Translate this page into:
Status of artificial sweeteners, glucose oxidase and some quality parameters of honey samples from the Asir region, Saudi Arabia
⁎Corresponding author at: Department of Chemistry, Faculty of Science, King Khalid University, Abha, Saudi Arabia. balshehre@kku.edu.sa (Badria M. AL-Shehri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Artificial sweeteners are low calorie polyhydric alcohols produced from carbohydrates or amino acids. Presence of artificial sweeteners in honey is not investigated before. Objective: this study measured the concentration of Xylitol and Sorbitol, the activity of glucose oxidase (GOX) and the concentration of glucose and acidity of honey samples from Asir region-Saudi Arabia. Furthermore, the values of pH and electric conductivity were determined.
Methods
The floral origin of the honey samples were confirmed through pollen analysis. The PH, electric conductivity (EC.), acidity, and glucose concentration were measured according to the methods of international honey commission. The glucose oxidase activity and the concentration of xylitol and sorbitol were determined according to the instructions of Megazyme International kits CAT.#: K-GLOX and CAT.#: K-SORB, respectively. The statistical package for social sciences (SPSS)-version 20 was applied to analyze the obtained results.
Results.
The floral origin analysis showed that the honey samples were Acacia (8) and Zizphus (4). The altitudes of the Acacia honey samples were 900 and 2000 m above sea level while the Zizphus honey samples were collected from 900 m above sea level. The studied quality parameters (Glucose, PH, Acidity, and EC.) were within the range of the CODEX standards and the Saudi Food and Drug Authority guide for honey. Irrespective of the altitude, the glucose oxidase activity in the Acacia honey samples was (4.7 ± 1.13) whereas its activity of the Zizphus honey was (5.0 ± 1.62). There was insignificant difference between the glucose activity of the Acacia and Zizphus honey samples (p-value = 0.7). The concentration of xylitol and sorbitol were not affected by the floral origin (p-value ≥ 0.17).
Conclusions.
The Acacia and Ziziphus honey are characterized by containing high amounts of sorbitol and trace xylitol content. The floral origin and year of harvest significantly affected pH, EC and Acidity of the honey samples.
Keywords
Sorbitol
Xylitol
Enzyme
Glucose
Altitude
Floral origin
- ANOVA
-
Analysis of Varience
- EC
-
Electrical Conductivity
- HMF
-
Hydroxymethylfurfural
- INT
-
Iodonitrotetrazolium
- NAD
-
Nicotineamide Adenine Dinucleotide
- SPSS
-
Statistical Package for Social Sciences
- W/ V
-
Weight/ Volume
Abbreviations
1 Introduction
Honey is a sweet substance that is modified and ripened by the honeybees from flower nectars, plant secretions or excretions of plant sucking insects in different states; liquid, viscous, semi crystalized or totally crystalized. The honey is composed majorly of sugars and water. Whereas, the minor component of honey includes organic acids, amino acids, proteins, dicarbonyl molecules, hydrogen peroxides, vitamins, phenolic acids, flavonoids and minerals. The quality parameters of honey include moisture, pH and free acidity, electric conductivity, glucose, fructose, sucrose, hydroxymethylfurfural (HMF), and diastase enzyme (Alimentarius 2019). The honey is well known to be active as an antioxidant, antimicrobial and anticancer. Furthermore, the honey is used for wounds and burns healing, immune system posting and for general wellbeing (Scepankova et al., 2017). The quality of honey and its biomedical activities are affected by the floral and geographical origins, climate conditions, altitude and honey harvest and treatment (Mohammed 2020).
Glucose oxidase (GOX) is an enzyme responsible for the conversion of glucose to gluconic acid and hydrogen peroxide. It is secreted by the honeybees and it contributes to the chemical composition and biomedical activity of honey. The glucose oxidase activity is affected by the honey storage and processing conditions beside the honeybee associated factors such as the species, health and nutrition of the honeybees (White et al., 1963, White Jr and Subers, 1964, Chen et al., 2012).
Xylitol and sorbitol are sweeteners produced from xylose and glucose by reduction reaction using the Raney nickel catalyst in alcoholic media, high temperature (130–190 °C) and a pressure of 9–26 bar (Poshala 1879, García et al., 2020). Moreover, sugar alcohols can be produced by plants and microorganisms such as bacteria, fungi and yeast (Sheet et al., 2014, Godswill 2017, Erian and Sauer 2021). It is reported that feeding honeybees with xylitol and sorbitol plays a role in the learning and memory of honeybees (Mustard et al., 2018).
This article investigated the effect of floral origin, year of harvest and altitude on the activity of glucose oxidase and the concentration of glucose, xylitol and sorbitol. Moreover, this article evaluated some honey quality parameters including moisture, conductivity, pH and acidity.
2 Material and methods
2.1 Honey samples
Twelve honey samples were collected from the honeybee farms and their floral origin was confirmed microscopically according the method of (Louveauxet al., 1978).The honey samples were from different floral origins (Acacia and Ziziphus), years of harvest and altitudes. The honey samples were collected through the years 2019, 2020 and 2021 (Fresh). The altitudes of the honey samples were 900 and 2000 m above sea level (Tables 1 and Table 2). *The values of the quality parameters of the honey samples were within the ranges of the honey standards of the International Honey Commission and the Saudi Food and Drug Authority. For the first time, this study reported the presence of sorbitol and xylitol in honey samples.
Mean ± STD
Honey samples
Moisture %
pH
EC µS/cm
Acidity meq NaOH /Kg
Glucose %
Sorbitol %
Xylitol %
GOX U/g
Acacia
Altitude
900n: 6
15.98 ± 1.19
4.35 ± 0.9
530 ± 456.2
39.5 ± 8.8
32.3 ± 3.9
1.14 ± 0.43
0.25 ± 0.9
5.26 ± 0.53
2000n: 2
15.75 ± 1.76
4.1 ± 0.42
42.5 ± 10.6
44 ± 12.7
31.8 ± 2.35
1.49 ± 0.03
0.33 ± 0.07
3.02 ± 0
Year
2019n: 2
15.75 ± 1.77
4.1 ± 0.42
42.5 ± 10.61
44 ± 12.7
31.81 ± 2.35
1.49 ± 0.03
0.33 ± 0.006
3.02 ± 0.0
2020n: 2
16.15 ± 1.91
5.5 ± 0.28
1045 ± 487.9
28.5 ± 4.95
27.61 ± 2.55
1.5 ± 0.7
0.33 ± 0.15
5.4 ± 0.05
2021n: 4
15.9 ± 1.07
3.78 ± 0.09
272.5 ± 47.87
45 ± 0.0
34.72 ± 1.14
0.96 ± 0.13
0.21 ± 0.03
5.19 ± 0.66
Ziziphus
Altitude
900n: 4
17.42 ± 0.98
5.19 ± 0.36
925 ± 93.27
34.6 ± 4.9
27.8 ± 2.68
1.45 ± 0.6
0.32 ± 0.012
5.0 ± 1.62
year
2019n: 2
17.65 ± 1.2
5.33 ± 0.25
985 ± 106.07
38 ± 0.0
27.56 ± 2.06
1.47 ± 0.06
0.32 ± 0.014
4.72 ± 1.54
2020n: 2
17.2 ± 1.12
5.05 ± 0.49
865 ± 21.21
31.25 ± 5.3
28.11 ± 4.12
1.43 ± 0.06
0.31 ± 0.014
5.28 ± 2.27
CODEX Standards
≤20–23
–
≤ 800–˃ 800
≤ 50
–
–
–
–
SFDA Standards
≤20–23
–
≤ 800–1200
≤ 50
–
–
–
–
Dependent variable
Sample 1
Sample 2
P-value
Mean pH value
Acacia 2019
Acacia 2020
0.002
Zizphus 2019
0.004
Zizphus 2020
0.014
Acacia 2021
Zizphus 2019
≤0.001
Zizphus 2020
0.001
Mean EC value
(µS/cm)Acacia 2019
Acacia 2020
0.001
Zizphus 2019
0.002
Zizphus 2020
0.004
Acacia 2020
Acacia 2021
0.002
Acacia 2021
Zizphus 2019
0.004
Zizphus 2020
0.009
Mean acidity value
(meq NaoH/Kg)Acacia 2019
Acacia 2020
≤0.001
Acacia 2021
≤0.001
Zizphus 2019
≤0.001
Zizphus 2020
≤0.001
2.2 Analysis of the quality parameters
The moisture, conductivity, pH and acidity of the honey were measured according to the method of International Honey Commission (Bogdanov, 2009). The moisture percentage was measured using the refractometer by putting one drop of honey on the prism and the moisture was read. The conductivity was measured in 20% (W/V) honey solution by the usage of conductometer after being calibrated with standard KCl solution (1413µS/cm). The pH of the honey samples was determined using a calibrated pH meter (pH 4, 7 and 9) in a 13.3% (W/V) honey solution. For the determination of the acidity the honey solution was titrated with 0.1 M sodium hydroxide solution and the end point of the titration was taken when the pH reached 8.30 (Bogdanov, 2009).
2.3 Measurement of GOX activity and the concentration of xylitol and sorbitol
The activity of the glucose oxidase enzyme was investigated following the instructions of the kit prepared by the megazyme international (code number CAT.#: K-GLOX). Likewise, the concentration of xylitol and sorbitol was measured according to kit of the megazyme international (code number CAT.#: K-SORB).
The basic idea of the Glucose oxidase kit is the reaction of the glucose oxidase in the honey samples with standard solution of glucose and the product (hydrogen peroxide) is reacted with peroxidase (POD) enzyme and a chromogen (p-hydroxybenzoic acid + 4-aminoantipyrine) to produce a colored precipitate (quinoneimine dye). Finally, the density of the dye color is determined at the wavelength of 500 nm. Solutions of 50% (W/V; one gram/ 2 mL) of honey samples were prepared and 0.5 mL from each sample was pipetted in a test tube. In as second tube 0.5 mL of standard glucose solution (90 mg/mL) and 2 mL of the peroxidase enzyme were mixed and their absorbance was read at 510 nm (A1) against blank tube (containing 0.5 mL of water instead of sample). After reading their absorbance the content of the first test tube were transferred to the content of the second test tube, mixed, incubated for 20 min and their absorbance was read at the same wavelength (A2). A standard curve was created using standard glucose oxidase solutions. From the standard curve, the line equation was calculated using the excel program and the equation was employed to calculate the glucose oxidase activity in the honey samples and multiplied by 200 to obtain the concentration in 100 g (%).
The sorbitol and xylitol kit contains sorbitol dehydrogenase and diaphorase enzymes. The sorbitol dehydrogenase converts the sorbitol and xylitol to fructose and xylose, respectively. The sorbitol dehydrogenase needs NAD as cofactor and reduces it to NADH. The diaphorase catalyzes the reduction of iodonitrotetrazolium chloride (INT) to an INT-formazan colored complex which is measured at the wavelength of 492 nm. The sample cuvette was containing 0.1 mL of 50% (W/V) honey solution, 2 mL of distilled water, 0.5 mL of buffer solution (pH 8.6), 0.2 mL of NAD+/INT and 0.02 mL of diaphorase. The blank cuvette contained all the contents of the sample cuvette except that the sample amount was replace by distilled water. The absorbance of the mentioned contents was measured at 492 nm (A1). Finally, 0.05 mL of sorbitol dehydrogenase was added to the sample and blank cuvettes and the absorbance (A2) was read after the end of the reaction (15 to 20 min). The concentration of sorbitol (g\L) in the 50% honey solution was calculated following the equation (0.2627 × ΔA) while the concentration of xylitol (g/L) was determined according to the equation (0.2194 × ΔA). The results of the sorbitol and xylitol were multiplied by 200 to obtain their amount in 100 g of honey (%).
2.4 Statistical analysis
The obtained data were analyzed using the statistical package for social sciences (SPSS). The ANOVA test was used to investigate the effect of floral and geographical origin, altitude and season. The hierarchical agglomerative cluster analysis was used to classify the honey samples according to the results of the studied parameters compared to their classification according to their floral origin, altitude and year of harvest.
2.4.1 Data availability statements
All data generated or analyzed during this study are included in this published article and its supplementary information files.
3 Result
3.1 3.1-Results of the floral origin
The microscopic analysis showed that the honey samples were from two floral origins; Acacia (8 samples) and Zizphus (4 samples) [Fig. 1].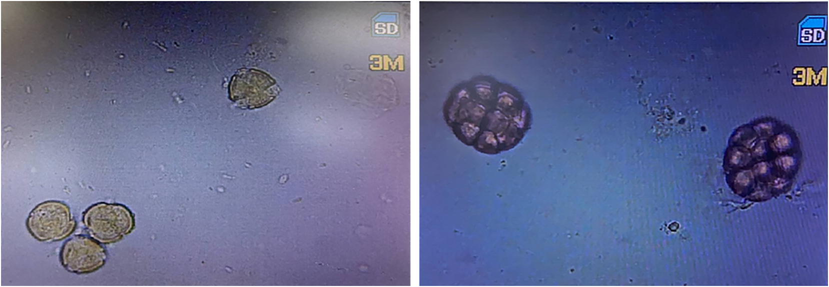
Representative figure for the Acacia and Ziziphus pollens in the honey samples.
3.2 Results of the studied parameters
Regarding the standard curve of the GOX, the equation of the straight line was (Y = 0.079X + 0.0024) and R2 was 0.98.
The concentration range of sorbitol and xylitol were (0.78–2.0%) and (0.17–0.44%), respectively while the range of the GOX activity was (3.02–6.89 U/g). The results of the quality parameters were within the standards of international honey commission and the Saudi standards for honey (Codex Alimentarius 2019, Msolla 2021).The obtained results showed that the floral origin and year of harvest had significant effects on the pH, EC and acidity (Table 1 and Table 2).
3.3 Hierarchical agglomerative cluster analysis
The hierarchical agglomerative cluster analysis showed that the classification of the honey samples according to the studied parameters was slightly different from the classification according the floral origin and year of harvest [Fig. 2].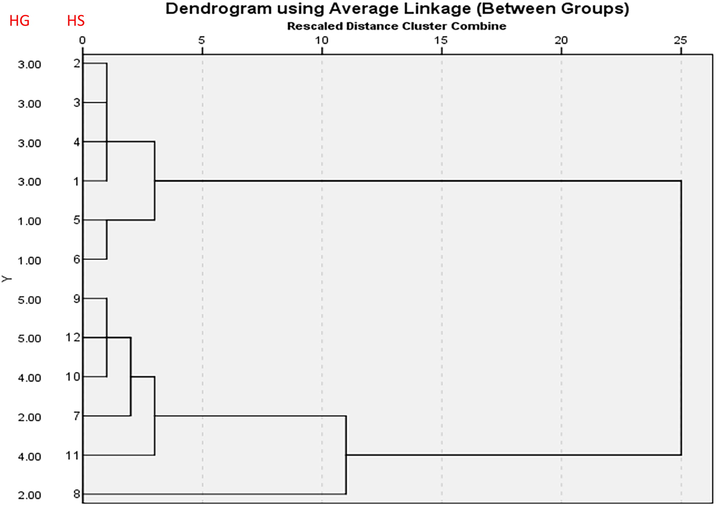
The hierarchical agglomerative clustering of the honey samples according the results of the studied parameters. The percentage of clustering was 83.3% (10 out of 12). HS: Honey Sample; HG: Honey Group.
At the first level all the samples were grouped similar to the grouping of the floral origin, altitude and year of harvest. At level 2, sample 12 (Ziziphus 2020) and sample 7 (Acacia 2020) were grouped together which is against the classification of the floral origin, altitude and year of harvest. At level three, there were two groups. Samples 4 and 5 are classified in one group and the two were Acacia honey samples from the 2021 and 2019, respectively. The second group of level three contained four samples, 10, 11, 12 and 7. The 10, 11 and 12 were Ziziphus honey samples harvested in 2019 and 2020 while the sample number 7 was Acacia harvested in 2020. Level 4 of the hierarchical agglomerative clustering was composed of one group containing six honey samples, 7, 8, 9, 10, 11 and 12. The honey samples number 7, 8 and 9 were Acacia honey samples whereas the samples number 10, 11 and 12 were Ziziphus honey samples. The last level contained all the honey samples except samples numbers 2 (Acacia 2021) and 6 (Acacia 2019).
The classification of the honey samples according to the results of the studied parameters was efficient by 83.3% (10 out of 12). The difference may be due to the fact that each honey sample contains more than one pollen type.
4 Discussion
The PH, electric conductivity, and acidity were significantly affected by the storage conditions and floral origin while the other parameters were not affected.
The range of the glucose oxidase activity of the Acacia honey was (3.02–5.93 U\g) while that of the Ziziphus honey samples was (3.63–6.89 U\g). The floral origin, altitude and year of harvest had insignificant effects on the mean values of the GOX activities. This study measured the enzyme activity of GOX in International units which measure the transformation of one micromole of glucose in one minute per gram of honey while the published literature measured the GOX activity by the micrograms of the hydrogen peroxide produced in one hour per gram of honey (Strelec et al., 2018, Sahin et al., 2020), or measured the amount of the enzyme in micrograms of enzyme per gram of honey (Bucekova et al., 2019).This study reported that the floral origin, year of harvest (storage at 4 °C) and altitude did not affect the glucose oxidase activity. Such these previously reports mentioned that the floral origin has major effect on the activity of glucose oxidase in honey (Belay et al., 2017, Strelec et al., 2018). Storage of honey at high or low temperature (50 °C or 5 °C) is reported to affect the activity of honey enzymes while storage at room temperature had no effect on the activity of honey enzymes (Tulandi, 2019).
To our knowledge, this the first study that evaluated the concentration of sorbitol and xylitol in honey samples. The concentration range of sorbitol and xylitol were (0.78–2.0%) and (0.17–0.44%), respectively. The sorbitol and xylitol may be of plant origin or produced by honey microbes (Sheet et al., 2014, Erian and Sauer 2021). Medically, sorbitol is used as laxative, cathartic and diuretic medicine while the xylitol is famous by its ability to reduce the dental caries through inhibition of Streptococcus mutant growth and adhesion to the tooth surface (Janakiram et al., 2017, Association 2020).The laxative effect of some honey samples may be due to their content of sorbitol and the honey could contribute to the inhibition of dental caries because it contains trace amount of xylitol.
The range of the moisture percentage in the honey samples of this study was (13.95%–20.25%). The results were within the moisture range of honey according to the Codex standards and according to the guide to the production, trading and import of honey and bee products issued by the Saudi Food and Drug Authority (2021) (Alimentarius 2019, Msolla 2021). The previously published articles showed that the moisture percentage of the Saudi honey was ranging from 8.8 to 18.5% (Mesallam and El-Shaarawy 1987, Ahamed et al., 2017, Al-Ghamdi et al., 2019). This study found that the floral origin in Jazan region had significant effect on the percentage of moisture similar the finding of (Corbella and Cozzolino 2006, Khan et al., 2021).
The range of the pH of the honey samples was (3.6–7.2). The upper limit of the pH range was slightly higher than the previously reported pH values in Saudi Arabia (5.7) (Ahamed et al., 2017, Khan et al., 2021). The US national honey Board (2005) adopted pH range for honey samples from 3.4 to 6.1 (Pavlova et al., 2018). With regard to the effect of floral and geographical origin on the pH of honey, this study reported that the Ziziphus honey of Jazan region had significantly increased pH value compared to the polyfloral honey of Jazan. The polyfloral honey of Asir region had significantly increased pH value compared to the pH of the polyfloral honey from Jazan region. Similar to our findings, reported significant effects of the floral and geographical origins on the value of the honey pH (Ahamed et al., 2017, Khan et al., 2021).
The conductivity range of the honey samples of this study was (35–1420µS\cm) and the floral and geographical origins had insignificant effects on the conductivity values. According to the Codex Alimentarius (2019), the conductivity of honey depends on the floral origin, some honey samples are with conductivity of not more than 800 µS\cm while others are with conductivity of not less than 800 µS\cm (Codex Alimentarius 2019). One previous study reported very high conductivity values for honey samples from Egypt and Yemen ranged from 0.53 ± 0.03 to 4.18 ± 0.05 mS/cm (El Sohaimy et al., 2015).
A recent study from India showed that storage of honey increased its pH, moisture, conductivity and HMF while it decreased the proline concentration and the activity of diastase and invertase enzymes (Bhalchandra et al., 2022).
The minimum and maximum values of the acidity of the honey samples were 25 and 53 meq NaOH /100 g. The upper limit of the honey acidity exceeded the range of the honey acidity approved by the Codex Alimentarius (2019), which may be due to the floral origin, climate or storage conditions. This study reported significant effects of the floral origin and storage on the honey acidity. Some of the previous studies concluded that the floral origin has significant effect on the acidity of honey (Shamsudin et al., 2019, Adgaba et al., 2020, Ng et al., 2021).
The range of the glucose concentration in the honey samples of this study was (25.2%–35.4%). Our results are similar to the previously published literature which showed that the glucose concentration in the honey is ≤ 38% (Nguyen et al., 2019, Ghramh et al., 2020).
This study is limited due the small number of samples as a whole and in the subgroups. Future studies should consider increasing the number of samples and parameters.
5 Conclusions
Acacia and Ziziphus honey samples contain small amounts of sorbitol and xylitol. The GOX activity and the concentration of sorbitol and xylitol were not affected by the floral origin or the year of harvest neither the altitude. The pH, EC and acidity were significantly affected by the floral origin and year of harvest.
Funding
The authors appreciate the support of the research center for advanced materials science (RCAMS) at King Khalid University Abha, Saudi Arabia through a project number KKU/RCAMS/22.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Physico-chemical, antioxidant and anti-microbial properties of some Ethiopian mono-floral honeys. Saudi J. Biol. Sci.. 2020;27(9):2366-2372.
- [Google Scholar]
- Some physiochemical properties of Acacia honey from different altitudes of the Asir Region in Southern Saudi Arabia. Czech J. Food Sci.. 2017;35(4):321-327.
- [Google Scholar]
- Comparison of physicochemical properties and effects of heating regimes on stored Apis mellifera and Apis florea honey. Saudi J. Biol. Sci.. 2019;26(4):845-848.
- [Google Scholar]
- Association, A. A. P. (2020). National center for biotechnology information. pubchem compound summary for CID 12699, N-Nitroso-N-methylurea. retrieved 24.
- Effect of storage on various honey quality parameters of Apis mellifera honey harvested from Kannad region, Aurangabad. J. Pharmacog. Phytochem.. 2022;11(1):239-246.
- [Google Scholar]
- Enzyme activity, amino acid profiles and hydroxymethylfurfural content in Ethiopian monofloral honey. J. Food Sci. Technol.. 2017;54(9):2769-2778.
- [Google Scholar]
- Harmonized methods of the International Honey Commission. Internat. Honey Comm. 2009:16-23.
- [Google Scholar]
- Antibacterial activity of different blossom honeys: New findings. Molecules. 2019;24(8):1573.
- [Google Scholar]
- The effect of standard heat and filtration processing procedures on antimicrobial activity and hydrogen peroxide levels in honey. Front. Microbiol.. 2012;3:265.
- [Google Scholar]
- Codex Alimentarius. (2019). “International Standard for Honey CXS 12-19811 Adopted in 1981.” Amended in 2019: 1-8.
- Classification of the floral origin of Uruguayan honeys by chemical and physical characteristics combined with chemometrics. LWT-Food Sci. Technol.. 2006;39(5):534-539.
- [Google Scholar]
- Physicochemical characteristics of honey from different origins. Ann. Agric. Sci.. 2015;60(2):279-287.
- [Google Scholar]
- Utilizing yeasts for the conversion of renewable feedstocks to sugar alcohols-a review. Bioresour. Technol.. 2021;126296
- [Google Scholar]
- Production of sorbitol via catalytic transfer hydrogenation of Glucose. Appl. Sci.. 2020;10(5):1843.
- [Google Scholar]
- Quality evaluation of Saudi honey harvested from the Asir province by using high-performance liquid chromatography (HPLC) Saud. J. Biol. Sci.. 2020;27(8):2097-2105.
- [Google Scholar]
- Sugar alcohols: chemistry, production, health concerns and nutritional importance of mannitol, sorbitol, xylitol, and erythritol. Int. J. Adv. Acad. Res. 2017;3:31-66.
- [Google Scholar]
- Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med.. 2017;8(1):16.
- [Google Scholar]
- Tolerance of Ziziphus and Acacia honeys to one year storage conditions and altitude. J. King Saud Univer.-Sci.. 2021;33(7):101577
- [Google Scholar]
- Factors affecting the physicochemical properties and chemical composition of bee’s honey. Food Rev. Internat. 2020:1-12.
- [Google Scholar]
- Honey Value Chain Development in Ruvuma Region. Tanzania: Sokoine University of Agriculture; 2021.
- Nutritional value and taste play different roles in learning and memory in the honey bee (Apis mellifera) J. Insect Physiol.. 2018;107:250-256.
- [Google Scholar]
- Botanical origin differentiation of malaysian stingless bee honey produced by Heterotrigona itama and Geniotrigona thoracica Using Chemometrics. Molecules. 2021;26(24):7628.
- [Google Scholar]
- Honey and its role in relieving multiple facets of atherosclerosis. Nutrients. 2019;11(1):167.
- [Google Scholar]
- Poshala, K. K. (1879). “Artificial Sweeteners: A Review.”.
- Investigation of variations of invertase and glucose oxidase degrees against heating and timing options in raw honeys. J. Chem. 2020
- [Google Scholar]
- Honey Health Benefits And Uses In Medicine. Bee Products-Chemical and Biological Properties. Springer; 2017. p. :83-96.
- A comparative characterization of physicochemical and antioxidants properties of processed Heterotrigona itama honey from different origins and classification by chemometrics analysis. Molecules. 2019;24(21):3898.
- [Google Scholar]
- Some alternative sweeteners xylitol, sorbitol, sucralose and stevia. Karaelmas Fen ve Mühendislik Dergisi. 2014;4(1):63-70.
- [Google Scholar]
- Glucose oxidase activity and hydrogen peroxide accumulation in Croatian honeys. Croat. J. Food Sci. Technol.. 2018;10(1):33-41.
- [Google Scholar]
- The effect of storage temperature on the quality of honey. Sanitas. 2019;10(1):59-71.
- [Google Scholar]
- Studies on honey inhibine. 4. Destruction of the peroxide accumulation system by light. J. Food Sci.. 1964;29(6):819-828.
- [Google Scholar]
- The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA)-Spec. Sect. Enzymol. Subj.. 1963;73(1):57-70.
- [Google Scholar]







