ν(C13–O1)
943
ν(C17–C19)
941
ν(C–C)
926 m
933
ν(C12–O1)
930
ν(C13–O1)
ν(C12–O1)923
ν(C13–C15)
ν(C12–O1)
ν(C12–C14)939
τO–H
917 sh
913
τCO4–H38
τO47–H38918
ν(C–O)
911 w
910 s
908
ν(C12–O1)
ν(C12–C14)905
ν(C13–O1)
901
ν(C19–C21)
911
τCO4–H38
τO47–H38909
ν(C–O)
881 sh
881
δ(C12O1C13)
884
ν(C18–C20)
890
ν(C19–C21)
892
τCO4–H38
δ(O4H38O47)879
τO–H
864 m,br
870
ν(C18–O8)
863
τO11–H45
τO4–H38856
τO–H
852 m
850 sh
859
ν(C18–O8)
ν(C16–C18)851
ρC15–H27
855
τO–H
842 m
839 vs
847
τO–H
816
ν(C21–O3)
820
τwCH2(C23)
βR1(A61)818
βR1(A62)
βR1(A61)
833
τwCH2
804
804 w
804
δ(O1C13O3)
δ(C14C12O1) δ(O1C13C15)812
ν(C21–O3)
τwCH2(C23)
807
δ(O1C13O3)
δ(O1C13C15)810
δ(OCO)
δ(OCC)
796 sh
798
ν(C20–O2)
τwCH2(C22)
798
ν(C21–O3)
791
τwCH2(C22)
806
τwCH2
777
ν(C20–O2)
τwCH2(C22)780
ν(C20–O2)
τwCH2(C22)785
ν(C21–O3)
τwCH2(C23)779
ρH2O
744
ν(C20–O2)
757
ν(C20–O2)
747
wagH2O
732 w,br
731 w
724
δ(O1C13O3)
728
τCO4–H38
τO47–H38728
τO–H
711 sh
709 sh
711
βR1(A61)
βR1(A62)
716
τCO4–H38
τO47–H38719
τO–H
698 w
697 m
690
ν(C14–C16)
689
ν(C15–C17)
699
τCO4–H38
τO47–H38706
βR1(A62)
686
ν(C14–C16)
682
ρH2O
673
δ(O2C12O1)
678
τR2 (A61)
672
ν(C15–C17)
674
δ(O11H45O46)
679
ρH2O
669 w,br
669 w
667
δ(O2C12O1)
668
δ(O1C13O3)
δ(O2C12O1)
δ(C17C19O9)
663
ρH2O
669 w,br
669 w
656
τCO4–H38
τO47–H38661
ρH2O
645 w,br
629 sh
631
ρC15–H27
624
ρC15–H27
643
ρH2O
614 w,br
617 w
616
δ(C14C12O1)
608
τCO4–H38
τO47–H38617
τO–H
601 m
600
δ(O6C16C18) δ(C20C22O10)
601
βR1(A61)
601 m
594
δ(C21C23O11)
593
δ(C21C23O11)
588
δ(C21C23O11)
598
δ(CCO)
583 w
576 w
574
δ(O1C13O3)
δ(O5C15C17)577
τO–H
560 sh
569
δ(O5C15C17)
δ(O1C13O3)563
δ(O1C13O3)
571
τO–H
550 sh
552
δ(O8C18C16)
547
τCO4–H38
τO47–H38552
τO–H
540 vs
542
δ(O1C13O3)
548
τR3(A61)
538 w
532
δ(C17C19O9)
537
δ(C17C19O9)
δ(C21C19O9)
534
τCO4–H38
τO47–H38538
τwH2O
518 w
520 s
524
τR1 (A61)
522
τCO4–H38
δ(O1C13O3)522
τwH2O
501 sh
506 sh
504
ρC15–H27
494
ρC15–H27
496
τR3(A61)
δ(O6C16C18) δ(O8C18C20)504
δ(OCC)
476
τO6–H40
τO4–H38475
δ(C20C22O10)
498
τO–H
473
δ(C20C22O10)
δ(C20C22O10)473
τO6–H40
469
τO10–H44
465
τO–H
459
δ(C21C23O11)
βR2(A62)
τR1 (A62)456
τO5–H39
459
τO–H
445 w
448 s
450
τO4–H38
445
τO5–H39
448
τO5–H39
τO7–H41451
ρH2O(W1)
δ(O4H38O47)443
τO–H
442 sh
440 sh
441
τO5–H39
440
τO8–H42
437
τO8–H42
442
βR2(A62)
430 w
429 sh
433
τO10–H44
433
δ(CCO)
428
βR2(A62)
425
τO5–H39
428
δ(C23C21O3)
430
βR2(A62)
425
τO5–H39
424
τO8–H42
δ(C23C21C19)428
δ(CCO)
423 sh
419
βR2(A62)
423
βR2(A62)
424
βR2(A62)
415
βR2(A62)
414
δ(O4C14C16)
415
δ(O4C14C16)
ν(C12–C14)413
τO6–H40
βR2 (A61)415
βR2 (A61)
410 w
407 vs
409
τO7–H41
407
τCO4–H38
τO47–H38406
βR2 (A61)
400 sh
399
βR3(A61)
405
τCO4–H38
τO47–H38404
βR2 (A61)
396
βR3(A62)
397
βR3(A62)
392
τO10–H44
398
βR3(A62)
400
βR2 (A62)
393 sh
394
τO10–H44
388
τO10–H44
389
τO9–H43
381
τO9–H43
380
τO10–H44
τO4–H38382
τO4–H38
375 sh
380
τO8–H42
τO6–H40377
τO9–H43
378
τO9–H43
τO7–H41381
βR3(A61)
370 s
366
δ(O6C16C14)
369
βR3(A62)
370
δ(C15C17O7)
370
δ(CCC)
365 sh
364
δ(C15C17O7)
362
δ(C15C17O7)
366
βR3(A61)
363
βR3(A61)
360 sh
361
βR3(A61)
358
τCO–H
354 sh
356
τO7–H41
352
τO7–H41
350
τO5–H39
354
τCO4–H38
352
τwH2O
342 w
340
δ(O5C15C17)
340
τwH2O
334
τO10–H44
333
δ(O4C14C16)
τO10–H44334
τCO4–H38
336
τCO–H
317 m
329
τO10–H44
322
τCO4–H38
τO47–H38320
τCO–H
307
δ(O8C18C20)
308
τO11–H45
ρH2O(W1)313
δ(OCC)
300 m
304
δ(C21C19O9)
306
δ(O6C16C14)
306
δ(O8C18C20)
307
τO11–H45
τCO4–H38307
ρH2O
293 sh
298
δ(O8C18C20) δ(O2C20C22)
293
δ(C21C19O9) δ(C23C21O3)
288
wagH2O(W1)
ν(O46–H45)297
δ(OCC)
281 sh
282
δ(C21C19O9)
284
δ(O4C14C12)
284
δ(CCO)
276
δ(O8C18C16)
276
wagH2O(W1)
278
wagH2O
269 sh
271
δ(O6C16C14)
271
τO47–H38
τCO4–H38273
δ(OCC)
258
δ(O5C15C13)
258
δ(OCC)
256 sh
254
δ(O5C15C17)
253
δ(O5C15C17)
255
δ(O5C15C17)
δ(O5C15C13)250
δ(OCC)
249 w
247
δ(O5C15C17) δ(O4C14C16)
250
δ(O6C16C18)
250
δ(O6C16C18)
248
τO46–H45
247
δ(OCC)
240
δ(C19C17O7)
238
δ(C19C17O7)
241
δ(O5C15C13)
δ(C19C17O7)237
τO47–H38
τCO4–H38237
δ(OCC)
235 sh
231
τR1 (A62)
235
τO11–H45
230
τR1 (A61)
223 sh
232
δ(O5C15C13)
τO11–H45229
ρC15–H27
230
τR1 (A61) δ(C19C17O7)
229
τR1 (A61)
216 m
216
δ(O4C14C12)
215
δ(O4C14C12)
214
τO46–H45
τO11–H45221
δ(CCC)
209 sh
211
δ(O4C14C12)
212
τR2 (A61) δ(C12O1C13)
206
τR2 (A61)
202 w
194
δ(O2C20C22)
193
δ(O5C15C13)
195
δ(O2C20C22)
197
ν(O–H)
189 sh
184
βR2 (A61)
180
τO11–H45
δ(C23C21O3)187
τO11–H45
182
ν(O47–H38)
185
ν(O–H)
178
τO11–H45
170
δ(C23C21C19)
178
τO4–H38
183
ν(O–H)
160 vw
161
δ(C18C20C22)
βR2 (A61)163
τO11–H45
164
ν(O–H)
160 vw
157
δ(C18C20C22)
151
δ(C18C20C22)
151
δ(C23C21C19)
157
ν(O–H)
132
τR3(A61)
135
τR3(A61)
τR1 (A61)144
τwC12–O1
τwC13–O1138
τR3(A61)
126
τR2 (A62)
122
τR2 (A62)
τR1 (A62)126
τR2 (A62)
127
τCO4–H38
ττO47–H38122
τR3(A62)
118
τwC20–C22
τR3(A61)
113
τR3 (A62)
118
τR2 (A62)
114
τR3(A62)
110 vw,br
111
τR3 (A62)
110
τR3 (A62)
100
τwC20–C22
110
τwC21–C23
τR3 (A62)112
τR3(A62)
97
τwC21–C23
96
τwC20–C22
97
τwC–C
80.1
86
τwC21–C23
86
τwC21–C23
88
τwC21–C23
90
τO47–H38
τCO4–H3890
τwC–C
72.4
68
τR3(A61)
τR2 (A61)74
τCO4–H38τO47–H38
74
τwC–C
61
τR2 (A61)
64
τR3(A61)
τR3 (A62)67
τR2 (A61)
61
τOC4–H38
τO47–H3865
τOC–H
54 vw,br
56.6
57
δ(C23C21C19)
58
τR2 (A61)
59
τOC–H
49.3
52
τO47–H38
τCO4–H3855
τOC–H
47.4
44
τO4–H38
47
τO–H
43.1
43
τO47–H38
44
τOC–H
38.1
39
τR2 (A61)
τR2 (A62)40
δ(C12O1C13) δ(C14C12O1) δ(O1C13C15)
41
δ(C12O1C13)
δ(O1C13C15)
38
τwC–O
29
τwC12–O1
30
τCO4–H38
τO47–H3832
δ(OCC)
23
τwC12–O1
28
τCO4–H38
τO47–H3827
δ(OCC)
18 sh
19
τwC13–O1
22
τwC13–O1
23
τwC13–O1
21
τCO47–H38
τO4–H3823
τwC–O
13 sh
16
τwC12–O1
15
τwC–O
Later, the assignments for some groups are presented below.
4.6.1 Bands assignments
4.6.1.1 O–H modes
For the four trehalose species, the B3LYP/6-31G∗ calculations predicted these stretching modes as nearly pure modes, hence, due to the form of the bands and to their intensities these vibration modes for the anhydrous species can be easily assigned to the broad IR and Raman bands located in the 3500–3160 cm−1 region, as observed in Table 4. Besides, in the dihydrated species in that region are also expected the four antisymmetric and symmetric OH stretching modes corresponding to the two water molecules. Thus, the IR bands at 3500, 3444 and 3335 cm−1 are associated with those vibration modes for the hydrated species while, for the dimer, the band at 3374 cm−1 and the shoulder at 3269 cm−1 in the same spectrum are also related to those stretching modes. Here, the different positions predicted for these vibration modes in the hydrated species could probably reveal forming the H bonds, as supported by the NBO and AIM calculations (Tables, S5, S6 and S7) and, as also was observed in the maltose species (Iramain et al., 2016). The intra-molecular O–H stretching modes are particularly sensitive to environmental modifications, as reported by Branca et al., 1999 from viscosity and Raman scattering studies and, as will be seen later, the force constants related to this stretching mode is lower in the dihydrated trehalose species, in reference to maltose and lactose.
Here, the OH deformation modes of the water molecules for the hydrated and the dimer species are clearly assigned to the IR bands at 1687 and 1639 cm−1. The OH deformation modes for all the species are predicted between 1419 and 1194 while in the anhydrous species of maltose those modes appear in the 1421–1171 cm−1 region (Iramain et al., 2016), therefore, these modes can be associated with observed bands in that region. In general, the calculations predict the out-of-plane deformation modes for the dihydrated species strongly mixed with other vibration modes, such as deformations, stretching and rocking modes of the OH, CH2, C–O and C–H groups, as shown in Table 4. Note that the out-of-plane deformation modes in the anhydrous forms appear at lower wavenumbers (476 up to 189 cm−1) while in the dihydrated species these modes are also predicted in the higher wavenumbers region (from 1389 up to 163 cm−1). In the anhydrous forms of maltose these modes are assigned between 537 and 182 cm−1 while for the monohydrated species the bands between 884 and 107 cm−1 are associated to those modes. The comparisons between the experimental IR and Raman spectra with the corresponding theoretical in the different regions (Figs. S4–S7) support the presence of more than one molecule in the primitive cell due to the increase in the numbers and intensities of the bands in both spectra for this species in relation to the observed in the dihydrated species.
4.6.1.2 CH modes
In sucrose, lactose and maltose these stretching modes were assigned to the bands observed in the 3094/2830, 3086/2881 and 2982/2845 cm−1 regions (Brizuela et al., 2012a, 2014; Márquez et al., 2015a,b; Iramain et al., 2016), respectively while in the trehalose species these modes are predicted between 2997 and 2821 cm−1. The Raman band of medium intensity at 2997 cm−1 is associated to the symmetric modes corresponding to the dihydrated and dimeric species. In the lactose species, the rocking modes are assigned between 1469 and 1212 cm−1 while in maltose are assigned to the bands between 1457 and 1063 cm−1. In the trehalose species, these modes are predicted between 1434 and 1180 cm−1.
4.6.1.3 CH2 modes
In maltose, the antisymmetric and symmetric stretching modes are assigned respectively to the pairs of bands at 3032/2970 and 2963/2862 cm−1 (Iramain et al., 2016) while in the four species of trehalose these modes are predicted at 3153/2881 cm−1. Obviously, the symmetric modes are observed with higher intensities in the Raman spectrum and, for this reason, they are assigned to the Raman band at 2997 cm−1. In lactose, the deformation or scissoring modes are assigned at 1457/1367 cm−1 (Márquez et al., 2015a,b), in the maltose species these modes are assigned at 1499/1455 cm−1 (Iramain et al., 2016) while in trehalose species they are assigned at 1469/1451 cm−1. The wagging, rocking and twisting modes are predicted by calculations between 1451/1405 and in some cases coupled at 1268 cm−1, 1265/1211 and 1069/777 cm−1, respectively as indicated in Table 4. This way, they are assigned accordingly.
4.6.1.4 Skeletal modes
The C–O stretching modes belonging to the two glucopyranose R1 and R2 rings and to the glycosidic C12–O1–C13 bonds are predicted by calculations in different regions and mixed with other vibration modes, as can be seen in Table 4. Thus, these modes for the anhydrous and dihydrated species can be observed from 1132 to 744 cm−1. Note that the C12–O2 and C13–O3 stretching modes are predicted at higher wavenumbers in the four species than the C12–O1 and C13–O1 stretching modes, as expected because these stretching modes are related to the glycosidic C12–O1–C13 bonds which present different values for the anhydrous and dihydrated species, as shown Table 2. In the maltose species, the C–C stretching modes are assigned to the bands between 1171 and 668 cm−1, as predicted by calculations, in this species, these modes are predicted and assigned in the 1131–672 cm−1 region, as can be seen in Table 4. The remaining vibration modes for the anhydrous, dihydrated and dimeric species are indicated in Table 4 and they correspond to the deformation and torsions of both glucopyranose rings and to the OCO, CCC, CCO and OCC deformation modes which are predicted in the lower wavenumbers region.
4.7 Force field
For the three anhydrous species of trehalose and their dihydrated form the force constants were computed using the SQMFF methodology (Rauhut and Pulay, 1995a) with the Molvib program (Sundius, 2002). The results in the gas phase at the B3LYP/6-31G∗ level of theory are presented in Table 5 together with those values reported for the maltose and lactose species (Márquez et al., 2015a,b; Iramain et al., 2016) at the same level of theory. Comparing first the values for the anhydrous species, we observed that the three species show practically the same values and there are no significant changes among them, however, when these values are compared with those corresponding to the dihydrated species all the values slightly increase for this species in relation to the anhydrous species with exception of the f(νO–H) force constant whose value decrease for the dihydrated species. This observation is probably related with the higher quantity of intra-molecular interactions observed for this species using NBO and AIM studies (Tables S5–S7). On the other hand, the anhydrous species of maltose and lactose show higher values in the f(δC–O–C) force constants related to the glycosidic bonds, between 2.78 and 1.88 mdyn Å−1 as compared with those corresponding to trehalose (1.19–1.14 mdyn Å−1) while the hydrated species of trehalose present a force constant value (2.31 mdyn Å−1) between those corresponding to maltose (2.78–2.27 mdyn Å−1) and lactose (1.92 mdyn Å−1). Here, the f(δC–O–C) force constants related to the glycosidic bonds evidence clearly the different characteristics in the three sugars: maltose > trehalose > lactose but these values are not justified because maltose and lactose are reducing sugars while trehalose is a non-reducing sugar. Hence, the behavior electrophilic of trehalose could also be related to their f(νO–H)H2O force constant because the value for the dihydrated trehalose is 6.88 mdyn Å−1 while for monohydrated lactose is 6.96 mdyn Å−1 and for monohydrated maltose 7.25–7.05 mdyn Å−1. Here, the low f(νO–H)H2O force constant value for trehalose are strongly related to the lowest BO values observed in particular for the O–H bonds corresponding to the water molecules. This way, the following relation is observed in the f(νO–H)H2O force constants: trehalose < lactose < maltose showing a low f(νO–H)H2O force constant value for trehalose which could justify: (i) the very fragile character from the trehalose–water system to the temperature and concentration changes, as experimentally was reported by Branca et al. (1999) from viscosity and Raman scattering studies, (ii) why the presence of the disaccharides inhibits the protein dynamical transition (Magazù et al., 1998) from studies of the influence of hydrogen bond connectivity on transport properties for the same trehalose–water system, (iii) the non-reducing property of this sugar and, (iv) the ability of trehalose to form H bonds and, this way, to stabilize the membranes, as reported by Crowe and Crowe (1984). Units are mdyn Å−1 for stretching and mdyn Å rad−2 for angle deformations.
B3LYP/6-31G*
Force constant
Trehalosea
Maltoseb
Lactosec
Anhydrous
Dihydrated
Anhydrous
Monohydrated
Anhydrous
Monohydrated
αα-
αβ-
ββ-
αα-
α-
β-
α-
β-
α-
β-
α-
f(νO–H)H2O
6.88
7.25
7.05
6.96
f(νO–H)
7.14
7.16
7.11
6.94
7.09
7.04
7.00
6.96
7.12
7.10
7.00
f(νCH2)
4.73
4.75
4.75
4.81
4.81
4.79
4.82
4.75
4.73
4.73
4.82
f(νC–H)
4.71
4.71
4.73
4.71
4.70
4.73
4.74
4.78
4.64
4.59
4.65
f(νC–O)C
4.42
4.41
4.38
4.54
4.73
4.78
4.45
4.75
4.67
4.68
4.65
f(νC–O)H
5.04
5.03
5.05
5.07
5.02
4.94
5.10
4.96
5.02
5.11
3.82
f(νC–C)
4.00
4.01
3.97
4.14
3.88
3.86
3.90
3.96
3.91
3.91
3.91
f(δC–O–C)
1.18
1.14
1.19
2.31
2.50
2.64
2.27
2.78
1.88
1.89
1.92
f(δC–O–H)
0.80
0.80
0.80
1.01
0.76
0.78
0.82
0.95
0.74
0.74
0.83
f(δH–C–H)
0.81
0.81
0.81
0.82
0.85
0.80
0.80
0.90
0.82
0.82
0.81
5 Conclusions
In this work, the dihydrated trehalose form was completely characterized using the FTIR and Raman spectroscopy combined with DFT calculations. The structures of three anhydrous species of trehalose, the dihydrated form and their dimer were theoretically determined in gas phase and aqueous solution using the hybrid B3LYP/6-31G∗ method. In aqueous solution, the calculations were computed with the PCM and SD models in order to predict the solvation energies. Both NBO and AIM studies reveal high stability for the dihydrated species in solution that could probably explain the formation of several intermolecular hydrogen bonds where are involved every hydroxyl group in the system generating, this way, higher ‘‘rigidity’’ in trehalose and a lower volume expansion and solvation energy for trehalose in solution, in relation to maltose and sucrose. Besides, the low f(νO–H)H2O force constant value for trehalose could justify the very fragile character from the trehalose–water system to the temperature and concentration changes among other properties. In addition, the studies using the frontier orbitals show the high gap values observed for the anhydrous and dihydrated species of trehalose which probably justify why it is a nonreducing disaccharide of glucose while the similar lower gap values predicted for maltose and lactose probably justify that these carbohydrates are reducing sugars. Finally, the complete vibrational assignments for the anhydrous and dihydrated form of trehalose were performed using the scaled mechanical force fields.
Acknowledgements
This work was subsidized with grants from CIUNT Project 26/D207 (Consejo de Investigaciones, Universidad Nacional de Tucumán). The authors thank Prof. Tom Sundius for his permission to use MOLVIB.
References
- Structural and vibrational properties of trehalose: a density functional study. J. Phys. Chem. B. 2000;104:6313-6317.
- [Google Scholar]
- Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys.. 1993;98:5648-5652.
- [Google Scholar]
- IR and Raman spectroscopic studies of the interaction of Trehalose with Hen Egg white Lysozyme. Biopolymers. 1994;34:957-961.
- [Google Scholar]
- AIM2000; a program to analyze and visualize atoms in molecules. Comput. Chem.. 2001;22:545-549.
- [Google Scholar]
- Water interaction with a, a-trehalose: molecular dynamics simulation, J. Chem. Soc. Faraday Trans.. 1998;94:2755-2762.
- [Google Scholar]
- The fragile character and structure-breaker role of α, α-trehalose: viscosity and Raman scattering findings. J. Phys. Condens. Matter. 1999;11:3823-3832.
- [Google Scholar]
- Vibrational and relaxational contributions in disaccharide/H2O glass formers. Phys Rev B. 2001;64:224204-224208.
- [Google Scholar]
- Destructuring effect of trehalose on the tetrahedral network of water: a Raman and neutron diffraction comparison. Phys. A. 2002;304:314-318.
- [Google Scholar]
- A complete characterization of the vibrational spectra of sucrose. Carbohydr. Res.. 2012;361:212-218.
- [Google Scholar]
- Experimental and theoretical vibrational investigation on the saccharinate ion in aqueous solution. Spectrochim. Acta Part A. 2012;95:399-406.
- [Google Scholar]
- A complete assignment of the vibrational spectra of sucrose in aqueous medium based on the SQM methodology and SCRF calculations. Carbohyd. Res.. 2014;388:112-124.
- [Google Scholar]
- The crystal structure of,-trehalose dihydrate from three independent X-ray determinations. Acta Cryst.. 1972;B28:3145-3158.
- [Google Scholar]
- Electromagnetic fields effects on the secondary structure of lysozyme and bioprotective effectiveness of Trehalose. Adv. Phys. Chem. 2012:6. Article ID 970369
- [Google Scholar]
- Quantum mechanical modeling of fluoromethylated-pyrrol derivatives a study on their reactivities, structures and vibrational propertiesJ. Phys. Chem. Biophys.. 2014;4(1):2-9.
- [Google Scholar]
- Vibrational spectroscopy and chemometrics to characterize and quantitate trehalose crystallization. Anal. Biochem.. 2010;399:48-57.
- [Google Scholar]
- Crowe, J.H. Crowe, L.M., 1984. Preservation of Membranes in Anhydrobiotic Organisms: The Role of Trehalose, 702–703.
- Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, Ö., Foresman, J.B., Ortiz, J.V., Cioslowski, J., Fox, D.J., 2009. Gaussian Inc, Wallingford CT.
- NBO3.1; Theoretical Chemistry Institute. Madison, WI: University of Wisconsin; 1996.
- Impact of freeze-drying on ionization of sulfonephthalein probe molecules in trehalose-citrate systems. Pharm. Sci.. 2006;95(7):1498-1509.
- [Google Scholar]
- FTIR, HATR and FT-Raman studies on the anhydrous and monohydrate species of maltose in aqueous solution. Carbohyd. Res.. 2016;428:41-56.
- [Google Scholar]
- Preparation and properties of polyaniline in the presence of Trehalose. Soft Nanosci. Lett.. 2011;1:71-75.
- [Google Scholar]
- Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev.. 1988;41:785-789.
- [Google Scholar]
- α,α-Trehalose-water solutions. II Influence of hydrogen bond connectivity on transport properties. J. Phys. Chem. B. 1998;102:2060-2063.
- [Google Scholar]
- Experimental simulation of macromolecules in trehalose aqueous solutions: a photon correlation spectroscopy study. J. Chem. Phys.. 1999;111(19):9086-9092.
- [Google Scholar]
- Study of the dynamical properties of water in disaccharide Solutions. Eur. Biophys. J.. 2007;36:163-171.
- [Google Scholar]
- FTIR spectroscopy studies on the bioprotective effectiveness of trehalose on human hemoglobin aqueous solutions under 50 Hz electromagnetic field exposure. J. Phys. Chem. B. 2010;114:12144-12149.
- [Google Scholar]
- Mean square displacements from elastic incoherent neutron scattering evaluated by spectrometers working with different energy resolution on dry and hydrated (H2O and D2O) Lysozyme. J. Phys. Chem. B. 2010;114:9268-9274.
- [Google Scholar]
- Thermal behaviour of hydrated lysozyme in the presence of sucrose and trehalose by EINS. J. Non-Cryst. Solids. 2011;357:664-670.
- [Google Scholar]
- Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B. 2009;113:6378-6396.
- [Google Scholar]
- A structural and vibrational investigation on the antiviral deoxyribonucleoside thymidine agent in gas and aqueous solution phases. Int. J. Quantum Chem.. 2014;114(3):209-221.
- [Google Scholar]
- Spectroscopic and structural studies on lactose species in aqueous solution combining the HATR and Raman spectra with SCRF calculations. Carbohyd. Res.. 2015;407:34-41.
- [Google Scholar]
- A comparative study on the structural and vibrational properties of two potential antimicrobial and anticancer cyanopyridine derivatives OJSTA. 2015;4:1-19.
- Electrostatic interaction of a solute with a continuum. J. Chem. Phys.. 1998;55:1117.
- [Google Scholar]
- Nielsen, A.B., Holder A.J., 2008. Gauss View 5.0, User’s Reference, GAUSSIAN Inc., Pittsburgh, PA.
- The Role of Trehalose for the Stabilization of Proteins. J. Phys. Chem. B. 2016;120:4723-4731.
- [Google Scholar]
- Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc.. 1983;105:7512-7516.
- [Google Scholar]
- Transferable scaling factors for density functional derived vibrational force fields. J. Phys. Chem.. 1995;99:3093-3099.
- [Google Scholar]
- Transferable scaling factors for density functional derived vibrational force fields. J. Phys. Chem.. 1995;99:14572.
- [Google Scholar]
- Structural and spectroscopic studies of two 1,3-benzothiazole tautomers with potential antimicrobial activity in different media. Prediction of their reactivities Comput. Theor. Chem.. 2015;1061:89-99.
- [Google Scholar]
- Structural, topological and vibrational properties of an isothiazole derivatives series with antiviral activities. J. Mol. Struct.. 2015;1100:279-289.
- [Google Scholar]
- Glass transition and time-dependent crystallization behavior of dehydration bioprotectant sugars. Carbohyd. Res.. 2010;345:303-308.
- [Google Scholar]
- Trehalose-incorporated organic–inorganic hybrid nanocomposites produced by thiol-ene photopolymerization. Polym. J.. 2016;48:111-116.
- [Google Scholar]
- Illuminating trehalose mediated inhibition of protein aggregation through lysozyme-silver nanoparticle interaction. Soft Matter.. 2015;11(37):7241-7249.
- [Google Scholar]
- The crystal and molecular structure of trehalose dihydrate. Acta Cryst.. 1972;B28:3258-3263.
- [Google Scholar]
- Low-frequency vibrations of crystalline a, a-trehalose dehydrate. Chem. Phys. Lett.. 2006;429:371-377.
- [Google Scholar]
- Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent. Chem. Rev.. 1994;94:2027.
- [Google Scholar]
- MOLDRAW Program. Torino, Italy: University of Torino, Dipartimento Chimica IFM; 1998.
- Neutron scattering studies on dUTPase complex in the presence of bioprotectant systems. Chem. Phys.. 2008;345:250-258.
- [Google Scholar]
- Fibroblast cell proliferation on photo-cured trehalose cinnamoyl ester thin films. J. Bioact. Compatible Polym.. 2015;30(1):87-98.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jksus.2017.01.009.
Appendix A
Supplementary data







