Translate this page into:
Structural analysis of green synthesized Fe doped TiO2 nanocomposites and their application to wastewater remediation
⁎Corresponding author at: Department of Physics, Hindu College, Moradabad, PIN-244001, Mahatma Jyotiba Phule Rohilkhand University, Bareilly, Uttar Pradesh, India. akumarmbd@hinducollege.edu.in (Anil Kumar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Pure and Fe-doped TiO2 nanoparticles were synthesized through green synthesis route for wastewater remediation application. Pure and Fe-doped TiO2 nanoparticles were further characterised through various techniques like XRD, SEM, and EDS. The XRD patterns indicate pure anatase phase with space group I 41/a m d through the Rietveld refinement. The average crystalline size of Fe-doped TiO2 nanoparticles decreases with the increment in the concentration of doping. The high agglomeration of pure and Fe-doped TiO2 nanoparticles is observed through SEM. EDS analysis conforms the presence of Fe, Ti, and O elements in the nanoparticles. The photo catalytic activities of pure and Fe-doped TiO2 under ultraviolet (UV) light with different concentration were investigated. The dissolved oxygen in the water increases with the increment of Fe doping and time of exposer of UV light.
Keywords
Green synthesis
Nanocomposites
Photocatalysis
Remediation
TiO2
Wastewater
- DO
-
Dissolved oxygen
- EDAX
-
Energy dispersive X-ray Analysis
- EDS
-
Energy-dispersive X-ray spectroscopy
- FESEM
-
Field effective scanning electron microscope
- FWHM
-
Full width at half maximum
- INNH
-
Iron nitrate nonahydrate (INNH)
- JCPDS
-
Joint committee on powder diffraction standards
- TEM
-
Transmission electron microscope
- TTIP
-
Titanium isopropoxide (TTIP)
- UV
-
Ultra violet
- XRD
-
X-ray diffraction
Abbreviations
1 Introduction
Nanomaterials are interesting because they exhibit different physical and chemical properties as compared with their macro-scale counterparts. The physical properties of a bulk material do not depend on size, but in case of nanomaterials, size dependent properties are often observed. The green synthesized nanomaterials are identified as environment friendly multifunctional materials having their higher water solubility, low toxicity and biodegradability (Nabi et al., 2018; Patil et al., 2016; Hoffmann et al., 1995). Therefore, green synthesis of nanomaterials is considered as an important method of synthesis which is commonly being used in laboratory and industry (Singh et al., 2018). Furthermore, green synthesized nanomaterials are frequently applied to human contact related areas and development of such synthesis processes is a growing need of time because harsh toxic chemicals are not used there. That is why, the green synthesis of nanomaterials is a better alternative method comparable to other synthesis methods (Hussain et al., 2016). In chemical methods generally several chemical species or molecules are used and they increase the particle reactiveness and toxicity and might decay the human health and environment. Among the metal oxide nanoparticles, TiO2 is interesting because it has remarkable photocatalytic and distinctive semiconducting properties (Huston et al., 2021). It is also non-toxic, biocompatible, and chemically stable material. Due to having strong oxidizing properties and has large surface area, Nano TiO2 shows high photo catalytic activities (Cong et al., 2007). The main applications of TiO2 are disinfection and purification of wastewater, self-cleaning coating for buildings and the production of hydrogen called green currency of energy by splitting water molecules (Kabra et al., 2004; Linsebigler et al., 1995). In green synthesis method the naturally occurring molecules are utilized to form the nanoparticles with distinctive properties. In green synthesis principles the use of unsafe reagents is minimized and the efficiency of chemical processes is maximized. The green synthesized nanoparticles induce a less determining effect on environment and human health compared with the other synthesis chemical methods. In green synthesis, the naturally occurring materials like plants extracts, plants and microorganism are used as a reducing and stabilizing agent (Friedmann et al., 2010).
The development of novel green synthetic methods for Fe-doped TiO2 nanocomposites has gained significant attention due to their potential for enhanced structural, electrical, and photo catalytic properties (Suárez et al., 2011). Fe doping in TiO2 enhances its optical and magnetic properties, which are crucial for various applications in photocatalysis, solar cells, and magnetic storage. The addition of Fe increases the bandgap energy, leading to improved photocatalytic activity and enhanced optical properties (Shalaby et al., 2024) The choice of Fe as a preferred dopant in green synthesized TiO2 is driven by its environmental friendliness, enhanced optical and magnetic properties, structural stability, cost-effectiveness, and biocompatibility. These advantages make Fe a suitable choice for various applications where TiO2 is used. These composites have shown promising results in various applications such as water purification and air pollution control, and they offer improved performance compared to pure TiO2, making them promising materials for various applications (Barkhade and Banerjee, 2019). Some researchers have reported that Fe-doped TiO2 composites exhibit better activity than pure TiO2, leading to the exploration of various synthesis methods (Wang et al., 2011). XRD and FESEM-EDS were performed for characterizing the produced samples. The photo catalytic activity of TiO2 and Fe-doped TiO2 under UV light was investigated (Mancuso et al., 2021). The doping of Fe is selected because radius of Fe3+ (0.69 Å) is like the radius of that of Ti4+ (0.75 Å). Here Iron traps the electron–hole pairs so that electron hole recombination is decreased. Fe3+ doped TiO2 nano crystallites prevent the accumulation of the particles because of its high surface area and promote photocatalytic activity with band gap of 2.6 eV (Wen et al., 2011).
Water is the most vital resource for all living things for their survival. Around 3 % of total water on earth is available for use, and most of this available water is frozen in glaciers and polar ice caps which make it unavailable (Oki and Kanae, 2006). Nowadays, the shortage of clean water is one of the greatest environmental problems before the entire human community. Because of the widespread industrialization and anthropogenic causes, the organic and inorganic contaminants, dyes, heavy metals, spilled oil, pharmaceutical wastes and other complex chemicals are found in the water (Johnson et al., 2017). These accumulated contaminants do serious damage to the entire environment and humans. The widespread industrialization and urbanization led to an increase in organic pollutants into surface water, groundwater, and soil. Water pollution is the contamination of water with harmful substances, including chemicals or microorganisms (Li et al., 2021). Water pollution is a serious problem nowadays which directly affects the human health and environment. Unsafe water is a cause of people death every year greater than that of any war and other forms of violence combined. Regarding the suitable and complete removal of organic pollutants, TiO2 has high photo catalytic activity among other semiconductors. Because of its high oxidizing power, cost-effectiveness, environmental friendliness, enhanced chemical stability, photo stability, and non-toxicity, TiO2 is a good candidate for photo catalytic and antibacterial applications (Kazuya and Akira, 2012; Daghrir et al., 2013).
Nano-photocatalysis is a globally and sustainable tool for treatment of wastewater and pretreatment of drinking water (Kumari et al., 2023). This method for wastewater treatment by nano-photocatalysis is very effective, non-selective and highly efficient. Nano-photocatalysis does the mineralization of complex contaminants which are capable of degrading and eliminating the complex and harmful pollutants (Gebre and Sendeku, 2019; Arularasu, 2019). Therefore, the iron doped TiO2 nanocomposites has attracted a lot of attention of researchers in field of different environmental remediation techniques specially for waste-water treatment. Dissolved oxygen is defined as the free and non-compound oxygen present in any liquid. It influences the organisms living inside water; therefore, it is a very important parameter applied for assessing the water quality. The quantity of dissolved oxygen in river water is very much greater than that of stagnant water, therefore, the aquatic life suffers in stagnant water. Bacteria presented in water consume oxygen for their organic matter decays (Ahmad et al., 2016; Mahlambi et al., 2015).
The wastewater includes the discharge of industrial wastes, organic matter of sewage, and runoff from agricultural lots. These discharges affect the quantity of dissolved oxygen in water bodies that is very important for the survival of aerobic organisms. Consequently, there discharge will be a cause to decrease the DO content. The measurement of the DO content is very important and describes about the water system to be predominantly aerobic or anaerobic which predicts the existence and survival of aquatic organisms (Helman et al., 2012; Kaenel et al., 2000). It predicts the working of aerobic biological processes to transform the biodegradable organic contaminants discharged in the water. When organic discharge takes place in the water, the DO decreases rapidly because the aerobic microorganisms consume oxygen produced in the process of metabolic degradation of organic matter. The DO content depends on water temperature, atmospheric pressure, dissolved salts, suspended matter, reducing compounds and living organisms (Reichert et al., 2009). The present work provides a comprehensive overview of wastewater remediation through nano-photocatalytic degradation.
2 Photocatalytic activity
UV radiation enhances the photo degradation which is helpful in breaking of the polymers and producing the radicals (Yousif and Haddad, 2009). Nano-sized titanium dioxide is an environmentally friendly photo semiconducting material. In the ground state of TiO2 both the electrons and holes lie in the valence band. The TiO2 nanoparticles are n-type semiconductors. When light is made incident over a semiconducting surface, electrons (
are transferred to the Conduction Band from the Valence Band by absorbing light of the wavelength and causes creation of holes (
) in the valence band (Fig. 1). Electrons reduce and holes oxidize the reactants absorbed by the semiconductor. The photo induced electrons and holes exhibit high reduction and oxidation potential in comparison of the hydrogen and ozone. That is why, the photo induced electron-hole pairs work as a strong redox device. In valence band, the holes produce hydroxyl radicals by the oxidation of H2O molecules adsorbed on the TiO2 surface and in the conduction band, the electrons perform reduction of O2 molecules present in the absorbed air and form peroxyl radicals. The hydroxyl and peroxyl radicals can oxidize and degrade organic and inorganic materials (Saini et al., 2020).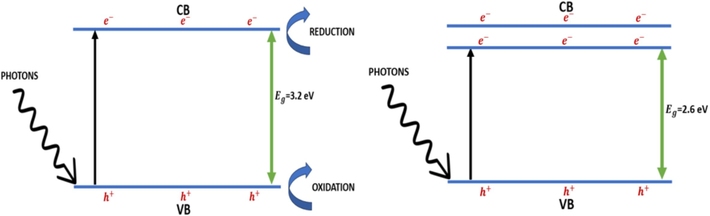
Photocatalytic action of pure and doped TiO2 nanocomposites.
The photocatalytic hydrogen production and wastewater remediation take place form reduction and oxidation process respectively. When photon of energy greater than energy gap
(3.23 eV for anatase, and 3.02 eV for rutile) is made incident on the TiO2 nanoparticles, by absorbing the energy of a photon, an electron makes transition to the conduction band from the valence band. This transition of electrons creates a hole in the valence band.
Under aerobic conditions, the adsorbed oxygen molecule captures the conduction band electron producing
ion which is readily protonated in acidic media (Manzoor et al., 2020).
This photon generated hole converts the hydroxide ion of surface adsorbed water into a hydroxyl radical in the absence of reducing species. This hydroxyl radical (HO*) can decompose the organic polymer into water and carbon dioxide.
The hydroxyl radical can be produced directly from the (O) ion under alkaline conditions and interacts with the hole as follows:
This hydroxyl radicals (HO*) are greater in numbers so that the photocatalytic ability becomes better. In acidic conditions the excess
interacts with the free electrons (
) and radicals H* are formed. The formation of radical H* causes a backlash with HO* and H* return to H2O.
The amount of the resulting hydroxyl radical in a photocatalytic activity is deduced in acidic conditions and the photocatalytic activity is decreased. Therefore, in the alkaline conditions, the photocatalytic activity is better than in neutral and acidic conditions.
3 Material and methods
All the ingredients and reagents required to prepare the TiO2 nanoparticles were purchased from Merck India Ltd. Aloe Vera leaves were collected from Hindu College campus in Moradabad. Aloe Vera leaves were cleaned before chopping into small pieces. 100 ml distilled water was boiled for 2 h at 100 °C with 50 gm of leaves. We have used Whatman filter paper for filtering the extract. The filtrate was stored for nanoparticle synthesis. The use of Aloe Vera extract as a reducing agent for the green synthesis of pure and Fe-doped TiO2 nanoparticles is rationally proposed based on its capability to reduce metallic ions to their respective metallic nanoparticles. 0.2 M solution of TTIP [Ti{OCH(CH3)}4] and INNH [Fe(NO3)3·9H2O] with 100 ml of distilled water was prepared. The 100 ml green extract of Aloe Vera prepared like this was mixed dropwise and stirred at 150 rpm for 2 h at 65 °C. Doping was performed from 2 % and 4 % by increasing the ratio of INNH in the TTIP solution.
The prepared samples were analysed through XRD using a Rigaku Ultima-IV X-ray diffractometer instrument situated at Nanoscale Research Facility, Manipal University, Jaipur, India equipped with a primary and fast accelerator detector operated at 40 kV and 40 mA. The surface morphological study of pure and Fe doped TiO2 NPs is performed using X-ray diffractometer with wavelength 1.54 Å at normal temperature and pressure where angle 2θ values ranges from 20° to 80°. FESEM micrographs and EDS maps were obtained in a JSM-7610FPlus of JEOL Ltd. The EDAX Energy Dispersive X-ray detector is installed in the JSM-7610F chamber to enable elemental analysis at the micro-nanoscale with high spatial resolution (1 kV 1.0 nm, 15 kV 0.8 nm).
Dissolved oxygen is a critical indicator of water quality. Monitoring DO levels is essential to assess the health of aquatic ecosystems and ensure the safety of human use. Understanding the factors that influence DO levels, such as water temperature, nutrient enrichment, and human activities, is crucial for maintaining good water quality. The dissolved oxygen in the sewage water as wastewater without sample is observed. Thereafter 100 mg of TiO2 sample was added to the 100 ml of the wastewater and again the dissolved oxygen value is observed. Now the water with TiO2 sample was exposed to UV lamp of 11 W for the duration of 4 h with the successive interval of 30 min and corresponding value of dissolved oxygen is measured with dissolved oxygen meter. This process was also performed with the 200 mg and 300 mg of TiO2 sample in 100 ml of waste water. Thereafter 100 mg, 200 mg and 300 mg of TiO2 sample with 2 % and 4 % doping of Fe were added to 100 ml of wastewater and same process is performed for the same time interval (Khalil et al., 2017; Madubuonu et al, 2020; Matinise et al., 2017).
4 Results and Discussion
4.1 Characterization
XRD patterns of green synthesized pure and Fe-doped TiO2 nanoparticles prepared with help of the Aloe Vera leaves extract are shown (Fig. 2). The nine distinct peaks at
= 25.308°, 37.791°, 48.047°, 53.885°, 55.073°, 62.692°, 68.756°, 70.304°, and 75.053° are observed corresponding to (101), (004), (200), (105), (211), (204), (116), (220), and (215) lattice planes belonging to the tetragonal phase (anatase) of TiO2 nanoparticles as per the database of JCPDS Card no. 21–1272. The crystallinity and high purity of the produced nanoparticles were confirmed by the sharpness of the peaks and the lack of unidentified peaks. The XRD pattern of TiO2 with 4 % Fe doping exhibits a minor angular shift of the (1 0 1) peak, which can be attributed mainly to strain and lattice distortion induced by Fe doping rather than the surface tension effect (Diallo et al., 2018; Hearne et al., 2004). Debye-Scherrer's equation was used to compute the average crystallographic size of TiO2 nanoparticles. To determine the average crystalline size of TiO2 nanoparticles the following Debye–Scherrer’s equation is used (Ramzanet et al., 2021).
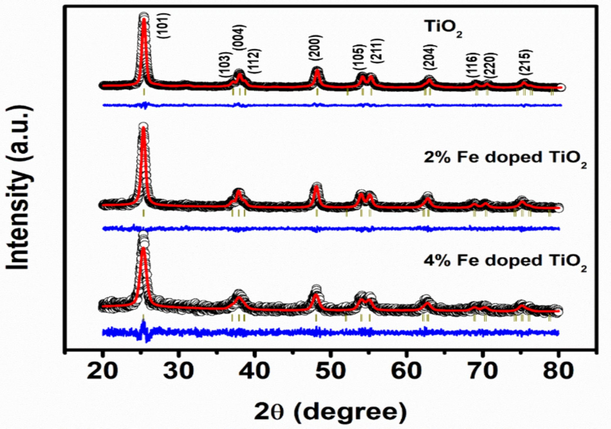
XRD spectra of TiO2 and Fe doped TiO2 with different ratio calcined at 550 °C for 3 h.
Sample
Particle size (nm) by Debye–Scherrer’s equation
TiO2
11.57
2 % Fe doped TiO2
11.26
4 % Fe doped TiO2
10.42
Elements
Weight%
Atomic %
Error %
C
11.5
20
9.8
N
2.7
4
20.3
O
44.6
58.3
11.4
Ti
36.3
15.8
2.2
Fe
5
1.9
6.6
4.2 FESEM-EDS
The FESEM micrographs and EDS analysis of the pure and Fe-doped TiO2 (Fe@2% and 4 %) photocatalyst are shown in Fig. 3(a)–3(f), respectively. The particles were found to be clumped together. According to earlier study, metal oxidation is the sole process that may cause these kinds of agglomerations.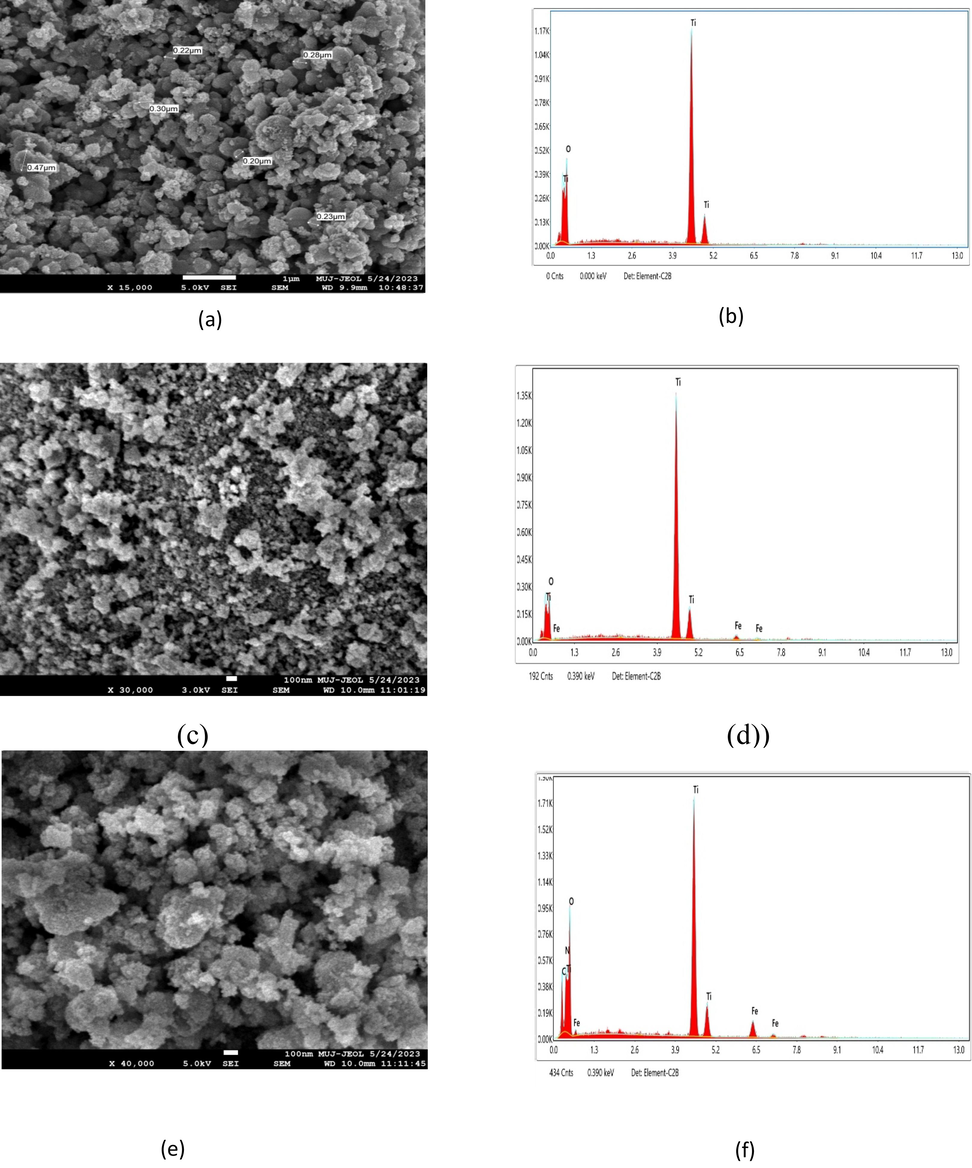
FESEM micrographs and EDS spectrum of (a) & (b) TiO2, (c) & (d) Fe −Ti @2%, (e) & (f) Fe −Ti@4%.
The particle structure of the TiO2 nanoparticles was found asymmetrical. The pure and Fe doped TiO2 nanocomposites with 2 % and 4 % doping average size was found to be roughly 18.88 nm, 46.98 nm and 69.94 nm respectively with help of histrograph (Fig. 4). Fe@TiO2 doping may be confirmed using SEM and EDS picture. EDS is a powerful tool for identifying and quantifying impurities in TiO2 materials, which is crucial for ensuring high purity and consistent performance in various applications (Škapin et al., 2015). Titanium (Ti), iron (Fe), oxygen (O), and other elements like N and C, which are derived from the water being used in thegreen synthesis process, are shown in Table 2 have different peaks in the compositional analysis (Crişan et al., 2018).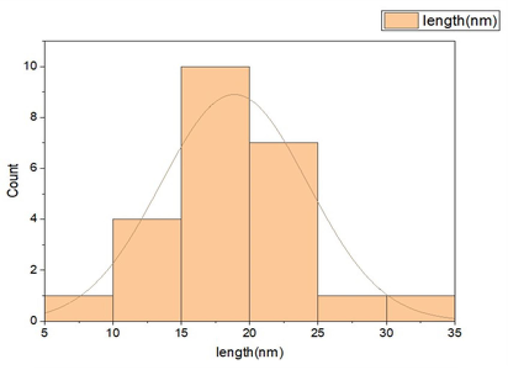
Histograph of particle size distribution.
4.3 Wastewater remediation
DO levels are critical for aquatic organisms, as they use oxygen for respiration. Different species have varying DO requirements. For example, bottom feeders and some invertebrates can tolerate lower DO levels (1–6 mg/L), while fish lying in shallow water need higher level of DO (4–15 mg/L). Microorganisms like bacteria and fungi also require DO for decomposition, which is essential for nutrient recycling. Firstly, the dissolved oxygen in the wastewater without sample is observed as 1.9 mg/l. Thereafter, 100 mg of TiO2 sample was added to the 100 ml of the wastewater and again the dissolved oxygen and value was observed. Now the water with TiO2 sample is exposed to UV lamp of 11 W for the duration of 4 h with successive interval of 30 min and the corresponding value of value of dissolved oxygen was observed. The same process was repeated by adding TiO2 with doping of 2 % and 4 % Fe (Fig. 5). Here Blue line is for pure TiO2, Red line for 2 % Fe doping and Gray line for 4 % Fe doping.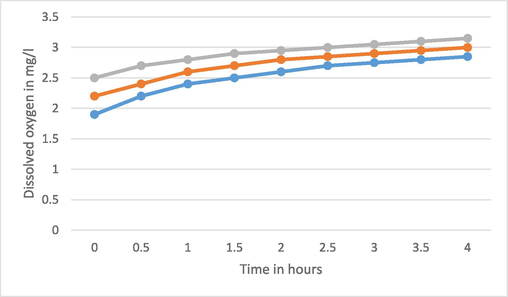
DO of water for pure and Fe doped TiO2 with 1 gm/lit.
The DO value changes from 1.85 to 2.85 mg/l whenever wastewater with pure TiO2 is exposed to UV light for the time from 0.5 to 4 h. With Fe-Ti@2% and 4 % these values vary from 2.2 to 3 and 2.5 to 3.15 mg/l respectively. Now the concentration of pure and doped TiO2 NPs is increased as 200 mg and 300 mg in 100 ml of wastewater and similar process of exposer with UV light is performed and corresponding DO is observed (Fig. 6, Fig. 7). Obviously, the dissolved oxygen in the water increases with the increment of Fe doping and time of exposer of UV light.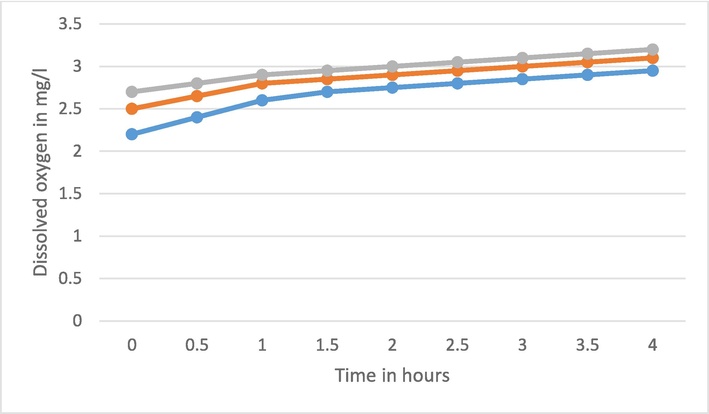
DO of water for pure and Fe doped TiO2 with 2 gm/lit.
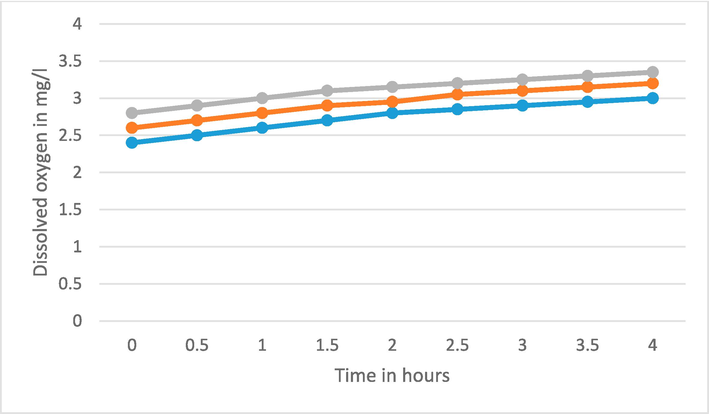
DO of water for pure and Fe doped TiO2 with 3 gm/lit.
5 Conclusion
The green synthesized pure and Fe doped TiO2 @2% and 4 % were prepared by flash pyrolysis method and thereafter the heat treatment for sintering is performed. Fe doping in TiO2 was confirmed using FESEM and EDS pictures. Debye-Scherrer's equation was used to compute the average crystallographic size of TiO2 nanoparticles. The Fe doped TiO2 nanocomposites partcles’ average size was determined with help of histogrph of particle size distribution.
Green synthesized titanium dioxide nanocomposites are ecofriendly photo semiconductors. UV radiation enhances the photodegradation process which is helpful in breaking of the polymers and produces hydroxyl and peroxyl radicals. The dissolved oxygen value in waste water increases when green synthesized nanosized TiO2 sample was added to it and further increases when the waste water was exposed to UV light. The impurities of waste water get reduced when green synthesized nanosized pure and Fe doped TiO2 composites were added and further reduced when this was exposed to UV light. By virtue of these results, we can say that green synthesized Fe doped TiO2 nanocomposites provide modern, eco-friendly, and cost effective, and high-efficiency wastewater treatment method.
CRediT authorship contribution statement
Sunder Singh: Methodology, Investigation, Data curation. Ravikant Divakar: Methodology, Investigation, Data curation, Conceptualization. Pratibha Maurya: Resources. Bhopal Singh: Resources, Formal analysis. Kahkashan Perveen: Writing – review & editing, Funding acquisition. Najat A. Bukhari: Writing – review & editing, Funding acquisition. Anil Kumar: Supervision.
Acknowledgement
The financial assistance provided by Research and Development Scheme of Uttar Pradesh Government (letter No. - 46/2021/603/sattar-4-2021-4(56)/2020 dated 30/03/2021) for this work is greatly acknowledged. The authors would like to acknowledge the support provided by Researchers Supporting Project Number RSP2024R358, King Saud University, Riyadh, Saudi Arabia
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic systems as an advanced environmental remediation: Recent developments, limitations and new avenues for applications. J. Environ. Chem. Eng.. 2016;4(4):4143-4164.
- [CrossRef] [Google Scholar]
- Effect of organic capping agents on the optical and photocatalytic activity of mesoporous TiO2 nanoparticles by sol–gel method. SN Appl. Sci.. 2019;1:393.
- [CrossRef] [Google Scholar]
- Optical Properties of Fe doped TiO2 Nanocomposites Synthesized by Sol-Gel Technique. Mater. Today:. Proc.. 2019;18:1204-1209.
- [CrossRef] [Google Scholar]
- Cong, Y., Zhang, J., Chen, F., Anpo, M., He, D., 2007. Preparation, Photocatalytic Activity, and Mechanism of Nano-TiO2 Co-Doped with Nitrogen and Iron (III). 111(28), 10618–10623. http://dx.doi.org/10.1021/jp0727493.
- Iron doped TiO2 films and their photoactivity in nitrobenzene removal from water. Appl. Surf. Sci.. 2018;455:201-215.
- [CrossRef] [Google Scholar]
- Modified TiO2 for environmental photocatalytic applications: a review. Ind. Eng. Chem. Res.. 2013;52(10):3581-3599.
- [CrossRef] [Google Scholar]
- Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem. Lett. Rev.. 2018;11(2):166-175.
- [CrossRef] [Google Scholar]
- TiO2 for water treatment: parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B-Environm.. 2010;99:398-406.
- [CrossRef] [Google Scholar]
- New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: an overview. SN Appl. Sci.. 2019;1:928.
- [CrossRef] [Google Scholar]
- A Raman spectroscopy study at high pressure. Phys. Rev. B. 2004;70(13):134102
- [CrossRef] [Google Scholar]
- A highly accurate method for determination of dissolved oxygen: Gravimetric Winkler method. Anal Chim Acta. 2012;741:21-31.
- [CrossRef] [Google Scholar]
- Environmental applications of semiconductor photocatalysis. Chem. Rev.. 1995;95(1):69-96.
- [CrossRef] [Google Scholar]
- Green synthesis of nanoparticles and its potential application. Biotechnol. Lett.. 2016;38:545-560.
- [CrossRef] [Google Scholar]
- Huston M., DeBella M., DiBella M., Gupta A., 2021. Green Synthesis of Nanomaterials. Nanomaterials (Basel). Aug 21;11(8):2130. doi: 10.3390/nano11082130.
- Johnson C., Affolter Matthew D., Inkenbrandt P., and Mosher C., 2017. Water Contamination from An Introduction to Geology, Salt Lake Community College, LibreTexts, 14.9. https://opengeology.org/textbook.
- Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: a review. Ind. Eng. Chem. Res.. 2004;43(24):7683-7696.
- [CrossRef] [Google Scholar]
- Effects of aquatic plant management on stream metabolism and oxygen balance in streams. Freshwater Biol.. 2000;45(1):85-95.
- [CrossRef] [Google Scholar]
- Kazuya Nakata; Akira Fujishima., 2012. TiO2 photocatalysis: Design and applications. 13(3), doi: 10.1016/j.jphotochemrev.2012.06.001.
- Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev.. 2017;10(4):186-201.
- [CrossRef] [Google Scholar]
- A review on photocatalysis used for wastewater treatment: dye degradation. Water Air Soil Pollut.. 2023;234(6):349.
- [CrossRef] [Google Scholar]
- Li P., Karunanidhi D., Subramani T., Srinivasamoorthy K., 2021. Sources and Consequences of Groundwater Contamination. Arch Environ Contam Toxicol. Jan;80(1):1-10. doi: 10.1007/s00244-020-00805-z.
- Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev.. 1995;95(3):735-758.
- [CrossRef] [Google Scholar]
- Bio-inspired iron oxide nanoparticles using Psidium guajava aqueous extract for antibacterial activity. Appl. Phys. A. 2020;126(1):72.
- [CrossRef] [Google Scholar]
- Recent developments in environmental photocatalytic degradation of organic pollutants: the case of titanium dioxide nanoparticles-a review. J. Nanomater. 2015:01-29.
- [CrossRef] [Google Scholar]
- Mancuso A., Sacco O., Vaiano V., Bonelli B., Esposito S., Freyria F.S., Blangetti N., Sannino D., 2021.Visible Light-driven photocatalytic activity and kinetics of Fe-doped TiO2 prepared by a three-block copolymer templating approach. Materials (Basel). 5;14(11):3105. doi: 10.3390/ma14113105.
- Investigation of relaxation phenomenon in lanthanum orthoferrite extracted through complex impedance and electric modulus spectroscopy. J. Appl. Phys.. 2020;128:064101
- [CrossRef] [Google Scholar]
- ZnO nanoparticles via Moringa oleifera green synthesis: Physical properties & mechanism of formation. Appl. Surf. Sci.. 2017;406:339-347.
- [CrossRef] [Google Scholar]
- A review on novel eco-friendly green approach to synthesis TiO2 nanoparticles using different extracts. J. Inorg. Organomet. Polym Mater.. 2018;28(4):1552-1564.
- [CrossRef] [Google Scholar]
- Global hydrological cycles and world water resources. Science. 2006;313(5790):1068-1072.
- [CrossRef] [Google Scholar]
- Nanoparticles for environmental clean-up: a review of potential risks and emerging solutions. Environ. Technol. Innov.. 2016;5:10-21.
- [CrossRef] [Google Scholar]
- Ilyas;Tariq Mahmood; Green synthesis of Cu@TiO2 via cedrus deodara leaf extract: A novel composite with high photocatalytic and antibacterial activity. Current Res. Green Sustain. Chem.. 2021;100137
- [CrossRef] [Google Scholar]
- Estimating stream metabolism from oxygen concentrations: effect of spatial heterogeneity. J Geophys Res. 2009;114:G03016.
- [CrossRef] [Google Scholar]
- A promising proton conducting electrolyte BaZr1-xHoxO3-Î́ (0.05 ≤ x ≤ 0.200.20) ceramics for intermediate temperature solid oxide fuel cells. Sci. Rep.. 2020;10(1):3461.
- [CrossRef] [Google Scholar]
- Fe dopant controlled the ferromagnetic, structural, thermal, optical, and electrical characteristics of CdO nanoparticles. Results Chem.. 2024;7:101260
- [CrossRef] [Google Scholar]
- ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechn.. 2018;16:84.
- [CrossRef] [Google Scholar]
- Photocatalytic activity of hierarchically structured, thermally stable, anatase particles. RSC Adv.. 2015;5(34):26769-26776.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of TCE in dry and wet air conditions with TiO2 porous thin films. Appl Catal B. 2011;108:14-21.
- [CrossRef] [Google Scholar]
- Carbon-sensitized and nitrogen-doped TiO2 for photocatalytic degradation of sulphanilamide under visible-light irradiation. Water Res.. 2011;45(16):5015-5026.
- [CrossRef] [Google Scholar]
- Preparation and visible light photocatalytic activity of Ag/TiO2/ graphene nanocomposite. Nano-Scale.. 2011;3(10):4411-4417.
- [CrossRef] [Google Scholar]







