Translate this page into:
Stroke preventing effect of resveratrol isolated from fungi and in vivo activity in male albino rats
⁎Corresponding author at: Department of Public Health, College of Applied Medical Sciences, Majmaah University, Majmaah 11952, Saudi Arabia. ai.ismail@mu.edu.sa (Ahmed Ismail)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The main objective of the study was to analyze the stroke preventing property of resveratrol from Alternaria alternata SK. The endophytic fungus A. alternata SK was isolated from the Brinjal plant. The isolated fungus was characterized based on morphological characters and molecular methods. It was cultured in submerged cultivation and resveratrol was isolated. The isolated resveratrol was used to test stroke preventing properties using animal model. The experimental male Albino rat fed with resveratrol decreased HDL cholesterol level in serum. The amount of malondialdehyde significantly lower in the cardiac tissues of Albino AD rat treated with resveratrol (P < 0.05). Likewise, the level of malondialdehyde in the haemolysate sample showed significant variation than control animal (P < 0.05). Administered resveratrol improved antioxidant potential. The amount of antioxidant enzymes such as, SOD, CAT and GPx significantly higher in resveratrol administered Albino rats (P < 0.05) than control rat. The amount of GSH, α-tocopherol and ascorbate in cardiac muscle of AD rats fed with resveratrol increased significantly than in AD Albino rats (P < 0.05). The amount of aspartate amino transferase, alanine amino transferase, alkaline phosphatase, and lactate dehydrogenate were reduced in experimental rats treated with resveratrol than control (P < 0.05). The experimental group treated with resveratrol decreased the level of LDH (1.63 ± 0.128 nmol NADPH/mg) than ischemia group (2.08 ± 0.11 nmol NADPH/mg) (P < 0.05). The amount of H2O2 was found to be high in ischemia group (428.2 ± 2.9 nmol) and it reduced (320.3 ± 10.2 nmol) in resveratrol treated animal (P < 0.05). In vivo analysis of cerebral ischemia/reperfusion in Albino rats revealed the protective role of resveratrol on LDH and H2O2 in hippocampus region.
Keywords
Cardiovascular diseases
Stroke
Fungi
Resveratrol
Antioxidant
Cardio-protective
1 Introduction
Resveratrol (3,5,4′-trihydroxystilbene) is widely distributed in fruits, plants, red wine, and various endophytic fungi. It has various pharmacological and biological activities and it regulates various pathways (Ahmadi and Ebrahimzadeh, 2020). Resveratrol can treat and prevent various diseases, including cardiovascular diseases, diabetes, cancer, stroke, acute respiratory distress, and pneumoniae (Filardo et al., 2020). Resveratrol can activate NK cells, and suppress the expression of pro-inflammatory factors and TLR4 and reduce the expression of MMP3 and MMP-1 proteinases. Resveratrol is widely used in cosmetic and food industry. It can also be applied as a food additive in yogurt, one of the components of cosmetics and skin care products because of its whitening, and antioxidant properties (Ratz-Łyko and Arct, 2019). Resveratrol is used as the analogues and it can be converted to piceatannol and pterostilbene by enzymatic action. The organisms such as Yarrowia lipolytica, Escherichia coli and Saccharomyces cerevisiae produced resveratrol. Recombinant technology and fermentation methods were used for the commercial production of resveratrol (Lu et al., 2016).
Endophytic fungi have the ability to produce various plant-based metabolites, including taxol, a functional compound from fungi (Venugopalan and Srivastava, 2015). These endophytes have the ability to produce various secondary metabolites similar to their host plant species during evolution. These endophytes show several advantages of accumulating rich amount of metabolites from the plants and these are toxic to various microorganisms. Fungi have the ability to produce resveratrol and characterized from various sources. Fungi such as, Lasiodiplodia, Arcopilus, Alternaria, Fusarium, Penicillium, Botryosphaeria, and Aspergillus produce resveratrol (Kiselev, 2011; Mei et al., 2015; Dwibedi and Saxena, 2018). Mushrooms such as, Suillus bellinii, Russula delica, Lactarius deliciosus, Pleurotus eryngii, Inonotus obliquus, Sparassis crispa and Flammulina velutipes produce resveratrol (Kang et al., 2014). Alternaria sp. MG1 is one of the endophytic fungi has been earlier screened from the cob of Vitis vinifera and has the potential to produce resveratrol in the liquid medium enriched with glucose (Shi et al., 2012).
Resveratrol has cardio-protective properties and is used to improve cardiovascular functions in experimental animals by improving the functional properties of progenitor cell/caridiac stem compartments and cardiac cells, recover ventricular function in diabetic heart (Delucchi et al., 2012). Resveratrol improved beneficial effect in left ventricle function in heart, decreased contractile dysfunction, reduced cardiac hypertrophy and interstitial fibrosis (Riba et al., 2017). It involves in various molecular action, including, improvement of myocardial Ca2+ handling, inhibition of pro-hypertrophic signalling molecules, stress signalling (MKP-1) pathways, phosphorylation of prosurvival and inflammation (Riba et al., 2017). In stroke, ROS are generally generated and these generated ROS trigger various reactions that affect the neuronal membrane. Recent findings revealed the role of ROS in the damage of cellular components in brain. These ROS involved in various pathophysiological phases and leads to neuron death. Increased stress caused by ROS involved malfunction of mitochondria in brain. Resveratrol prevent the expressions of nitric oxide synthase, suppressing phosphorylation of p38 in rats associated with myocardial infarction and vascular endothelial growth factor (Yan et al., 2018). Administration of resveratrol reduced myocardial infarction and subsequently reduced triglyceride level, aspartate transaminase (AST)/alanine transaminase ratio, and heart rate were observed (Öztürk et al., 2017). Resveratrol treatment smoothen cardiovascular function by reducing vasodilation, ischemia–reperfusion injury and atherosclerosis (Hung et al., 2000). Polygonum cuspidatum was considered as one of the traditional sources of resveratrol and it has been used to prevent arteriosclerosis and hyperlipidemia. Resveratrol in the biological system induces vasodilation and decreases hypertension and associated cardiovascular risk (Das et al., 2007). Resveratrol is consider as one of the metabolites has various molecular targets and useful to treat atherosclerosis, ischemia/reperfusion, metabolic syndrome and heart diseases (Rauf et al., 2017). Resveratrol improved stress resistance and improved the life span of many organisms including, yeast, and vertebrates (Valenzano et al., 2006). Fungi from the genera, namely Alternaria, Mucor, Geotrichum, Aspergillus, Cephalosporium, Penicillium, and Botryosphaeria produced resveratrol (Shi et al., 2012). In this study, resveratrol was screened from the fungus, A. alternata SK and cardio-protective activities were analyzed in vivo to reduce the risk of stroke.
2 Materials and methods
2.1 Source of endophytes
Brinjal plant was used for the isolation of resveratrol producing endophytes (Baazeem et al., 2021a). Brinjal plant was grown in green house condition for three months and the mature leaves, stem and root were used for the isolation of endophytes. Freshly collected leaves were stored at 2 °C until sample preparation (Baazeem et al., 2021b).
2.2 Culture media and isolation of endophytes
Potato dextrose agar (PDA) medium was used for the isolation of fungi. The culture medium composed of peeled potato: 200 g, dextrose 20 g, water 1 L and agar 20 g. Plant leaves, stem and root were used for the isolation of endophytes. The plant part was cultured on PDA medium after sterilization of plant parts (Baazeem et al., 2021b) and finally rinsed with double distilled water. It was serially diluted and cultured on PDA medium for 8 – 10 days. After 10 days of incubation, it was sub-cultured frequently.
2.3 Screening of resveratrol production
The isolated fungal spores of the individual strain (104/mL) were inoculated in potato dextrose broth medium and incubated for 8 – 10 days at 28 ± 2 °C. The Erlenmeyer flask was placed on a rotary shaker incubator at 150 rpm. The liquid broth and fungal culture were separated by centrifugation at 10000 rpm for 10 min. It was freezed in liquid nitrogen and further extracted with ethanol. The frozen sample was crushed into powder and to the every 1 gm fungal cells, 15 mL ethanol (80 %) was added. The cell free supernatant and the extracts of fungal cells were mixed and concentrated using a vacuum evaporator. To the 50 mL sample, 25 mL ethyl acetate was added and mixed. Then the ethyl acetate phase was collected and mixed with 3 % NaHCO3 (15 mL). The ethyl acetate fraction was evaporated and dried at 45 °C for 2 h. The dried sample was dissolved in HPLC grade methanol (1 mL) and filtered using a syringe filter and resveratrol content was calculated using a High Performance Liquid Chromatography. The HPLC system was equipped with LC-15C pumps, and injector, equipped with C18-column. About 10 µL sample was injected and acetonitrile was used for the determination of resveratrol. trans-resveratrol was used at various concentrations and used as the standard.
2.4 Characterization of fungal strains
Fungal colony morphology and sporulation of the fungal strains were carried out based on the growth performance on the PDA medium. The morphological characters were analyzed using a light microscopy with 100X magnification. Further, molecular characterization was performed based on internal transcribed spacer (ITS) sequencing. ITS1 and ITS2 regions were sequenced and molecular characterization of strain A. alternata SK was performed. The fungal strain was cultured in liquid medium and the freeze-dried mycelium was used for the extraction of DNA. The genomic DNA was extracted using a DNA extraction kit and PCR amplification was performed using PCR amplification kit. The primers used were, 5′-GAGCGGATAACAATTTCACACAGG-3′forward) and 5′ - CGCCAGGGTTTTCCCAGTCACGAC-3′(reverse) and amplification was carried out based on the instruction of the manufactures.
2.5 Bacterial growth and resveratrol biosynthesis
The fungal strain A. alternata SK was used for the determination of growth and resveratrol biosynthesis. The spores of the strain were inoculated in 250-mL Erlenmeyer flask containing 150 mL potato dextrose broth medium and incubated for 8 days at 28 ± 2 °C. Dry weight was measured for every 24 h. The fungal cells were extracted with ethanol, followed by ethyl acetate. The liquid broth was extracted with ethyl acetate and dried using NaHCO3 and suspended in methanol. The amount of resveratrol content and biomass were determined in triplicate analysis.
2.6 In vivo experimental model
Male albino rat (n = 18) was maintained in cages and allowed to access for water and food. It was maintained with adequate temperature and day/night cycle (12 h). The experimental animals applied in this study were maintained according to the institutional guidelines and approved by institutional animal ethical committee (Approval number- RSG-10928/03/2021). The experimental animals were divided into three groups. The control group consists of six male albino rats and allowed free access of water and control diet. To analyze the impact of resveratrol the experimental animal treated with standard diet, a group (n = 6) was fed with 5 mg/L resveratrol and supplemented with drinking water. The selected dose was based on preliminary experimental trials and the dose was approximately 2 mg/kg/day. The influence of resveratrol in atherogenic diet (AD) was studied in experimental animals. The atherogenic diet was prepared as suggested previously. After one month of the experimental trials, the AD male Albino rats were divided into two groups. To one subgroup, atherogenic diet and water was provided. To another subgroup, resveratrol was supplemented at 5 mg/L concentration. The experiments were continued for two weeks further with AD- resveratrol and AD group. The control group of animal was continued for 45 days with the same treatment.
2.7 Preparation of sample for analytical experiments
The experimental animals were sacrificed and blood was collected. Serum sample was separated from the blood sample and further haemolysate was prepared. Lipid analysis was carried out on the serum sample of the experimental animals and haemolysate samples were used for the determination of antioxidant content. Serum and haemolysate samples were store at −20 °C until analysis. Cardiac tissue (200 mg) was homogenized with phosphate buffer (2 mL) using a glass homogenizer. It was centrifuged for 10 min at 3000 rpm and the supernatant was used for biochemical assay. Total protein content of the sample was estimated as described previously by Bradford using bovine serum albumin (100 – 1000 µg/mL) standard.
2.8 Lipid profile analysis
The lipid profile of the Albino rat blood, including, triglycerides (TG), total cholesterol (TC), very low-density lipoprotein cholesterol (VLDL), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) were estimated. Biochemical kits were used until otherwise stated and all units were expressed as mg/dL. Lipid profile was determined using commercial diagnostic kit (Aspen Laboratories, India).
2.9 Lipid peroxidation assay
The lipid peroxidation assay was performed in both haemolysate and tissue sample. The amount of malondialdehyde (MDA) was evaluated as a measure of lipid peroxidation.
2.10 Antioxidant enzymes in experimental animals
The amount of antioxidant enzymes from the haemolysate and homogenate was evaluated as suggested previously. Catalase (CAT) activity of the sample was determined and the specific activity was expressed as U/mg protein. One enzyme unit was defined as µmol of H2O2 consumed/min/mg protein. Superoxide dismutase (SOD) activity of the sample was evaluated as described previously by Marklund (1984) and the result was expressed as U/mg protein. The antioxidant enzyme, Glutathione peroxidase (Gpx) was evaluated as suggested previously (Rotruck et al., 1973) and enzyme activity was expressed as GSH consumed/min/mg protein.
2.11 Antioxidant properties of reduced glutathione, ascorbate and α-Tocopherol
The amount of non-enzymatic antioxidants in cardiac muscle samples were evaluated as suggested previously. The amount of reduced glutathione (GSH) (µg/mg protein) was evaluated as suggested by Moron et al. (1979). Ascorbate content of the sample was estimated (µg/mg protein) as described previously (Omaye et al., 1979). α-Tocopherol content of the cardiac muscle was assayed as described earlier by Desai and Scott (1965) and the result was expressed as µg/mg protein.
2.12 Analysis of cardiac enzymes
Determination of cardiac enzymes is one of the suitable methods to analyze the cardiac damage. The cardiac enzymes such as, aspartate transaminases (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and alanine transaminases (ALT) were determined. The lipid profile was estimated using commercial diagnostic kit (Aspen Laboratories, India; Bhat Biotech, India).
2.13 In vivo analysis of cerebral ischemia/reperfusion in Albino rats
In this study, right middle cerebral artery occlusion was made for the analysis of ischemia/reperfusion. Briefly, the experimental Albino rats were acclimatized and anesthetized using chloral hydrate (500 mg/kg), a 4–0 nylon monofilament was introduced and an one end was coated with poly-l-lysine was carefully introduced. After two hours of ischemia, the experimental animal was withdrawn and introduced into the cages. In the case of sham-rats, the carotid artery was open and the filament was not inserted. To the experimental group, resveratrol was administered through water and to the control group only standard diet provided.
2.14 Analysis of lactate dehydrogenase and hydrogen peroxide in the hippocampus of rat brain
The hippocampus of the brain of Albino rat was ground with Tris buffer (pH 7.0) and the sample was obtained after 48 h of reperfusion. The reaction mixture consists of sodium pyruvate (0.01 M), NADH (0.02 M), and sodium phosphate buffer (pH 7.4, 0.1 M) and the final volume of the reaction mixture was 2.5 mL. Lactate dehydrogenase activity was assayed as nmol NADH oxidized/min/mg protein. The amount of hydrogen peroxide in the hippocampus region was estimated as suggested by Pick and Kesari (1981) with little modifications.
2.15 Statistical analysis
The results were expressed as mean ± standard deviation (SD) for experimental and control groups. One-way analysis of variance was performed to determine the significant level and the “P” value < 0.05 was considered as statistically significant.
3 Results
3.1 Isolation of endophytes for the secretion of resveratrol
In this study, 13 endophytes were obtained from Brinjal leaves, stem and root. A total of ten endophytes were isolated from the leaves and stem, and the remaining three were isolated from the root. The amount of resveratrol content was estimated by HPLC using standard transresveratrol chromatogram. These isolates produced resveratrol content and it widely varied between 17 and 206 μg/L. Among the fungal strains, the strain A. alternata SK showed increased resveratrol yield than other strains (Table 1). SK*: Resveratrol production was maximum (206 ± 2.8 µg/L) by the endophytic strain SK. The results are expressed as mean ± SD of three experimental trials.
Fungi strain
Dry weight of cells (g/L)
Resveratrol (µg/L)
AX
1.5 ± 0.4
84 ± 1.02
AD
2.5 ± 0.2
18.4 ± 0.85
AS
1.42 ± 0.1
10.5 ± 1.05
AM
3.69 ± 0.4
149 ± 1.03
DE
2.5 ± 1.1
1.42 ± 2.02
MZ
2.7 ± 0.32
187 ± 1.03
SK*
3.3 ± 0.21
206 ± 2.8
MJ
1.62 ± 0.95
155.4 ± 1.3
OI
1.53 ± 0.42
20.4 ± 1.2
PK
2.87 ± 0.48
18.3 ± 3.1
LU
3.04 ± 0.42
17 ± 2.4
TY
2.59 ± 1.032
0 ± 0
GE
3.085 ± 0.349
12.5 ± 0.5
3.2 Characterization of strain
The resveratrol-producing fungal strains were characterized based on cultural characters on potato dextrose broth and agar. It was identified based on sporulation properties and morphological characters. In our study, the resveratrol-synthesizing fungi belonged to Aspergillus, Penicillium, and Alternaria. The strain SK was isolated from the leaf of Brinjal and characterized as A. alternata SK which showed maximum amount of resveratrol than other fungal strains. It was brown colour colony and was dark in colour in reverse side of the culture medium (PDA) after 5 days of incubation. The mycelium was smooth sub-hyaline branches consists of septate hyphae and was approximately 3.5–4 μm in wide. Conidiophores of the strain were erect, sub-hyaline and ascending from both aerial and submerged hyphae, branched and simple septate. Based on 18 S rDNA gene sequencing the strain SK was characterized as A. alternate.
3.3 Growth profile and resveratrol production
The growth of fungal strain in potato dextrose broth medium was attained after five days of incubation. The dry weight of fungal strain decreased after seven days of culture. The biosynthesis of resveratrol was observed after 48 h of culture and attained maximum after five days of incubation. The growth and the biosynthesis of resveratrol increased simultaneously and decreased sharply after fifth day of incubation. The present finding revealed that the amount of resveratrol was 204.5 ± 12.8 μg/L after five days of culture and it was 158.4 ± 3.2 μg/L after seven days (Table 2). The growth was monitored for eight days and the standard deviation is an average of three different replicates.
Day
Dry weight (g/L)
Resveratrol (μg/L)
1
0.42 ± 0.06
0.34 ± 0.0
2
0.69 ± 0.04
28.3 ± 1.1
3
1.3 ± 0.52
138.2 ± 1.5
4
1.6 ± 0.62
149.4 ± 1.2
5
2.91 ± 0.1
204.5 ± 12.8
6
2.6 ± 0.42
187.4 ± 10.1
7
2.04 ± 0.18
158.4 ± 3.2
8
1.59 ± 0.27
142.3 ± 1.1.
3.4 Influence of resveratrol on serum lipid profile
In our study, elevated level of serum triglyceride, total cholesterol, VLDL and LDL was observed. The cardiac risk ratio was evaluated in control and AD fed Albino rats. Biochemical results revealed significant increase of serum triglycerides, total cholesterol and LDL and VLDL cholesterol. These increased level of biochemicals indicated increased risk of cardiovascular diseases in the AD administered Albino rats (P < 0.005). Moreover, AD experimental animal fed with resveratrol effectively decreased HDL cholesterol. Experimental Albino rats treated with resveratrol decreased lipid profile than normal Albino rats (Table 3) (P < 0.005).
Biochemical test
Control
AD
Res
AD + Res
Cholesterol (mg/dL)
47.5 ± 2.5
389.4 ± 10.3
42.4 ± 2.2
132.5 ± 1.23
Triglyceride (mg/dL)
78.4 ± 3.1
191.3 ± 2.4
74.2 ± 1.8
122.4 ± 3.9
LDL cholesterol (mg/dL)
19.3 ± 1.1
187.4 ± 1.5
18.3 ± 0.5
76.4 ± 1.2
HDL cholesterol (mg/dL)
73.2 ± 2.7
40.2 ± 2.9
67.4 ± 2.9
57.3 ± 2.2
VLDL (mg/dL)
21.04 ± 3.5
67.3 ± 1.6
14.3 ± 1.2
23.5 ± 1.7
3.5 Resveratrol precludes lipid peroxidation in haemolysate and cardiac tissue
The lipid peroxidation was evaluated by analyzing the amount of MDA from the sample. The haemolysate and cardiac tissue of AD Albino rats showed increased level of MDA (P < 0.05). The amount of MDA was higher in MD rats than in resveratrol administered Albino rats. The amount of MDA significantly lower in the cardiac tissues of Albino AD rat treated with resveratrol (P < 0.05). Likewise, the level of MDA in the haemolysate sample showed significant variation. Moreover, the resveratrol fed rats significantly increased LPO than control Albino rats and the result was described in Fig. 1.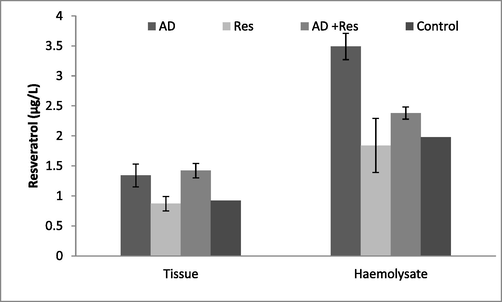
Resveratrol and lipid peroxidation in haemolysate and cardiac tissue of Albino rat.
3.6 Antioxidant properties of resveratrol
Administration of resveratrol to the experimental animal improved antioxidant potential. The amount of antioxidant enzymes such as, SOD, CAT and GPx significantly higher in resveratrol administered animal than control animal. Moreover, the antioxidant enzyme activities did not vary significantly in the AD group + resveratrol fed albino rat and control animals. This antioxidant pattern was similar in haemolysate sample of the Albino rats (Fig. 2a and b). The average amount of GSH, α-tocopherol and ascorbate in cardiac muscle of AD rats fed with resveratrol increased significantly than in AD Albino rats. Moreover, the average amounts of α-tocopherol and GSH in cardiac tissues of AD Albino rats treated with resveratrol did not show any significant variation (P > 0.05) (Fig. 2c).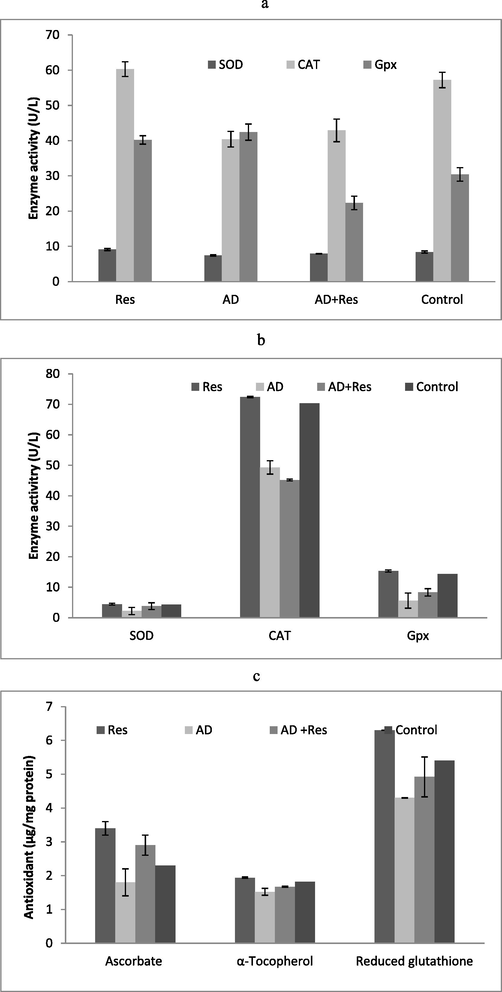
Antioxidant properties of resveratrol in in vivo experiment. SOD, CAT, Gpx activity of in cardiac muscle (a) and haemolysate (b) and non-enzymatic biomarkers (c) in experimental animal.
3.7 Role of resveratrol on cardiac enzymes
The cardiac enzymes such as, aspartate amino transferase (ALT), alanine amino transferase (AST), alkaline phosphatase (ALP), and lactate dehydrogenate (LDH) were elevated in AD Albino rats. However, the experimental AD Albino rats treated with resveratrol decreased the cardiac enzyme level than AD rats revealed cardioprotective activity. Administration of resveratrol reduced the level of cardiac enzymes in the cardiac muscle of the experimental animal like control Albino rat (Table 4). ALP: Alkaline phosphatase; AST: Aspartate transaminase; ALT: Alanine transaminase; LDH: lactate dehydrogenase.
Enzymes
AD
Res
AD + Res
Control
ALP
0.21 ± 0.03
0.08 ± 0.0
0.17 ± 0.02
0.11 ± 0.002
AST
0.49 ± 0.06
0.43 ± 0.05
0.33 ± 0.08
0.23 ± 0.003
ALT
0.012 ± 0.09
0.01 ± 0.0
0.09 ± 0.02
0.09 ± 0.0
LDH
76.2 ± 2.8
54.3 ± 2.9
60.3 ± 1.9
39.4 ± 2.2
3.8 Effect of resveratrol on LDH activity
The amount of LDH in the hippocampus region of injured experimentalanimal showed elevated LDH activity. The control animal (sham) showed decreased level of LDH in the hippocampus region (1.02 ± 0.01 nmol NADPH/mg). The experimental group treated with resveratrol decreased the level of LDH (1.63 ± 0.128 nmol NADPH/mg) than ischemia group (2.08 ± 0.11 nmol NADPH/mg) (P < 0.05). The amount of H2O2 was found to be high in ischemia group (428.2 ± 2.9 nmol) than sham group (276.3 ± 2.5 nmol). Moreover, the experimental animal treated with resveratrol showed decreased level of H2O2 (320.3 ± 10.2 nmol) (P < 0.05).
4 Discussion
Endophytic fungi are gaining much more attention recently because of their ability to produce various secondary metabolites with bioactive potentials. These metabolites have various applications, including, agriculture and medicine (Baazeem et al., 2021b; El-Sheikh et al., 2020). In this study, metabolites producing fungi were isolated from various plant species and resveratrol-producing fungi were screened from plant parts. Endophytes have the ability to produce resveratrol and the present result was consistent with previous findings on fungal endophytes. The number of available endophytic fungal strain in the plant parts widely varied. The endophytic fungi isolated from the Brinjal leaf showed potential resveratrol activity. Sun and Guo (2012) have been reported several endophytic fungi from various parts of the same plants. The screened strain A. alternata SK has the ability to produce resveratrol in large amount in submerged fermentation. Similarly, Zhao et al. (2010) reported the decreased level of metabolites by the other endophytes screened from plants. The decreased level of resveratrol biosynthesis was mainly due to the lack of suitable culture conditions. The production of biomass and resveratrol was similar with previoius reports (Chomcheon et al., 2009). Fungi from the genus, Alternaria have the ability to produce various toxins. The genus Alternaria specis as endphytic origin and produced valuable secondary metabolites (Shi et al., 2012).
The present finding revealed the possible application of resveratrol as novel therapeutic lead molecules for cardiovascular diseases and reduced blood cholesterol level. The high cholesterol diet influenced cholesterol deposition in various tissues in the form of cholesterol esters. The present findings revealed that the Albino rats fed with control diet showed elevated lipid content in the cardiac tissues. In our study, results revealed that resveratrol decreased HDL cholesterol in experimental animal fed with resveratrol. In Albino rats, one of the important impacts of resveratrol on lipid profile was its mode of action on in vivo ox-LDL. In experimental animal model, resveratrol reduced ox-LDL in different dietary condition. Ox-LDL induced atherosclerosis both by improving lipids signals that effectively activate macrophates and by inducing the formation of foam cells (Schaffer, 2003). In biological system, oxidative stress is one of the important causative factors that associated with increased cholesterol content and atherogenesis. In our study, improved MDA level and lipid peroxidation was observed in atherogenic diet treated experimental animal. The increased level of MDA and lipid peroxidation was mainly due to generation of free radicals. In the process of lipid peroxidation enhanced production of MDA has been reported (Lee et al., 2007). Red wine contains resveratrol and the available resveratrol in the red wine has been significantly decreased alcohol-induced lipid peroidation of heart muscle (Bradamante et al., 2004). Resveratrol has lipophilic property and this is very much associated with prevention of unsaturated fatty acids (Spanier et al., 2009). Administration of resveratrol increased the level of glutathione peroxidise, catalase and superoxide dismutase and reduced the generation of free radicals and protecting the endothelial cell from injury (Spanier et al., 2009). The present finding was agreed with previous reports. In our study, administration of resveratrol stimulated non-enzymatic and enzymatic antioxidant system when supplemented with atherogenic diet. These antioxidant effects were almost similar with haemolysate and heart tissue and this finding was consistent with previous studies in experimental mice with resveratrol treatment for 7 days and upregulation of glutathione peroxidase, superoxide dismutase and catalase in heart muscle of mice (Xia et al., 2010). ALT, AST, ALP and LDH have been considered as the important liver and cardiac enzymes and were observed in this study. The damaged cardiac tissues released these enzymes and the elevated level has also observed in the plasma. In our study, administered resveratrol prevented liver and cardiac damage in Albino rats. The experimental animals administered to atherogenic diet has been elevated the level of various cardiac enzymes such as, ALT, AST, LDH and ALP (Meng et al., 2014). The increased level of these enzymes has been correlated with hypertension among human population. The amount of serum AST and ALT has been high in hypertension individuals. These enzymes are primarily used to determine liver functions (Rahman et al., 2020). The experimental animal exposed to atherogenic diet elevated the level of cardiomakers and the muscular damage results elevated the levels in the plasma (Mitani et al., 2003). Recently, Djakpo et al. (2020) have been hypothesized the elevated level of AST/ALT ratio in cardiovascular disease patients. The increased level of alkaline phosphatise are also associated with an increased risk of cardiovascular diseases (Wang et al., 2018).
5 Conclusions
To conclude, administration of resveratrol with water effectively prevented liver and cardiac damages caused by atherogenic diet in experimental Albino rats. The present finding showed that resveratrol has cardio protective property by improving the antioxidant system, reducing cholesterol content of serum, balancing lipid metabolism and preventing cardiac tissue damage either in aorta or myocardium in experimental animals. Consumption of dietary resveratrol from natural sources is useful to prevent the risk of cardiovascular diseases.
Acknowledgement
The authors extend their appreciation to the deputyship for Research & Innovation, Ministry of education in Saudi Arabia for funding this research work through the project number (IFP-2020-29)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Resveratrol–A comprehensive review of recent advances in anticancer drug design and development. Eur. J. Med. Chem.. 2020;200
- [Google Scholar]
- In vitro antibacterial, antifungal, nematocidal and growth promoting activities of Trichoderma hamatum FB10 and its secondary metabolites. J. Fungi. 2021;7(5):331.
- [Google Scholar]
- Paecilomyces formosus MD12, a Biocontrol Agent to Treat Meloidogyne incognita on Brinjal in Green House. J. Fungi. 2021;7(8):632.
- [Google Scholar]
- Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev.. 2004;22(3):169-188.
- [Google Scholar]
- Aromatase inhibitory, radical scavenging, and antioxidant activities of depsidones and diaryl ethers from the endophytic fungus Corynespora cassiicola L36. Phytochemistry. 2009;70(3):407-413.
- [Google Scholar]
- Experimental evidence for the cardioprotective effects of red wine. Exp. Clin. Cardiol.. 2007;12(1):5.
- [Google Scholar]
- Resveratrol treatment reduces cardiac progenitor cell dysfunction and prevents morpho-functional ventricular remodeling in type-1 diabetic rats. PLoS ONE. 2012;7(6)
- [Google Scholar]
- Mode of action of selenium in relation to biological activity of tocopherols. Arch. Biochem. Biophy.. 1965;110(2):309-315.
- [Google Scholar]
- The significance of transaminase ratio (AST/ALT) in acute myocardial infarction. Arch. Med. Sci. Atheroscler. Dis.. 2020;5:279.
- [Google Scholar]
- Arcopilus aureus, a resveratrol-producing endophyte from Vitis vinifera. Appl. Biochem. Biotechnol.. 2018;186(2):476-495.
- [Google Scholar]
- Paecilomyces sp. ZB is a cell factory for the production of gibberellic acid using a cheap substrate in solid state fermentation. Saud. J. Biol. Sci.. 2020;27(9):2431-2438.
- [Google Scholar]
- Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. therapeut.. 2020;214
- [Google Scholar]
- Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovas. Res.. 2000;47(3):549-555.
- [Google Scholar]
- Compositional analysis of the fruiting body of transgenic Flammulina velutipes producing resveratrol. Food Chem.. 2014;164:211-218.
- [Google Scholar]
- Perspectives for production and application of resveratrol. Appl. Microbiol. Biotechnol.. 2011;90(2):417-425.
- [Google Scholar]
- Hypocholesterolemic and antioxidant properties of 3-(4-hydroxyl) propanoic acid derivatives in high-cholesterol fed rats. Chem. Biol. Interact.. 2007;170(1):9-19.
- [Google Scholar]
- Strategies for enhancing resveratrol production and the expression of pathway enzymes. Appl. Microbiol. Biotechnol.. 2016;100(17):7407-7421.
- [Google Scholar]
- Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J.. 1984;222(3):649-655.
- [Google Scholar]
- Biocatalysis and biotransformation of resveratrol in microorganisms. Biotechnol. Lett.. 2015;37(1):9-18.
- [Google Scholar]
- Cardioprotective effect of resveratrol on atherogenic diet-fed rats. Int. J. Clin. Exp. Pathol.. 2014;7(11):7899-7906.
- [Google Scholar]
- HMG-CoA reductase inhibitor, fluvastatin, has cholesterol-lowering independent “direct” effects on atherosclerotic vessels in high cholesterol diet-fed rabbits. Pharmacol. Res.. 2003;48(5):417-427.
- [Google Scholar]
- Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophy. Acta. Gen. Sub.. 1979;582(1):67-78.
- [Google Scholar]
- Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In: Methods in Enzymology. Vol Vol. 62. Academic press; 1979. p. :3-11.
- [Google Scholar]
- Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharm.. 2017;95:230-234.
- [Google Scholar]
- Superoxide anion and H2O2 production by chemically elicited peritoneal macrophages-induction by multiple non-phagocytic stimulus. Cell. Immunol.. 1981;59:301-308.
- [Google Scholar]
- Association between serum liver enzymes and hypertension: a cross-sectional study in Bangladeshi adults. BMC Cadiovascul. Disorder.. 2020;20(1):1-7.
- [Google Scholar]
- Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther.. 2019;21(2):84-90.
- [Google Scholar]
- A comprehensive review of the health perspectives of resveratrol. Food Fun.. 2017;8(12):4284-4305.
- [Google Scholar]
- Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid. Med. Cell. Longev.. 2017;2017:1-10.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-590.
- [Google Scholar]
- Alternaria sp. MG1, a resveratrol-producing fungus: isolation, identification, and optimal cultivation conditions for resveratrol production. Appl. Microbiol. Biotechnol.. 2012;95(2):369-379.
- [Google Scholar]
- Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J. Physiol. Pharmacol.. 2009;60(Suppl 4):111-116.
- [Google Scholar]
- Endophytic fungal diversity: review of traditional and molecular techniques. Mycology. 2012;3(1):65-76.
- [Google Scholar]
- Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol.. 2006;16(3):296-300.
- [Google Scholar]
- Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol. Adv.. 2015;33(6):873-887.
- [Google Scholar]
- Association between increased serum alkaline phosphatase and the coronary slow flow phenomenon. BMC Cardiovascul. Disorder.. 2018;18(1):1-6.
- [Google Scholar]
- Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther.. 2010;335(1):149-154.
- [Google Scholar]
- Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Exp. Ther. Med.. 2018;15(2):1728-1734.
- [Google Scholar]
- Endophytic fungi for producing bioactive compounds originally from their host plants. Curr. Res. Technol. Educ. Trop. Appl. Microbiol. Microbiol. Biotechnol.. 2010;1:567-576.
- [Google Scholar]







