Translate this page into:
Stirring and mixing in ethylic biodiesel production
⁎Corresponding author. lucas.meili@ctec.ufal.br (Lucas Meili)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This study aimed to analyze the influence of stirring and mixing on the production of soybean biodiesel. It was verified the influence of the baffle, different stirring speeds and two types of impellers (turbine and blade). The experiments were conducted by full experimental design 23 with the following fixed parameters: temperature (70 °C), oil/ethanol molar ratio (1/10), catalyst amount (1.5%), and reaction time (30 min). The independent variables were impeller type (turbine or blade), baffle (presence or absence), and stirring speed (150 or 350 rpm). Chromatographic analyses helped to determine the yield of biodiesel production at different operating times. The optimized parameters turbine impeller, absence of baffle, and stirring speed of 350 rpm provided high biodiesel yields within the first minutes of reaction. The analysis of the effect over time allows to verify in which period the variables had more influence on the biodiesel production and in which time there is no increase of the yield.
Keywords
Optimization
Stirring
Biofuels
Production
1 Introduction
Energy is indispensable for maintaining a high standard of living. Fossil fuels have an unstable cost and generate large amounts of pollutants, which motivates researchers to pursue the development of renewable energy technologies (Bokhari et al., 2017, 2016). Biodiesel has a strong potential of alternative fuel to replace diesel fuel because it is renewable, biodegradable, non-toxic and reduces sulfur oxide (SOx) emissions, contributing to environmental protection (Asif et al., 2017; Chuah et al., 2017).
The wide range of feedstocks available worldwide (Adewale et al., 2015) has been one of the factors driving researchers’ attention toward biodiesel production. For a feedstock to be applied in biodiesel production, it must have two very important characteristics: low production cost and potential for high-scale manufacture. The availability of feedstocks depends on regional weather, geographical location, local conditions of the soil, and agricultural practices (Atabani et al., 2012). The use of cheaper feedstocks allow for economical and competitive biodiesel production. Oil seeds have been constantly investigated as a source of biodiesel. In this context, soybean stands out because it does not pose any technical limitations and there is enough arable land worldwide to support a program that includes blends of biodiesel and conventional diesel (Ma and Hanna, 1999).
Biodiesel is most commonly produced by transesterification. This method is based on the conversion of triglycerides to fatty acid esters and glycerin by reaction of triglycerides with a low-molecular-weight alcohol, such as methanol and ethanol, in the presence of homogeneous (Atadashi et al., 2013; Hossain et al., 2010) or heterogeneous (Xie et al., 2015a, 2015b; Xie and Fan, 2014; Xie and Wang, 2014; Xie and Zhao, 2013) catalysts. The reaction is sensitive to variations in alcohol type, alcohol proportion, catalyst, stirring/stirring, temperature, and time (Stamenkovic et al., 2007).
Many factors can affect biodiesel production, so optimizing the process is important to achieve better yields with lower costs (Dharma et al., 2016; Xie et al., 2015a, 2015b; Xie and Fan, 2014; Xie and Wang, 2014; Xie and Zhao, 2013). The experimental design method is usually employed to guide optimization studies—assessing the influence of operating variables on the responses aids optimization of the experimental variables and produces effective results. This method is widespread and has been adopted in many research papers dealing with biodiesel production, such as the papers by Dharma et al. (Dharma et al., 2016), Sakdasri et al. (Sawangkeaw and Ngamprasertsith, 2016), Maneechakr et al. (Maneechakr et al., 2015), Maran and Pryia (Prakash Maran and Priya, 2015), Liu et al. (Liu et al., 2015), Yu et al. (Yu et al., 2015), Huerga et al. (Huerga et al., 2014), Orives et al. (Orives et al., 2014), Patle et al. (Patle et al., 2014), Costa et al. (Costa et al., 2013), and Silva et al. (Da Silva et al., 2011), among others. Nevertheless, few of these papers have evaluated the kinetics of the transesterification reaction by experimental design.
This study aimed to assess the dynamics of biodiesel production from soybean oil by experimental design. We assessed the effect of the following parameters on soy biodiesel production: impeller type (turbine or blade), presence or absence of baffle, and stirring speed (150 or 350 rpm). The experiments were carried out by using the full experimental design 23; the fixed parameters were as follows: temperature (70 °C), oil/ethanol molar ratio (1/10), catalyst amount (1.5%), and reaction time (30 min).
2 Methods
2.1 Soybean oil characterization
Soybean oil was submitted to physical-chemical analyses. The following parameters were examined:
-
Viscosity (measured according to ASTM D 445 (ASTM, 2006)).
-
Freezing Point (IAL, 2011).
-
Density (measured according to ASTM D 4052 (ASTM, 2011)).
-
Acidity value (IAL, 2011).
-
Saponification Index (IAL, 2011).
-
Moisture (measured according to ASTM D 6304 (ASTM, 2007)).
2.2 Biodiesel production
To determine the experimental conditions that maximized the synthesis of biodiesel by transesterification and to evaluate the influence of selected variables, a full factorial design with two levels and three variables was performed. The variables studied in this phase were impeller type, presence or absence of baffle, and stirring speed. The fixed parameters were temperature (70 °C), oil/ethanol molar ratio (1/10), catalyst amount (1.5%), and reaction time (30 min). These variables were fixed because we were only interested in verifying how stirring influenced the process; we were not interested in the influence of any other parameters. Table 1 lists the limits associated with each stirring variable. The lower and upper limits are represented by −1 and +1, respectively.
Level
Baffle
Impeller
Stirring Speed
−1
With
Turbine
150 rpm
+1
Without
Pitched blade
350 rpm
The following types of impellers were used: turbine and pitched blade. Table 2 shows the number of blades and the size of each impeller. Fig. 1 illustrates the dimensions of the impellers used in the experiments. Table 3 depicts the experimental design matrix 23. The experiments were performed randomly, so the numbering does not match the order in which the experiments are conducted. Soy ethyl biodiesel will be produced.
Impeller
n
L
w
d
D
Turbine
6
1/4D
(1.25 cm)1/5D
(1 cm)2/3D
(3.33 cm)5 cm
Pitched blade
4
3/10D
(1.5 cm)1/5D
(1 cm)–
5 cm
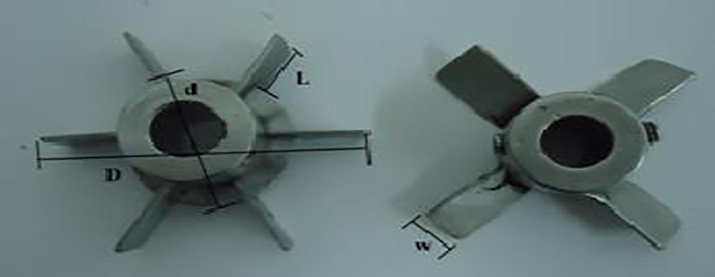
Turbine impeller (left) and blade impeller (right).
Experiment
Baffle
Impeller
Stirr. Speed
1
−1
−1
−1
2
1
−1
−1
3
−1
1
−1
4
1
1
−1
5
−1
−1
1
6
1
−1
1
7
−1
1
1
8
1
1
1
Soy biodiesel was obtained by transesterification according the process flowsheet displayed in Fig. 2; ethanol was the alcohol agent, and sodium hydroxide was the catalyst. Transesterification was accomplished by using 800 g of soybean oil (molecular weight = 884 g/mol), 416.3 g of anhydrous ethanol (oil/alcohol molar ratio = 1:10), and 12 g of NaOH (equivalent to 1.5% of oil, m/m). First, NaOH and ethanol were mixed to form a homogeneous mixture. Meanwhile, soybean oil was placed in a 2-L glass reactor equipped with a water circulating jacket and a mechanical stirrer. The reactor was connected to a thermostatic bath (Model TE-184) operating at a temperature of 70 °C. After the reactor reached this temperature, the mixture containing the catalyst and ethanol was added to the oil under stirring (150 or 350 rpm), as monitored by a digital multimeter (Model ET-14000). The reaction lasted 30 min. During the experimental run, samples were collected at t1 = 0.5 min, t2 = 1 min, t3 = 1.5 min, t4 = 2 min, t5 = 3 min, t6 = 4 min, t7 = 5 min, t8 = 10 min, t9 = 20 min, and t10 = 30 min. All the analyses were performed in duplicate, and the percentage error was below 5%.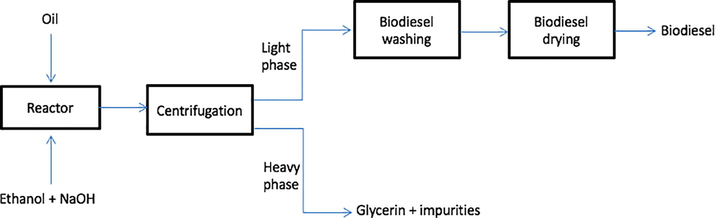
Process flowsheet.
At the desired time, 10 mL of the reaction mixture was withdrawn from the reactor and added to a tube containing 2.5 mL of diluted (1:100) sulfuric acid, which promoted phase separation. The less dense phase contained ethyl esters (biodiesel), unreacted excess alcohol, and catalyst. The denser phase consisted of glycerin, soap, unreacted excess alcohol, and catalyst.
The tube was centrifuged in the centrifuge Petro Teste Model 15H-6. The biodiesel phase was retrieved, and its pH was measured. When the pH value did not fall between 5 and 7, the biodiesel was washed with distilled water until the target pH was achieved. After the washings, magnesium sulfate was added to the biodiesel, to remove moisture. The drying agent was then separated from the biodiesel by simple filtration.
The yield of biodiesel production from soybean oil was determined by gas chromatography conducted on a chromatograph VARIAN, model CP-3800 equipped with an FID detector and a 2.3-m capillary column. The detector and injector temperatures were 250 and 240 °C, respectively. The oven temperature was programmed to rise from 150 to 260 °C at a heating rate of 10 °C/min. Glyceryl trioctanoate (tricaprylin) was used as internal standard; high-purity hydrogen gas (99.95%) was used as a carrier gas. The samples injected into the chromatograph were prepared from a mixture of approximately 0.15 mL of biodiesel and 1 mL of standard solution (tricaprylin and hexane). One microliter of the sample was injected into the chromatograph. Analyses were performed in duplicate. The yield of esters was calculated using Eq. (1).
mtricaprylin is the internal standard mass;
As is the sum of the areas of the peaks due to esters present in the sample;
f is the response factor;
Atricaprylin is the area of the peak corresponding to the internal standard;
ms is the mass of the sample.
2.3 Soy biodiesel characterization
The same methodologies described in Section 2.3. for soybean oil characterization were used to characterize the biodiesel. Viscosity, density, moisture content, and acidity were determined.
3 Results and discussion
3.1 Soybean oil characterization
Table 4 summarizes the physical-chemical properties of soybean oil. The results resembled literature data. The saponification index was slightly below the literature value, whereas viscosity and moisture were slightly above. The dynamic viscosity of the oil was higher than the viscosity of diesel fuel, from 1.9 to 4.1 cst (Ma and Hanna, 1999). To analyze the freezing point, soybean oil was refrigerated to −8 °C. No crystals emerged. The differences between the values observed herein and literature data may lie on differences in soybean oil purity and in measurement methods.
Propriety
Obtained Values
Literature Values
Viscosity. 40 °C (cst)
39.01
32.7 [31]
Freezing Point (°C)
<−8
−12.2 [11]
Relative density. 20 °C
0.919
0.919–0.925 [32]
Relative density. 25 °C
0.916
0.916–0.922 [32]
Acidity index (g of oleic acid/100 g)
0.16
< 0.3 [32]
Saponification index
180
189–195 [32]
Humidity (%)
0.068
<0.05 [33]
3.2 Soy biodiesel characterization
Table 5 displays the physical-chemical parameters of the soy biodiesel obtained in experiment 2, which afforded yield of 99.8%. The viscosity and density values agreed with the values advocated by the Brazilian Agency of Petroleum, Natural Gas and Biofuels (ANP) (Brasil, 2012), the regulatory agency in Brazil. The acidity index and moisture content were high.
Propriety
Obtained Values
Literature Values (32)
Viscosity. 40 °C (cst)
5.0625
3.0–6.0
Density. 20 °C (g/cm3)
0.8734
0.850–0.900
Acidity index (mg KOH/g)
3.08
<0.5
Humidity (%)
0.1
<0.05
3.3 Biodiesel production
Table 6 shows the biodiesel yields obtained at different reaction times. Fig. 3 displays the biodiesel yields as a function of time for the eight test runs. At the beginning of transesterification, the yields varied. Approximately after four minutes of the start of the reaction, high yields were achieved. Yields did not change significantly thereafter. To obtain more reliable results for the interaction between variables, we performed a full factorial design 23 involving three variables (impeller type, presence or absence of baffle, and stirring speed) in two levels, which amounted to eight experiments. All the analyses were conducted in duplicate, with 95% confidence interval.
Experiment Number
t1
0.5t2
1t3
1.5t4
2t5
3t6
4t7
5t8
10t9
20t10
30
1
94.2
98.8
99.2
98.5
98.2
98.4
98.9
99.2
99.8
99.2
2
66.2
74.1
83.2
92.2
98.9
98.5
99.2
99.6
99.9
99.8
3
77.5
89.2
93.2
93.2
92.9
93.5
93.8
95.1
94.9
95.3
4
80.3
89.3
96.2
99.8
99.6
99.5
99.8
99.8
99.9
99.9
5
86.4
98.5
98.3
98.9
98.9
99.2
99.1
99.0
99.2
99.2
6
86.5
92.4
97.3
98.9
99.8
99.5
99.8
99.2
99.6
99.8
7
71.7
79.8
85.8
90.5
95.9
98.7
99.8
99.5
99.6
99.6
8
79.5
85.8
88.3
90.2
93.1
94.9
96.2
98.8
99.1
99.6
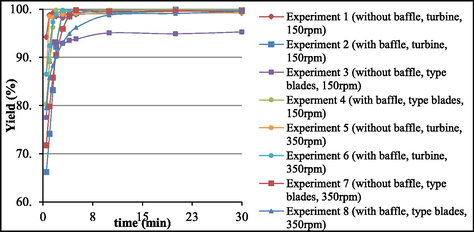
Yield x Time graph for all the 8 experimental runs.
The main effects were calculated (see Fig. 4). Interactions between the variables baffle (presence or absence) and impeller type, baffle (presence or absence) and stirring speed, and impeller type and stirring speed were determined. The effects were evaluated at t1 = 0.5 min, t2 = 1 min, t3 = 1.5 min, t4 = 2 min, t5 = 3 min, t6 = 4 min, t7 = 5 min, t8 = 10 min, t9 = 20 min, and t10 = 30 min.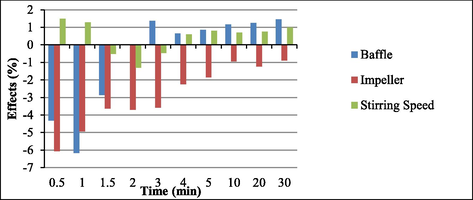
Main effects over time for baffle, impeller and stirring speed.
At 0.5, 1, and 1.5 min, the presence of baffle decreased soy biodiesel yield by 4.5%, 6%, and 3%, respectively. After two minutes, the presence of baffle exerted no further effect on soy biodiesel yield.
As for the impeller type, going from the lower level (turbine impeller) to the upper level (paddle impeller) diminished soy biodiesel yield at all times. At 0.5 min (30 s), the yield decreased 6% on average. Hence, the turbine impeller afforded better soy biodiesel yields throughout the reaction. Frascari et al. (Frascari et al., 2009) obtained the same result when they evaluated three types of impellers—turbine (radial flow), inclined blades (axial flow), and turbine with angled blades (radial-axial flow). They found that, depending on the impeller type, the reaction time could reduce drastically, and different reaction phases could emerge.
Changes in the stirring speed elicited very small alterations (Fig. 5). Increasing stirring speed raised soy biodiesel yields because higher stirring speeds favored collisions between soybean oil and alcohol molecules during the reaction. This result agreed with literature reports. For example, Noureddini and Zhu (Noureddini and Zhu, 1997), Ma et al. (Ma et al., 1999), and Stamenkovic et al. (Stamenkovic et al., 2007) described that the stirring speed significantly affected the transesterification reaction. Here, the interaction between the variables baffle (presence or absence) and impeller type affected transesterification to a larger extent than baffle alone (presence or absence).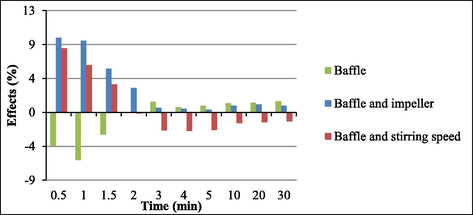
Main effect and interaction effects for baffle.
As for interactions between variables (see Fig. 5), going from the lower level (without baffle and 150 rpm) to the higher level (with baffle and 350 rpm) affected transesterification positively, but this effect decayed over time: at 0.5, 1, and 1.5 min the soy biodiesel yields increased by 8%, 6%, and 4%, respectively. After two minutes, this interaction no longer had a significant effect on transesterification. In other words, the presence of baffle and stirring speed of 350 rpm led to higher yields during the first minutes of reaction only.
Fig. 6 depicts the effects of the interactions between the variables baffle (presence or absence) and impeller type and between the variables impeller type and stirring speed as compared to the effect of impeller type alone, all of which provided significant results. Changing the variable impeller from the lower level (turbine) to the upper level (blade) and the stirring speed from the lower level (150 rpm) to the upper level (350 rpm) impacted soy biodiesel yields negavely—5%, 8%, 7%, and 5% at 0.5, 1, 1.5, and 2 min, respectively. The negative effect of the impeller enhanced at a stirring speed of 150 rpm.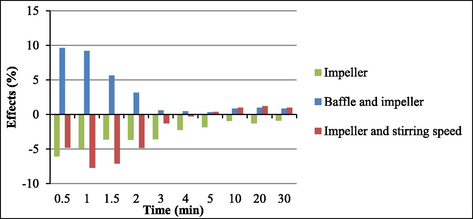
Main effect and interaction effects for impeller.
Fig. 7 illustrates the effects of the interactions between the variables stirring speed and baffle (presence or absence) and between the variables stirring speed and impeller type as compared to the effect of stirring speed alone. For the variable stirring speed, interaction effects were more significant than the main effect. The presence of baffle increased the positive effect of stirring speed at 0.5 and 1 min. At 1.5, 2, and 3 min, stirring speed of 150 rpm raised the negative effect of impeller type.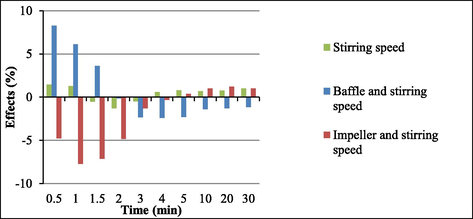
Main effect and interaction effects for stirring speed.
3.4 Reaction kinetics
Reaction kinetics were evaluated using first and second order models. The second order model (Eq. (2)) was the one that best fit the experimental data. The reaction rate was calculated based on the reduction of the fraction of oil mass.
Fractions of oil mass used to calculate the kinetic constants are presented in Table 7. The kinetic constants and R2 are presented in Table 8. For all reactions the R2 was equal or greater than 0.99. Higher values of kinetic constants indicate that the maximum oil yield for biodiesel was obtained faster. It was verified that the experiment 1 (without baffles, impeller turbine and 150 RPM) had the highest value of kinetic constant. Evaluating Table 6 it is possible to corroborate the result since a yield of 94.2% was obtained in t1. On the contrary, in experiments 2 (with baffles, impeller turbine and 150 RPM) and 7 (without baffles, Pitched blade and 350 RPM) the lowest kinetic constant values were obtained. Despite the fact that in the majority of the experiments more than 99% of yields were obtained at the end, the data of kinetic constant reinforce the conclusions already commented on full factorial design about the stirring and mixture.
Experiments
Time (min)
1
2
3
4
5
6
7
8
0
0.6548
0.6548
0.6548
0.6548
0.6548
0.6548
0.6548
0.6548
0.5
0.0376
0.2204
0.1465
0.1282
0.0884
0.0878
0.1844
0.1335
1
0.0076
0.1687
0.0702
0.0695
0.0095
0.0493
0.1315
0.0923
1.5
0.0050
0.1093
0.0441
0.0245
0.0108
0.0174
0.0923
0.0760
2
0.0095
0.0506
0.0441
0.0011
0.0069
0.0069
0.0617
0.0636
3
0.0115
0.0069
0.0460
0.0024
0.0069
0.0011
0.0265
0.0447
4
0.0102
0.0095
0.0421
0.0030
0.0050
0.0030
0.0082
0.0330
5
0.0069
0.0050
0.0402
0.0011
0.0056
0.0011
0.0011
0.0245
10
0.0050
0.0024
0.0317
0.0011
0.0063
0.0050
0.0030
0.0076
20
0.0011
0.0004
0.0330
0.0004
0.0050
0.0024
0.0024
0.0056
30
0.0050
0.0011
0.0304
0.0004
0.0050
0.0011
0.0024
0.0024
Experiments
K
R2
1
57.54
0.999
2
6.13
0.992
3
10.57
0.995
4
15.33
0.997
5
27.62
0.998
6
22.51
0.998
7
7.48
0.997
8
9.88
0.998
4 Conclusion
In the first few minutes of reaction, high oil yields in biodiesel have already been verified. In theory, baffles should improve mixing and stirring by enhancing contact between the reactants. However, baffles had no influence or even elicited undesired effects on transesterification. Turbine impeller was more effective than paddles. Higher stirring speed promoted better transesterification yields. The optimal conditions for soy biodiesel production were turbine impeller in the absence of baffle and stirring speed of 350 rpm.
Evaluation of soy biodiesel production process by dynamic experimental design provides valuable information about process optimization. Operation can be monitored during the transient and permanent regimes, to show in which of these periods the variables influence the process the most. Reaction kinetics reinforce the conclusions already commented about the influence of the stirring and mixture.
Acknowledgement
The authors thank National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Level -or Education- Personnel (CAPES), and Foundation for Research Support of the State of Alagoas (FAPEAL) for financial support.
References
- Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renewable Sustainable Energy Rev.. 2015;45:574-588.
- [CrossRef] [Google Scholar]
- Methyl ester synthesis of Pistacia khinjuk seed oil by ultrasonic-assisted cavitation system. Ind. Crops Prod.. 2017;108:336-347.
- [CrossRef] [Google Scholar]
- D 4052: Density and relative density of liquids by digital density meter. Am. Soc. Test. Mater. 2011
- [Google Scholar]
- D 6304: Test method for determination of water in petroleum products, lubricating oils, and additives by coulometric karl fisher titration. Am. Soc. Test. Mater. 2007
- [Google Scholar]
- D 445: Kinematic viscosity of transparent and opaque liquids (and the calculation of dynamic viscosity) Am. Soc. Test. Mater. 2006
- [Google Scholar]
- A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renewable Sustainable Energy Rev.. 2012;16:2070-2093.
- [CrossRef] [Google Scholar]
- The effects of catalysts in biodiesel production: a review. J. Ind. Eng. Chem.. 2013;19:14-26.
- [CrossRef] [Google Scholar]
- Cleaner production of rubber seed oil methyl ester using a hydrodynamic cavitation: optimisation and parametric study. J. Clean. Prod.. 2016;136:31-41.
- [CrossRef] [Google Scholar]
- Pilot scale intensification of rubber seed (Hevea brasiliensis) oil via chemical interesterification using hydrodynamic cavitation technology. Bioresour. Technol.. 2017;242:272-282.
- [CrossRef] [Google Scholar]
- Brasil, 2012. Resolução n° 7, de 19.03.2008 – DOU 20.3.2008. ANP Agência Nac. do Petróleo, Gás Nat. e Biocombustíveis.
- A review of cleaner intensi fi cation technologies in biodiesel production. Clean. Prod.. 2017;146:181-193.
- [CrossRef] [Google Scholar]
- Biodiesel production using oil from fish canning industry wastes. Energy Convers. Manag.. 2013;74:17-23.
- [CrossRef] [Google Scholar]
- Use of experimental design to investigate biodiesel production by multiple-stage Ultra-Shear reactor. Bioresour. Technol.. 2011;102:2672-2677.
- [CrossRef] [Google Scholar]
- Optimization of biodiesel production process for mixed Jatropha curcas–ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag.. 2016;115:178-190.
- [CrossRef] [Google Scholar]
- Optimization of mechanical agitation and evaluation of the mass-transfer resistance in the oil transesterification reaction for biodiesel production. Ind. Eng. Chem. Res.. 2009;48:7540-7549.
- [Google Scholar]
- Impacts of alcohol type, ratio and stirring time on the biodiesel production from waste canola oil. Afr. J. Agric. Res.. 2010;5:1851-1859.
- [CrossRef] [Google Scholar]
- Biodiesel production from Jatropha curcas: Integrated process optimization. Energy Convers. Manag.. 2014;80:1-9.
- [CrossRef] [Google Scholar]
- Métodos Físico-Químicos Para Análise de Alimentos. Inst. Adolfo Lutz; 2011.
- An optimization study on transesterification catalyzed by the activated carbide slag through the response surface methodology. Energy Convers. Manag.. 2015;92:498-506.
- [CrossRef] [Google Scholar]
- The effect of mixing on transesterification of beef tallow. Bioresour. Technol.. 1999;69:289-293.
- [CrossRef] [Google Scholar]
- Biodiesel production: a review1Journal Series #12109, agricultural research division, institute of agriculture and natural resources, university of nebraska-lincoln. 1. Bioresour. Technol.. 1999;70:1-15.
- [CrossRef] [Google Scholar]
- Experimental design and kinetic study of ultrasonic assisted transesterification of waste cooking oil over sulfonated carbon catalyst derived from cyclodextrin. J. Ind. Eng. Chem.. 2015;32:128-136.
- [CrossRef] [Google Scholar]
- Kinetics of transesterification of soybean oil. J. Am. Oil Chem. Soc.. 1997;74:1457-1463.
- [CrossRef] [Google Scholar]
- Experimental design applied for cost and efficiency of antioxidants in biodiesel. J. Am. Oil Chem. Soc.. 2014;91:1805-1811.
- [CrossRef] [Google Scholar]
- Multi-objective optimization of two alkali catalyzed processes for biodiesel from waste cooking oil. Energy Convers. Manag.. 2014;85:361-372.
- [CrossRef] [Google Scholar]
- Modeling of ultrasound assisted intensification of biodiesel production from neem (Azadirachta indica) oil using response surface methodology and artificial neural network. Fuel. 2015;143:262-267.
- [CrossRef] [Google Scholar]
- Response surface methodology for the optimization of biofuel production at a low molar ratio of supercritical methanol to used palm olein oil. ASIA-PACIFIC. J. Chem. Eng. 2016
- [CrossRef] [Google Scholar]
- The effect of agitation intensity on alkali-catalyzed methanolysis of sunflower oil. Bioresour. Technol.. 2007;98:2688-2699.
- [CrossRef] [Google Scholar]
- Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA-15 as a solid catalyst. Chem. Eng. J.. 2014;239:60-67.
- [CrossRef] [Google Scholar]
- Basic ionic liquid supported on mesoporous SBA-15 silica as an efficient heterogeneous catalyst for biodiesel production. Ind. Eng. Chem. Res.. 2015;54:1505-1512.
- [Google Scholar]
- Enzymatic production of biodiesel from soybean oil by using immobilized lipase on Fe3O4/Poly(styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy Fuels. 2014;28:2624-2631.
- [Google Scholar]
- Novel solid base catalyst for biodiesel production: mesoporous SBA-15 silica immobilized with 1,3-dicyclohexyl-2-octylguanidine. Renew. Energy. 2015;80:230-237.
- [CrossRef] [Google Scholar]
- Production of biodiesel by transesterification of soybean oil using calcium supported tin oxides as heterogeneous catalysts. Energy Convers. Manag.. 2013;76:55-62.
- [CrossRef] [Google Scholar]
- Optimal experimental design for an enzymatic biodiesel production system. IFAC-PapersOnLine. 2015;48:1258-1263.
- [CrossRef] [Google Scholar]







