Translate this page into:
Statistical analysis of the hydrogeochemical evolution of groundwater in hard and sedimentary aquifers system of Gadilam river basin, South India
*Corresponding author. Tel.: +60 85 443928; fax: +60 85 443837 geoprasanna@rediffmail.com (M.V. Prasanna),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The study of groundwater hydrogeochemistry of hard and sedimentary aquifers system in Gadilam river basin has resulted in a large number of geochemical dataset. Groundwater samples were collected at 41 sites over spread of 1380 km2 study area and analysed for major ions. The large number of data can lead to difficulties in the integration, interpretation and representation of the results. Application of statistical analysis of the data helps us to unravel the hidden relationship between ions. Correlation analyses and factor analyses were applied to classify the groundwater samples, and to identify geochemical processes controlling groundwater geochemistry. The correlation analysis helps in the determination of the spinal and the seasonal species (ions). Calcium (Ca2+), Magnesium (Mg2+), Sodium (Na+), Chloride (Cl−), Bicarbonate and Sulphate were determined as spinal species and Potassium (K+), Phosphate and Silica (H4SiO4) as the seasonal species. Factor analysis shows that dissolution and leaching of secondary salts, weathering and anthropogenic impacts are the dominant controlling factors in the study area. Though several factors were extracted for different seasons to identify the dominant hydrogeochemical regime of the study area, first three dominant factors were spatially distributed by their factor scores. This spatial representation of the factor scores show that part of the region is hydrogeochemically active.
Keywords
Hydrogeochemistry
Correlation
Factor analysis
Factor score
Gadilam river basin
1 Introduction
The chemistry of water is an important factor determining its use for domestic, irrigation or industrial purposes. The quality of groundwater is controlled by several factors, including climate, soil characteristics, manner of circulation of groundwater through the rock types, topography of the area, saline water intrusion in coastal areas, human activities on the ground, etc (Rajesh et al., 2002; Lakshmanan et al., 2003; Srivastava, 2005; Das Brijraj and Kaur, 2007; Cloutier et al., 2008). As the study area consists of both hard and sedimentary rocks, the groundwater potential is governed by several factors like weathering, anthropogenic impact and saline water intrusion. Groundwater samples were collected and analysed for major ions in different seasons. In order to handle this large data set and to get a reliable interpretation, application of statistics is essential. Multivariate statistical analysis has been successfully applied in a number of hydrogeochemical studies. Steinhorst and Williams (1985) used multivariate statistical analysis of water chemistry data in two field studies to identify groundwater sources. In their application of multivariate analysis to chemical data, Usunoff and Guzmán-Guzmán (1989) demonstrated the usefulness of the approach in hydrogeochemical investigations when considering the geological and hydrogeological knowledge of the aquifer. Melloul and Collin (1992) used PCA to supplement classical geochemical methods such as Scholler and Piper diagrams and successfully identified major water groups and factors affecting groundwater quality in an aquifer. Schot and Van der Wal (1992) applied principal components and clusters analysis to hydrochemical data to show the regional impact of human activities on groundwater composition. In the study of Farnham et al. (2003), the application of multivariate statistical analysis to trace element chemistry of groundwater helped identify rock–water interaction processes and groundwater redox conditions. All the hydrogeochemical studies mentioned above show that multivariate statistical analyses significantly help to classify groundwater and identify major mechanisms influencing groundwater chemistry. When the hydrogeochemical interpretation is combined with the knowledge of the geological and hydrogeological setting, multivariate statistical methods can also help to understand groundwater flow in complex aquifer systems (Farnham et al., 2000; Stetzenbach et al., 2001).
Few geochemical studies were carried out in the Gadilam river basin by Prasanna et al. (2008a) and (2009) to understand the hydrogeochemical nature of the groundwater. There has been limited attempt to study the statistical nature of these groundwaters. Hence, the main objective of this study is to enumerate the usage of factor score in identification of the hydrogeochemically active regimes represented by the major factors. Relationship among dissolved ions present in water may vary with seasons and different lithologies. Certain ions maintain the relationship with season and some do not. Such relationships have been enlightened by the use of the correlation analysis. An attempt has also been made to note the seasonal variation of the factor representations in the study area. This study also illustrates the usefulness of statistical analysis to improve the understanding of groundwater systems.
2 Study area
Area chosen for study is Gadilam river basin, which is located in Cuddalore and Villupuram district, Tamilnadu, India. The area is bounded between latitudes 11°30′n–11°55′n and longitudes 79°0′e–79°47′e. It covers a total area of about 1394 sq km (Fig. 1). The basin lies between the Ponnaiyar river basin in the north and Velar river basin in the south. Gadilam river basin covers different stratigraphic units viz. Archaean, Cretaceous, Tertiary to recent Alluvium (Fig. 2) (Muthukrishnan, 1993; CGWB, 1997a, b). Archaean complex consists of garnetic gneiss, charnockite, leptinites and schists. Limestone constitutes the cretaceous formation and Cuddalore sand stone of the tertiary age are the litho units of the area. The western part of the study area is composed of hard rock and the eastern part by sedimentary formation. It is inferred as a faulted contact between the hard rock and the sedimentary formation (Aravindan et al., 2004). The average annual rainfall of the basin is about 1643 mm. The water level ranges from 3.10 to 98.85 mbgl (below ground level) with an average of 62.37 bgl. Gadilam river originates in the hard rock region and flows through the sedimentary terrain. The important large scale extraction sites in this basin are Neyveli Lignite Corporation (NLC) and the boreholes of New Veeranam Scheme (NVS). The open cast mining of Lignite requires heavy pumping at the rate of 9000–10,000 m3 hr−1 as water table condition has to be brought down below the level of mining (Anandhan, 2005). Water from the Veeranam Lake is supplied through transmission line to Chennai city. To augment the supply during summer season, 48 deep bore wells were drilled and operations are done alternatively to pump the groundwater from deep aquifer and the pumped water is connected to the New Veeranam Scheme (NVS) pipelines. Apart from these large scale extraction features, an industrial estate SIPCOT (Small Industries Promotion Corporation of Tamilnadu) with groups of industries, which generate multi facet chemicals and raw materials are distributed along the downstream of the river Gadilam, near the coast at Cuddalore (Prasanna, 2008).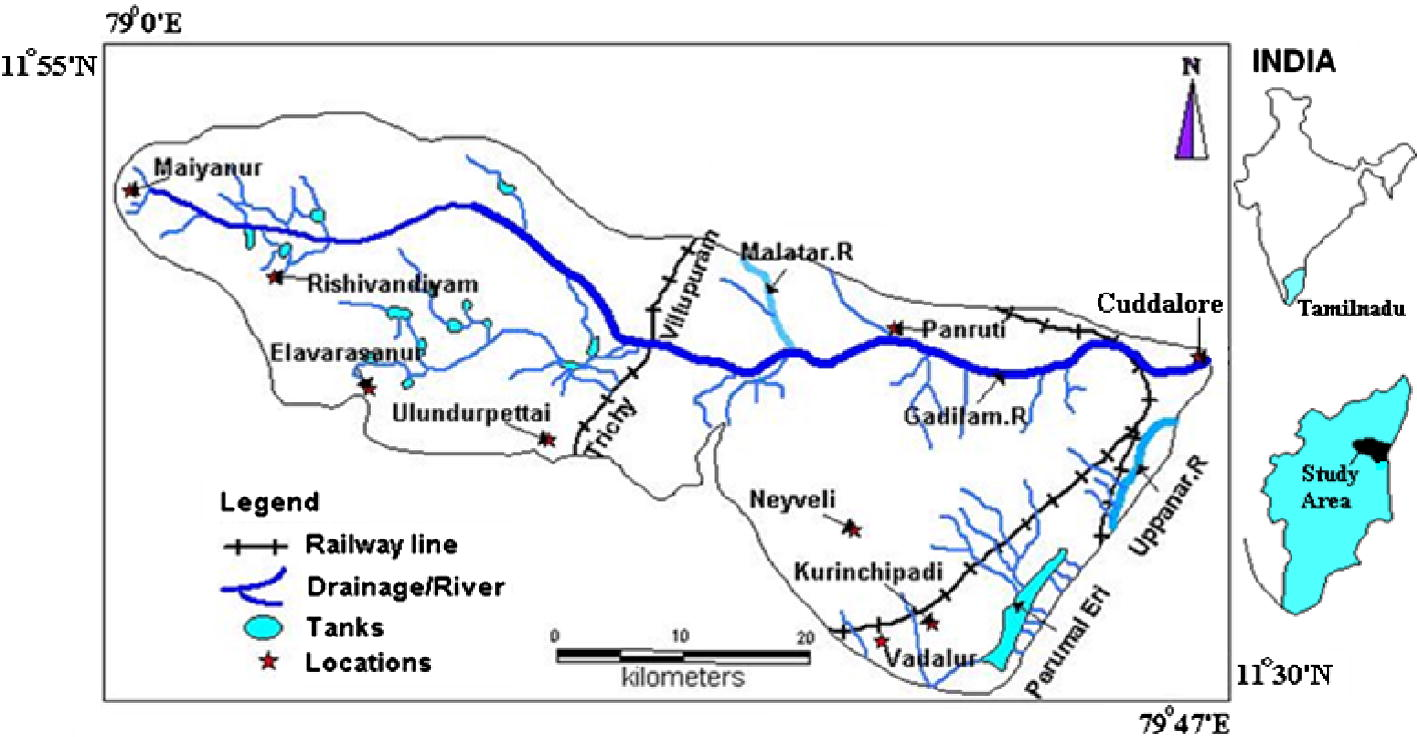
Location map of the study area.
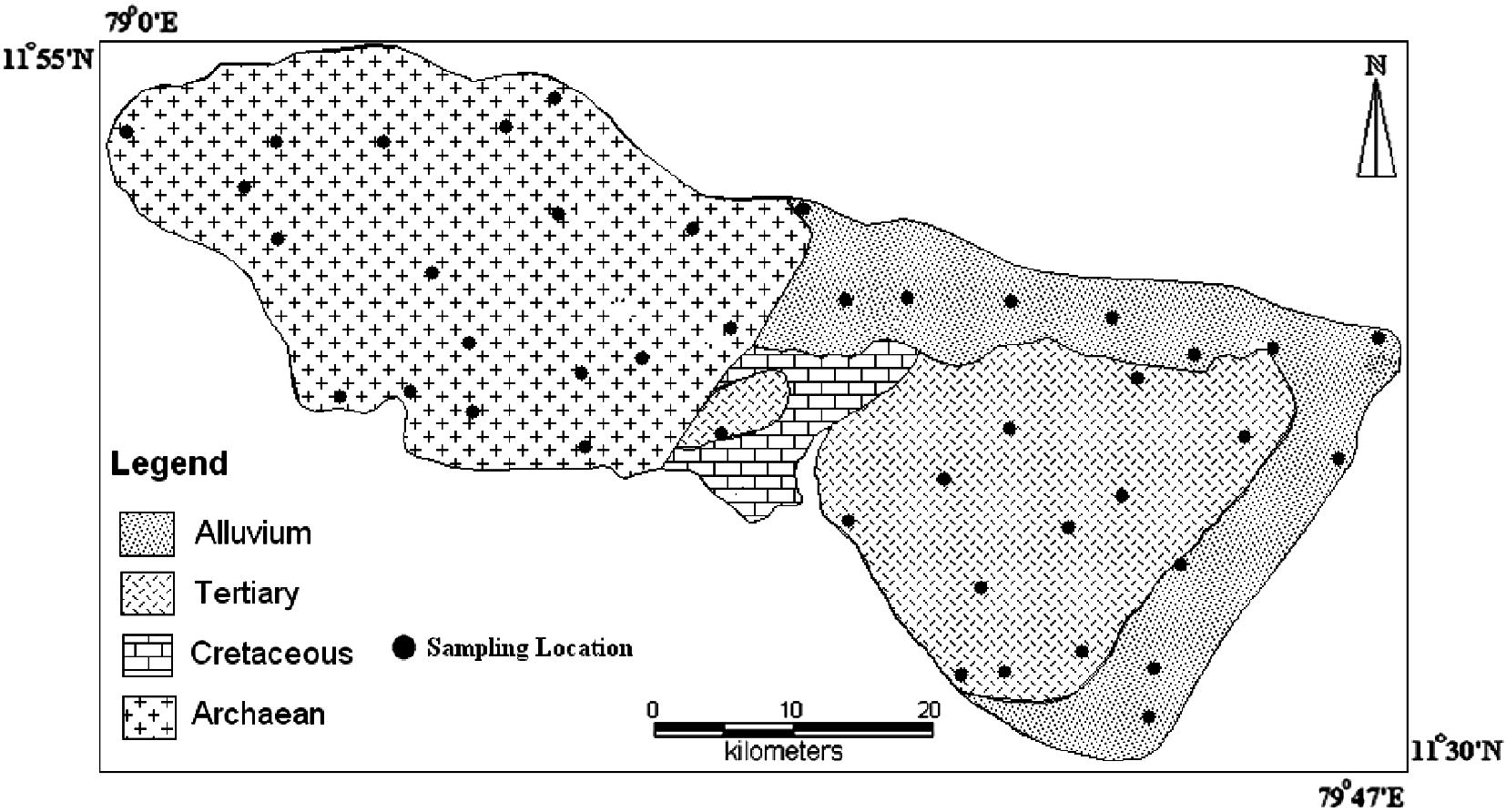
Geology and sampling location map.
3 Materials and methods
Groundwater samples were collected during March 2005, July 2005, January 2006 and November 2006 to broadly cover seasonal variation. A total of 156 groundwater samples were collected from bore wells (Fig. 2) in four different seasons (November 2006 represents NE monsoon; January 2006 represents Post monsoon; March 2005 represents Summer; July 2005 represents SW monsoon) to cover different litho units of the study area. In summer (SUM) and south west monsoon (SWM), 12 groundwater samples was collected from Alluvium, 10 groundwater samples from Tertiary and 14 groundwater samples from Archaean. In north east monsoon (NEM) and post monsoon (POM), 12 groundwater samples was collected from Alluvium, 12 groundwater samples from Tertiary and 17 groundwater samples from Archaean. The samples collected were analysed for major cations like, Ca and Mg by Titrimetry, Na and K by Flame photometer (CL 378); anions, Cl and HCO3 by Titrimetry, SO4, PO4, and H4SiO4 by Spectrophotometer (SL 171 minispec). EC and pH were determined in the field using electrode (Eutech). TDS was also measured in situ by TDS portable electrode model TDS Testr11+ (multi range). The analyses were done by adopting standard procedures (APHA, 1998). The average temperature at the time of sampling varies from 25 to 31 °C. The analytical precision for the measurements of cations and anions was determined by calculating the ionic balance error that varies by about 5–10% (Freeze and Cherry, 1979).
3.1 Data analysis
Each sampling site is characterized by a large number of chemical and physical variables, making the regional hydrogeochemical study a multivariate problem. The multivariate statistical analysis is a quantitative and independent approach of groundwater classification allows to group of groundwater samples and the making of correlations between chemical parameters and groundwater samples. In this study correlation analyses and factor analyses were applied using Statistical Package of Social Studies (SPSS) version 10.
Data obtained from the laboratory analysis were used as variable inputs for factor analysis. Preparation of a correlation matrix of the data from which initial factor solutions were extracted by the principal component analytical method. Factor extraction was done with a minimum acceptable Eigen value as 1 (Kaiser, 1958; Harmann, 1960). Orthogonal rotation of these initial factors to terminal factor solutions was done with Kaiser’s varimax scheme. This method maximizes the variance of the loadings on the factors and hence adjusts them to be either near ±1 or near 0 (Davis, 1973). Factor score coefficients are derived from the factor loadings. Factor scores are computed for each sample by a matrix multiplication of the factor score coefficient with the standardized data. The value of each factor score represents the importance of a given factor at the sample site. A factor score >+1 indicates intense influence by the process. Very negative values (<−1) reflect areas virtually unaffected by the process while near zero scores reflect areas with only moderate effect of the process. The spatial distribution of the factors can be assessed by a contour of the factor scores representing each factor.
4 Results and discussion
The summary of the analytical results of groundwater in the study area is represented in Table 1, which shows the average, maximum, minimum and standard deviation values for different seasons. The total cations (TZ+) and total anion (TZ−) balance (Freeze and Cherry, 1979) is considered to shows the charge balance error percentage. The error percentage in the samples of the present study ranges between ±1% and ±10%. Occurrence of errors in chemical analysis of groundwater is also due to the reagents employed, limitations of the methods and the instruments used presence of impurities in distilled water etc. The correlation coefficient between TZ+ and TZ− is around 0.6–0.9. TDS/EC ratio was ranging from 0.5 to 0.9. The role played by other ions than those considered here for the cations and anions charge balance is less significant. The groundwater in the study area is colorless and odorless in most of the places. Results of the four seasons were preceded by WATCLAST (Chidambaram et al., 2003). The dominant hydrogeochemical facies in the entire lithounits is Na–Cl indicating saline nature in the groundwater (Prasanna, 2008). Max – maximum, Min – minimum, Avg – average, Std – standard deviation, F. Avg – final average.
Summer
South West monsoon
North East monsoon
Post monsoon
F. Avg.
Max
Min
Avg
Std
Max
Min
Avg
Std
Max
Min
Avg
Std
Max
Min
Avg
Std
Alluvium
pH
7.30
6.60
6.99
0.21
8.65
6.40
7.29
0.65
7.83
6.01
7.17
0.61
9.20
6.83
8.15
0.91
7.40
EC
6337.31
420.18
1424.31
1605.68
6278.93
387.00
1543.82
1538.59
6728.00
468.00
1829.58
1645.38
6005.00
345.00
1652.59
1621.56
1612.58
Cl
2384.01
62.03
347.77
646.29
1994.06
26.59
357.67
526.91
2782.82
96.42
498.17
734.87
2375.15
51.00
393.60
639.42
399.30
HCO3
677.10
48.79
289.84
241.36
1293.20
91.50
397.77
334.67
634.39
54.30
270.75
151.37
1500.59
12.20
271.44
399.28
307.45
SO4
341.00
23.00
96.28
84.30
189.00
27.00
90.23
39.78
368.00
1.00
69.62
106.03
312.20
5.00
107.21
85.42
90.83
PO4
10.10
0.25
6.08
3.76
6.80
0.10
4.20
2.55
6.60
0.01
1.25
1.92
6.80
0.00
0.90
1.97
3.11
H4SiO4
34.00
8.10
19.02
9.15
50.00
1.60
30.06
14.15
147.50
8.80
58.60
36.30
160.00
27.00
73.92
40.62
45.40
Ca
261.33
11.00
64.11
69.41
264.00
16.00
103.14
76.12
352.00
6.00
64.33
93.63
168.00
10.00
50.16
41.09
70.44
Mg
91.20
1.00
14.47
25.00
62.39
0.00
17.09
17.51
134.40
1.00
27.51
35.31
124.80
4.79
19.50
33.78
19.64
Na
1210.03
81.00
279.94
310.56
1400.20
49.30
267.35
365.38
1091.95
74.71
317.34
274.98
1149.43
52.00
293.71
307.29
289.59
K
42.00
3.00
11.71
11.11
48.71
3.00
13.61
12.21
36.40
1.00
15.14
10.46
108.40
1.00
25.21
32.74
16.42
TDS
4436.44
267.81
994.45
1122.35
4395.25
271.00
1080.07
1077.38
4710.00
328.00
1291.44
1149.91
4204.08
242.00
1157.33
1134.97
1130.82
Tertiary
pH
7.30
6.10
6.69
0.35
8.52
6.00
7.36
0.87
7.50
5.56
6.68
0.57
8.60
6.21
7.76
0.72
7.12
EC
867.00
250.00
492.93
214.50
1280.00
197.00
543.72
330.01
822.00
165.00
448.75
220.10
981.00
286.21
476.58
198.16
490.49
Cl
241.05
26.59
84.19
64.26
239.29
8.86
89.69
73.97
194.97
8.00
81.67
52.13
255.23
44.00
95.32
61.99
87.71
HCO3
274.50
18.30
72.51
74.60
323.30
36.60
100.65
81.60
195.19
12.20
84.36
55.25
195.10
24.40
72.52
46.72
82.51
SO4
171.00
28.00
89.10
52.89
247.00
18.00
98.60
66.33
196.00
0.01
50.29
63.45
141.00
7.00
46.89
38.38
71.22
PO4
10.10
0.07
3.92
4.69
6.40
0.15
2.36
2.40
7.45
0.09
1.83
2.57
1.40
0.00
0.30
0.42
2.10
H4SiO4
69.00
18.10
31.62
17.51
54.00
7.30
27.16
15.29
72.40
8.00
39.37
18.93
120.00
18.00
68.38
27.02
41.63
Ca
55.99
8.00
24.90
14.18
88.00
10.00
36.00
27.36
40.00
6.00
23.16
11.83
34.00
10.00
22.08
7.78
26.54
Mg
9.00
0.00
3.40
3.13
33.60
1.00
8.48
10.03
14.40
0.00
6.51
4.35
14.40
1.00
6.40
4.20
6.20
Na
195.40
45.98
91.53
51.13
272.00
1.80
82.49
83.15
191.00
6.00
69.36
61.11
183.91
22.99
72.30
45.89
78.92
K
14.00
0.00
4.60
4.70
21.90
1.00
7.01
6.71
14.60
1.00
7.68
3.89
23.90
1.00
7.41
5.71
6.68
TDS
646.27
175.00
349.06
158.05
897.22
138.76
380.88
231.05
633.30
122.60
319.83
162.71
687.00
200.35
329.56
140.48
344.83
Archaean
pH
8.10
6.73
7.14
0.33
8.00
6.80
7.14
0.32
8.60
6.78
7.46
0.41
9.20
8.10
8.52
0.32
7.56
EC
2610.32
657.13
1500.32
580.51
2725.89
618.29
1498.53
642.83
2810.00
617.00
1364.24
591.00
2574.00
490.37
1129.36
583.25
1373.11
Cl
771.03
59.03
342.01
225.96
771.03
53.17
361.46
248.08
638.10
35.44
233.55
152.64
620.37
35.45
229.38
168.76
291.60
HCO3
524.60
284.50
425.76
90.61
579.50
274.50
416.11
92.70
414.79
183.00
278.44
84.44
268.39
109.80
185.15
48.27
326.36
SO4
124.60
43.00
70.97
21.56
158.20
53.40
87.03
28.66
336.22
1.50
67.27
98.55
288.18
4.00
116.98
82.67
85.56
PO4
15.00
4.80
8.76
2.75
6.40
3.80
5.36
0.81
3.60
0.20
0.75
1.01
1.60
0.00
0.18
0.40
3.76
H4SiO4
63.00
5.80
27.10
21.81
53.00
21.20
39.63
10.19
94.00
6.60
50.08
28.69
120.00
22.00
72.82
28.03
47.41
Ca
225.99
35.99
108.14
50.11
240.00
55.99
143.00
61.72
111.99
23.99
56.47
27.78
96.00
18.00
49.05
24.64
89.16
Mg
40.80
4.79
20.50
13.82
52.80
4.79
24.25
18.19
33.59
4.80
16.35
7.99
24.00
0.00
12.98
6.95
18.52
Na
405.00
55.20
231.60
90.11
491.00
50.30
188.58
122.44
581.20
69.80
192.60
129.67
551.72
54.90
188.04
128.68
200.21
K
208.00
1.00
56.11
66.48
115.30
3.30
31.24
34.95
17.40
9.40
10.12
1.93
78.00
6.60
14.95
17.78
28.11
TDS
1827.23
459.99
1050.22
406.35
1908.12
432.80
1048.97
449.98
1967.00
436.00
972.94
403.76
1802.47
343.26
795.56
409.11
966.92
4.1 Correlation analyses
The correlation analysis for groundwater in different litho units of all seasons was studied in detail.
4.2 Alluvium
In SUM, good correlation exists between Cl–Ca, Cl–Mg, Cl–Na, HCO3–Ca, HCO3–Na, HCO3–K, HCO3–Mg, Ca–Mg, Ca–Na and Mg–Na (Fig. 3). Poor correlation exists between SO4, PO4 and H4SiO4 with all other ions. Cl shows good correlation with Ca, Mg and Na indicates dissolution and leaching of secondary salts and a significant correlation of HCO3 with Ca, Mg, Na and K indicates chemical weathering. Poor positive correlation of SO4, PO4 and H4SiO4 shows the lesser possibility of anthropogenic influence into the system. Ions like Cl and HCO3 show significant correlation with Ca, Mg, Na and K indicating the predominance of chemical weathering along with leaching of secondary salts during this season. For SWM, good to excellent correlation is obtained between Cl–HCO3, Cl−–Ca, Cl−–Na+, Cl–Mg, HCO3–Mg, HCO3–Ca, HCO3–Na, PO4–H4SiO4, PO4–Ca, PO4–Mg, Ca–Mg and Ca–Na indicating the dominance of weathering and leaching processes. PO4 shows good correlation with H4SiO4 indicates anthropogenic impact from agricultural practices. Opaline Silica, from the rice plants provides a readily soluble source of Silica and frequent oxidation and reduction cycle of paddy soil may accelerate weathering of Smectite, thereby making silica more soluble (Wang et al., 1993). Poor correlation exists between SO4 with other ions, might be because of the effect of dilution. During monsoon period, the recharge of fresh meteoritic water with lesser ions dilutes the existing groundwater with higher ion concentrations. The intense dilution or dissolution process may reduce the concentration of the SO4 in the groundwater system (i.e. under saturation condition of sulphates in the system), which is reflected poor correlation with other ions (Prasanna, 2008). Generally weathering along with anthropogenic impact are the dominant processes during this season. In NEM, good correlation exhibits between Cl–Ca, Cl–Mg, Cl–Na, HCO3–K, PO4–H4SiO4, Ca–Mg, Ca–Na and Mg–Na. Poor correlation exists between HCO3, SO4 and PO4 with all other ions except K and H4SiO4. Cl shows good correlation with Na, Ca and Mg indicates leaching of secondary salts. PO4 is well correlated with H4SiO4 indicating anthropogenic impact from agricultural practices as similar as in SWM. HCO3 shows good correlation to poor correlation with K and Na indicates weathering of potash feldspar. In POM, good correlation exhibits between Cl–Ca, Cl–Mg, Cl–Na, H4SiO4–HCO3, PO4–K, Ca–Mg, Ca–Na and Mg–Na. Poor correlation exhibits between SO4 and H4SiO4 with other ions might be because of dilution. SO4, PO4 and K shows good to poor positive correlation indicates anthropogenic activities from fertilizers. The contribution of HCO3, H4SiO4 and Na indicates albite weathering from the source rock. Cl shows good correlation with Mg, Ca and Na indicates leaching of secondary salts. Generally chemical weathering, leaching of salts and anthropogenic impact are the major contribution for this terrain.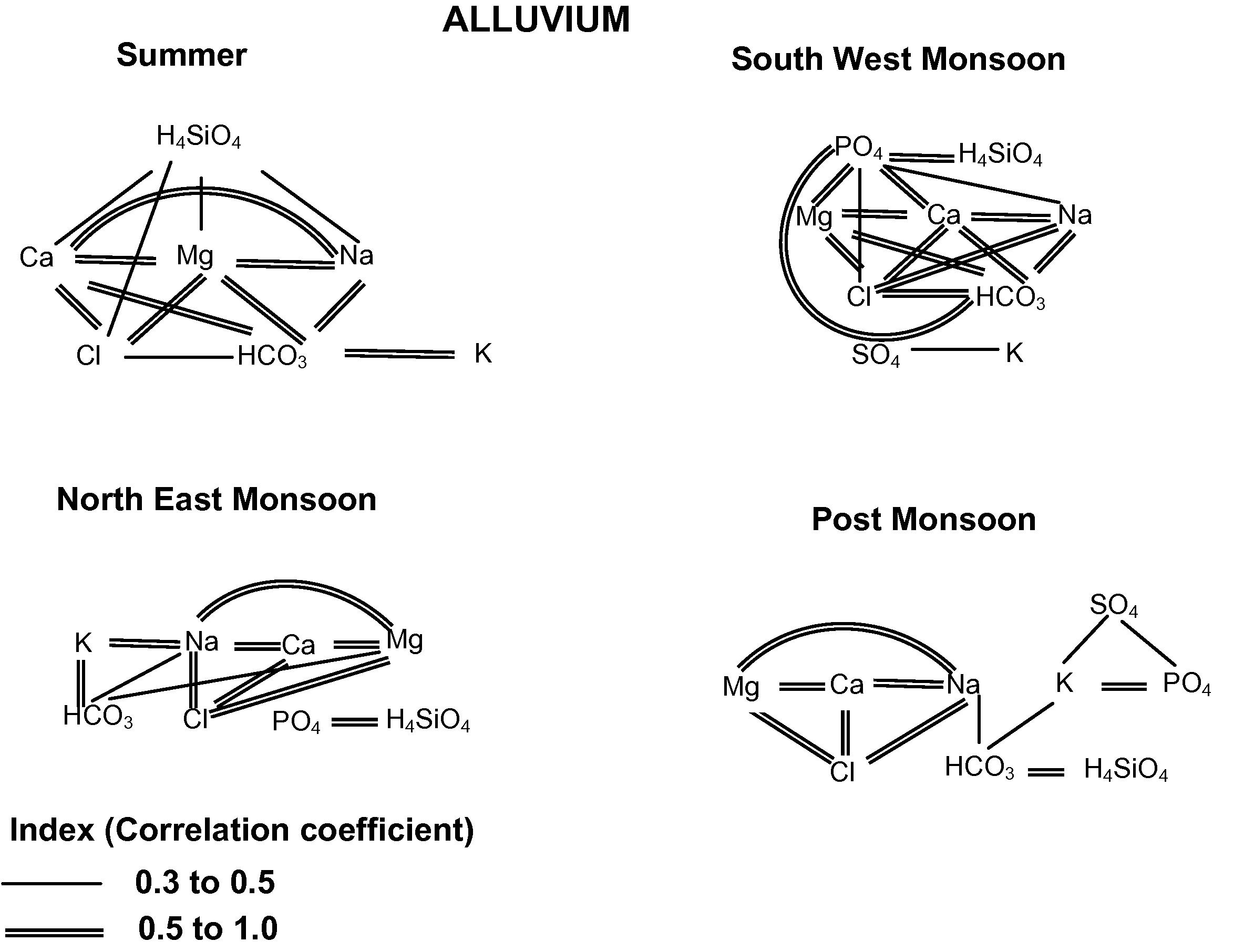
Correlation between the ionic species in groundwater of Alluvium formation.
4.2.1 Tertiary
In SUM, good correlation exhibits between Cl–Mg, Cl–Na, Cl–K, HCO3–Ca, SO4–Mg, SO4–Na, SO4–K, PO4–Ca, Mg–Na and Na–K (Fig. 4) Poor correlation exhibits between H4SiO4 with other ions indicates lesser influence of silica during this season. SO4 shows good poor positive correlation with Mg, Na and K indicates the leaching of marchasite present in this region (Sivalingam, 2005). HCO3 is well correlated with Ca indicates weathering of Carbonate minerals. In SWM, good to excellent correlation is obtained between Cl−–SO4, Cl−–Na+, Cl−–K,
, SO4–Na, PO4–Ca, PO4–H4SiO4, PO4–Mg, H4SiO4–Ca, H4SiO4–Mg and Ca–Mg. Poor correlation exists between HCO3 and SO4 with other ions except Ca and Na. Cl shows good correlation with SO4, Na and K indicates leaching of secondary salts as in SUM season. Good correlation between HCO3, Na and H4SiO4 indicates albite weathering. H4SiO4 is well correlated with Ca and Mg indicating intensive weathering reaction enhancing H4SiO4. PO4 is well correlated with H4SiO4 indicating anthropogenic impact from agricultural practices. In NEM, good correlation exhibits between Cl–Na, SO4–Na, H4SiO4–Ca, H4SiO4–K and Ca–K, indicating chemical weathering and leaching of secondary salts. Poor correlation exhibits between HCO3 and PO4 with other ions. In POM, good correlation exhibits between Cl–SO4, Cl–Ca, Cl–Na, Cl–K, HCO3–Ca, HCO3–Mg, SO4–Na, H4SiO4–Mg and Ca–Na, indicates leaching of secondary salts with anthropogenic impact. Poor correlation exhibits between PO4 with other ions indicating lesser influence of anthropogenic impact might be the effect of dilution. HCO3 is well correlated with Ca and Mg indicating weathering of carbonate minerals. Cl shows good correlation with SO4, Ca, Na and K indicating leaching of salts. In general, SO4 is well correlated with Na in all the seasons indicates high sodium sulfate concentration is derived from the local fertilizer plants at (NLC) which is located in this terrain. Generally weathering and leaching of secondary salts are the dominant processes during this season.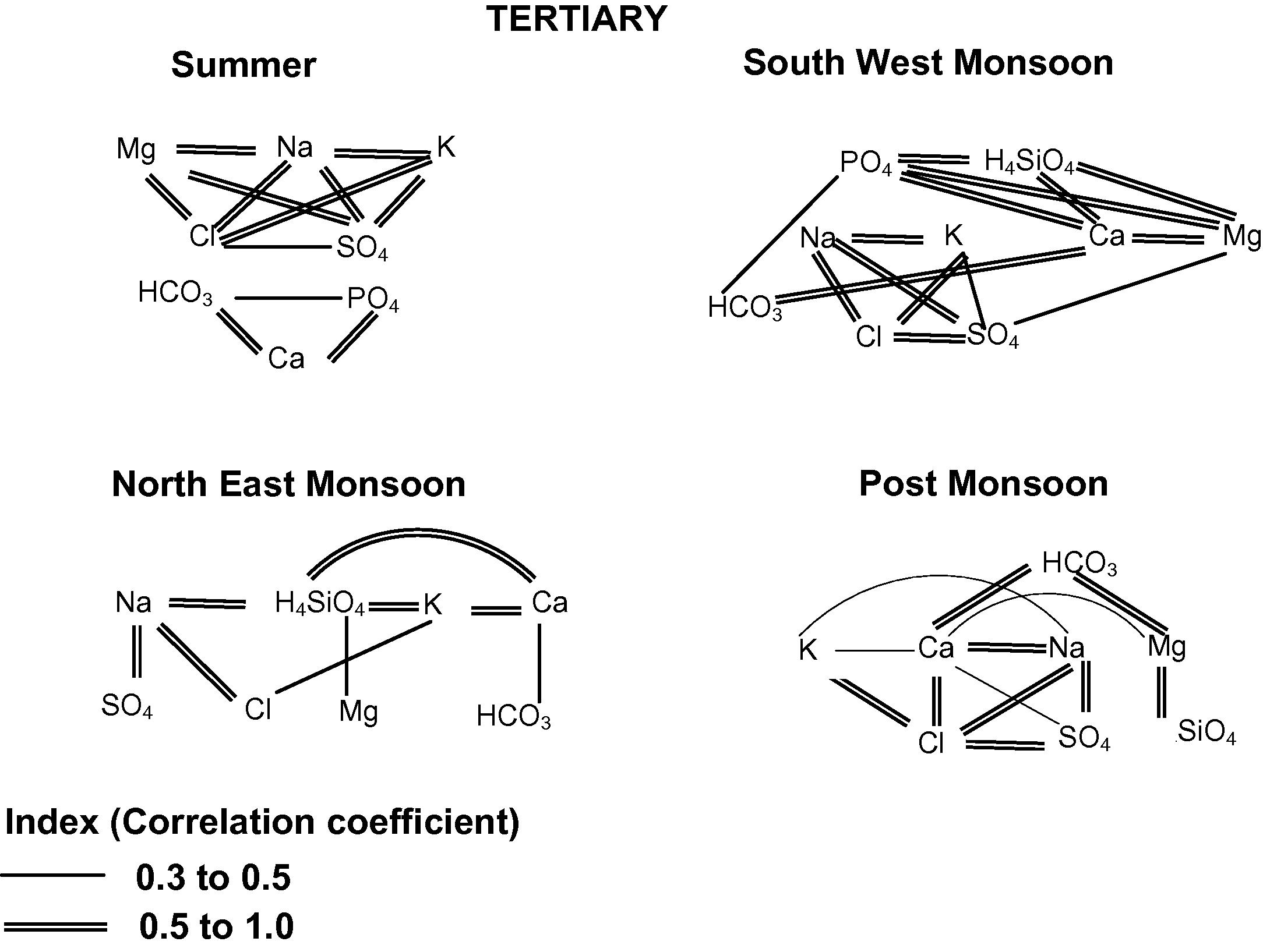
Correlation between the ionic species in groundwater of Tertiary formation.
4.2.2 Archaean
In SUM, good correlation exhibits between Cl–Ca, Cl–Mg, Cl–Na, Cl–K HCO3–Na and Na–K indicates the leaching of secondary salts (Fig. 5). Poor correlation exhibits between SO4, PO4 and H4SiO4 with other ions indicates lesser influence of anthropogenic impact. Cl shows good correlation with Ca, Mg, Na and K indicates secondary leaching of salts. HCO3 is well correlated with Na indicating weathering of sodic feldspar from the source rock. In SWM, good correlation exists between Cl–Ca, Cl–Na, Cl–K, HCO3–Na, PO4–H4SiO4, Ca–Mg and Na–K. Poor correlation exhibits between SO4 and H4SiO4 with other ions. Good to poor positive correlation of HCO3, Na, K and H4SiO4 indicates albite weathering from the source rock. Cl shows good correlation with Ca, Na and K indicating secondary leaching of salts. H4SiO4 is well correlated with PO4 indicates anthropogenic impact from the agricultural practices. In NEM, good correlation exhibits between Cl–Ca, Cl–Na, SO4–Na and H4SiO4–Ca indicates secondary leaching of salts. Good to poor positive correlation between H4SiO4, Ca, Na and K indicates potash feldspar weathering from the source rock. Poor correlation exists between HCO3 and PO4 with other ions. In POM, good correlation exhibits between Cl–HCO3, Cl–SO4, Cl–Ca, Cl–Na, HCO3–SO4, HCO3–Ca, HCO3–Na, SO4–Na and Ca–Na, indicating the leaching of salts along with weathering process. Poor correlation exhibits between PO4 and H4SiO4 with other ions indicates lesser influence of anthropogenic impact. Cl shows good correlation with Ca, Na and SO4 indicates leaching of secondary salts. Generally weathering and leaching of ions are the prominent processes during this season.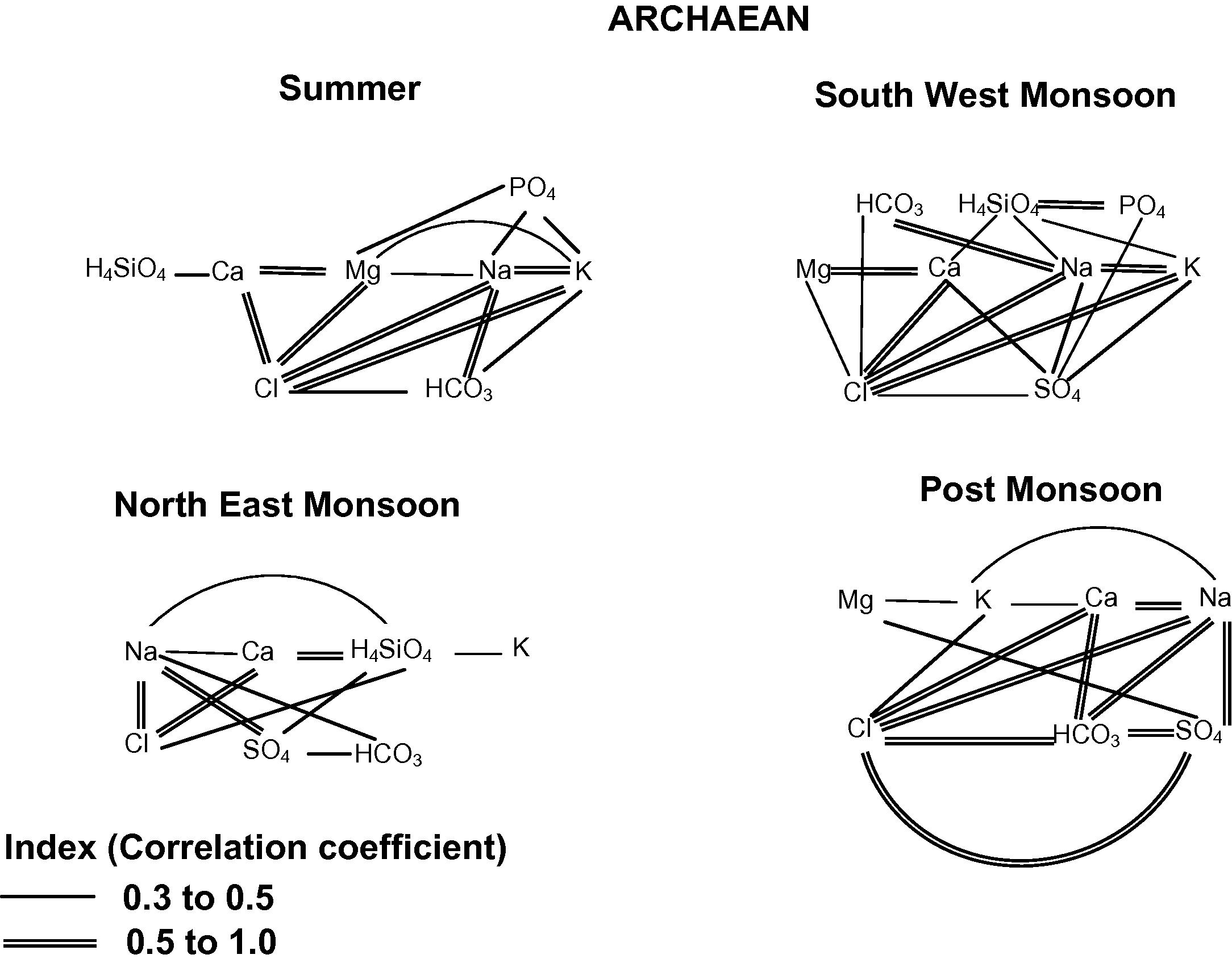
Correlation between the ionic species in groundwater of Archaean formation.
In general Ca, Mg, Na, Cl, HCO3 and SO4 remain to be the chief ionic species throughout the seasons and other play a less dominant role with respect to seasons. Hence correlation study reveals Ca, Mg, Na, Cl, HCO3 and SO4 form the spinal species (Chidambaram et al., 2007) and others are seasonal. This also shows that spinal species are the chief ions, which are playing a key role in controlling water chemistry of the region.
4.3 Factor analysis
After computation of the correlation matrix and the correlation coefficient, measures of interrelationship for all pairs of constituents are determined. Then by the examination of the factor loadings, factors are interpreted by rotation. Factor analysis is a widely used statistical technique in groundwater studies because it reduces the number of variables and enables the detection of structure in the relationships between variables. In this study principal component method is used as a parameter estimation method to transform a set of observed independent variables into an orthogonal set of variables called principal components. The first principal component accounts as much as possible of variance of the observed variants. The second principal component accounts for as much as possible of the residual variances not accounted by the first principal component. Each succeeding principal component accounts for as much as possible for the residual variance not accounted for by all the previous principal components. Initial factor loadings unlikely to reveal the underlying structure of the observed variants, because of certain mathematical conditions such as the variance properties of the principal components has to be understood in detail. To have a more clear view of this structure, common factors associated with initial set of loadings are linearly transformed into a new set of common factors, associated with new set of loadings, factor rotation. Though numerous schemes have been proposed, Kaiser Scheme called varimax rotation is used in this study. Varimax rotation yields set of loadings such as the variances of loadings are maximum. The factor analyses for four seasons were carried out and the results (Table 2) reflect complexities in chemical nature of the region.
Factor 1
Factor 2
Factor 3
Varimax rotated (n = 36) (SUM)
Cl
0.96
−0.05
0.01
HCO3
0.66
0.40
0.34
SO4
−0.06
−0.06
−0.87
PO4
0.00
0.85
0.32
H4SiO4
0.04
−0.84
0.33
Ca
0.82
0.04
0.41
Mg
0.91
0.14
0.10
Na
0.94
0.05
−0.19
K
0.36
0.49
0.15
Eigen values
3.88
1.86
1.33
Variance (%)
43.14
20.69
14.79
Cumulative (%)
43.14
63.84
78.63
Varimax rotated (n = 36) (SWM)
Cl
0.96
0.13
0.12
HCO3
0.80
0.47
-0.13
SO4
−0.02
−0.10
0.65
PO4
0.26
0.87
0.09
H4SiO4
−0.22
0.80
0.31
Ca
0.60
0.68
−0.12
Mg
0.20
0.63
−0.35
Na
0.95
−0.06
0.18
K
0.26
0.25
0.74
Eigen values
3.06
2.58
1.28
Variance (%)
33.99
28.64
14.27
Cumulative (%)
33.99
62.63
76.90
Varimax rotated (n = 42) (NEM)
Cl
0.98
0.10
−0.02
HCO3
0.29
0.77
−0.12
SO4
−0.19
0.68
−0.09
PO4
−0.14
−0.16
0.73
H4SiO4
0.08
0.02
0.81
Ca
0.95
0.00
0.02
Mg
0.93
0.12
−0.07
Na
0.80
0.51
−0.06
K
0.29
0.69
0.03
Eigen values
3.60
1.84
1.22
Variance (%)
39.94
20.46
13.56
Cumulative (%)
39.94
60.40
73.96
Varimax rotated (n = 42) (POM)
Cl
0.99
0.02
−0.07
HCO3
0.28
0.26
0.80
SO4
0.08
0.75
0.04
PO4
−0.06
0.67
−0.24
H4SiO4
−0.16
−0.25
0.83
Ca
0.88
0.06
0.11
Mg
0.92
−0.04
−0.08
Na
0.92
0.24
0.22
K
0.13
0.82
0.16
Eigen values
3.57
1.88
1.48
Variance (%)
39.69
20.84
16.47
Cumulative (%)
39.69
60.53
77.00
In SUM, three factors were extracted with 78.62% of total data variability (TDV). Factor 1 was represented by Cl−, HCO3, Ca, Mg and Na indicating leaching of secondary salts (Prasanna et al., 2009). The concentration of Na and Cl can be ascribed to the intrusion of seawater into the aquifer system which increases the concentrations of these ions. The presence of HCO3, Ca and Mg reflects the signatures of natural water recharge and rock–water interaction. Surface water charged with atmospheric and biogenic CO2 infiltrates into the subsurface and aggressively attack aluminosilicates including feldspars and micas present in the formation liberating cations such as Ca and Mg into the water and leaving residues of clay minerals. A consequence of this incongruent dissolution is a rise in pH and in HCO3 concentration of the water (Freeze and Cherry, 1979). Factor 2 represented by PO4 and K indicating the anthropogenic impacts from the agricultural practices. Factor 3 represented by HCO3 and Ca indicating water–soil/rock interaction.
In SWM, three factors were extracted with 76.90% of total data variability (TDV). Factor 1 was represented by Cl, HCO3, Ca and Na follow the same trend of factor 1 in SUM. Factor 2 representing PO4, H4SiO4, Ca and Mg indicating anthropogenic impact from the agricultural practices. Factor 3 represented by SO4 and K also indicating anthropogenic impact.
In NEM, three factors were extracted with 73.96% of total data variability (TDV). Factor 1 representing Cl, Ca, Mg and Na indicating leaching of secondary salts. Factor 2 representing HCO3, SO4, Na and K indicating intensive weathering. Factor 3 represented by PO4 and H4SiO4 indicating anthropogenic impacts from the agricultural practices (Prasanna et al., 2009).
In POM, three factors were extracted with 77% of total data variability (TDV). Factor 1 represented by Cl, Ca, Mg and Na follow the same trend of factor 1 in NEM. Factor 2 represented by SO4, PO4 and K indicating anthropogenic impacts from fertilizers. Factor 3 represented by HCO3 and H4SiO4 indicating weathering of silicate minerals.
In general leaching of secondary salts, weathering and anthropogenic impacts are the dominant controlling factors in the study area.
4.4 Factor scores
The factor score were also estimated to find out the spatial variation of the factor representation and to identify the zone of its representation. They are commonly obtained by two approaches weighted least square method and the regression method. The regression method is used in the study to compute the factor scores. The positive zones indicate the dominance of that factor (Hydrogeochemical regime). The spatial variation by using the factor score values of each sampling points were plotted by SURFER software. The spatial representation for factor 1 (Fig. 6) irrespective of seasons fall along the coast with higher industrialized region, where the influence of SIPCOT industry is preferably noted. The areal distribution of the factor score show that the intensity of salt water activity is well noted along the coastal area. This suggests that salt water ingress into the aquifer was predominantly related to water recharge from this tide influenced river. The high score area is the most densely populated and consequently witnesses higher groundwater abstraction. This probably establishes local freshwater depression cones, which induces saltwater infiltration into this region. The spatial representation of factor 2 (Fig. 7) is mainly represented in the Archaean region and along the contact of lithology with few in Alluvium region. In SUM and SWM seasons, higher scores are located in the upstream side of Archaean region. They represent leaching of secondary salts and anthropogenic impact by agricultural practices. In NEM, they fall in the region with prominent lithological contact and structural controls and a small patch in the SE side of the region. In POM, factor 2 is represented in the NE side of the region influenced by anthropogenic impacts. The spatial representation of factor 3 (Fig. 8) is mainly represented in the upstream side of Archaean formation with few along the contact of lithology and in Tertiary formation. In SUM, higher values are located in the upstream side of Archaean formation indicating the effect of leaching of secondary salts with some anthropogenic impacts. In SWM, they fall in the NW side and central part of the study area similar to SUM season. In NEM, factor 3 is well represented in the regions covered by sulphide ore bodies (Sivalingam, 2005) in Tertiary formation and agricultural practices. In POM, factor 3 is well represented by the lithology and structural control.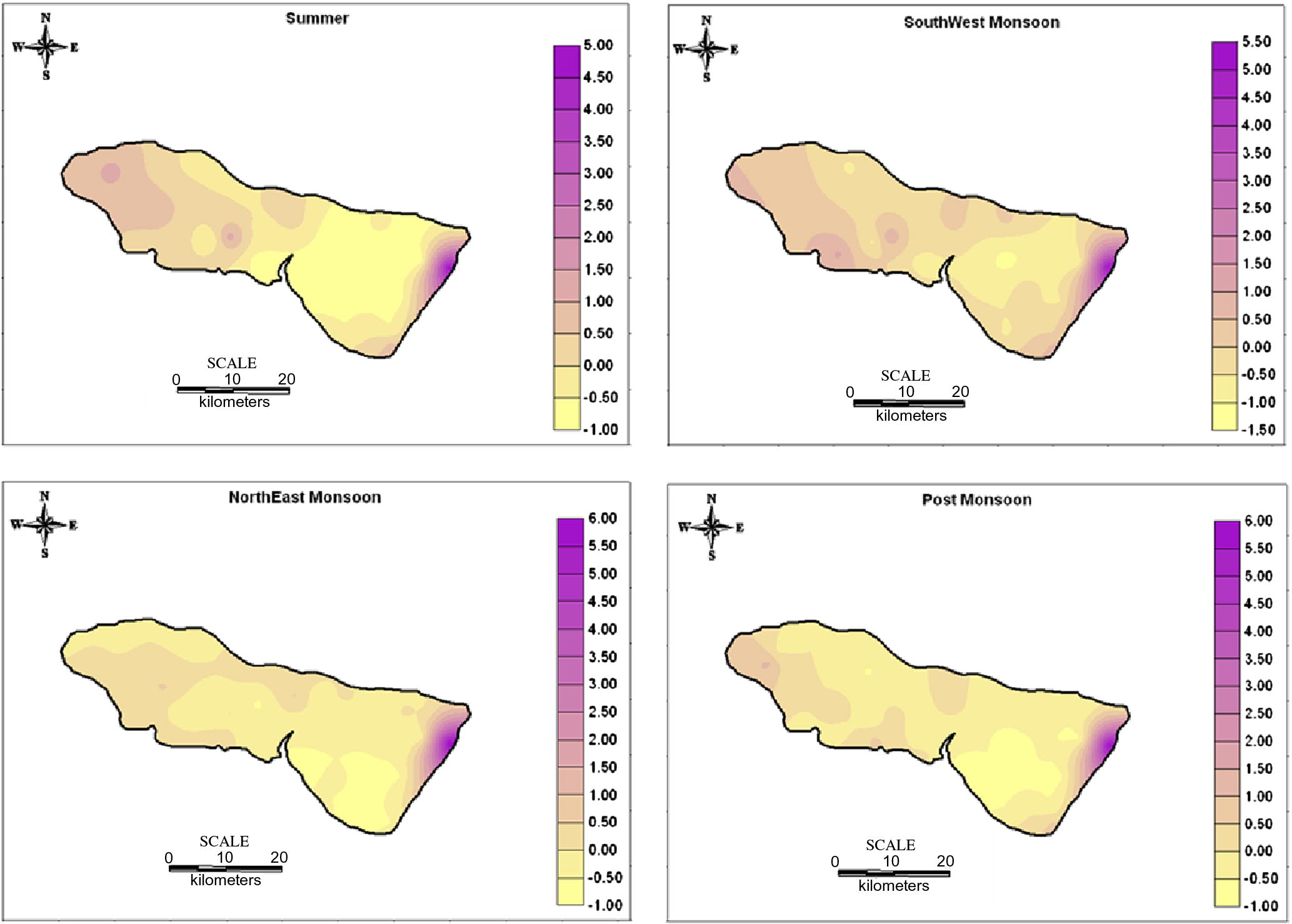
Spatial distribution of Factor 1 in groundwater.
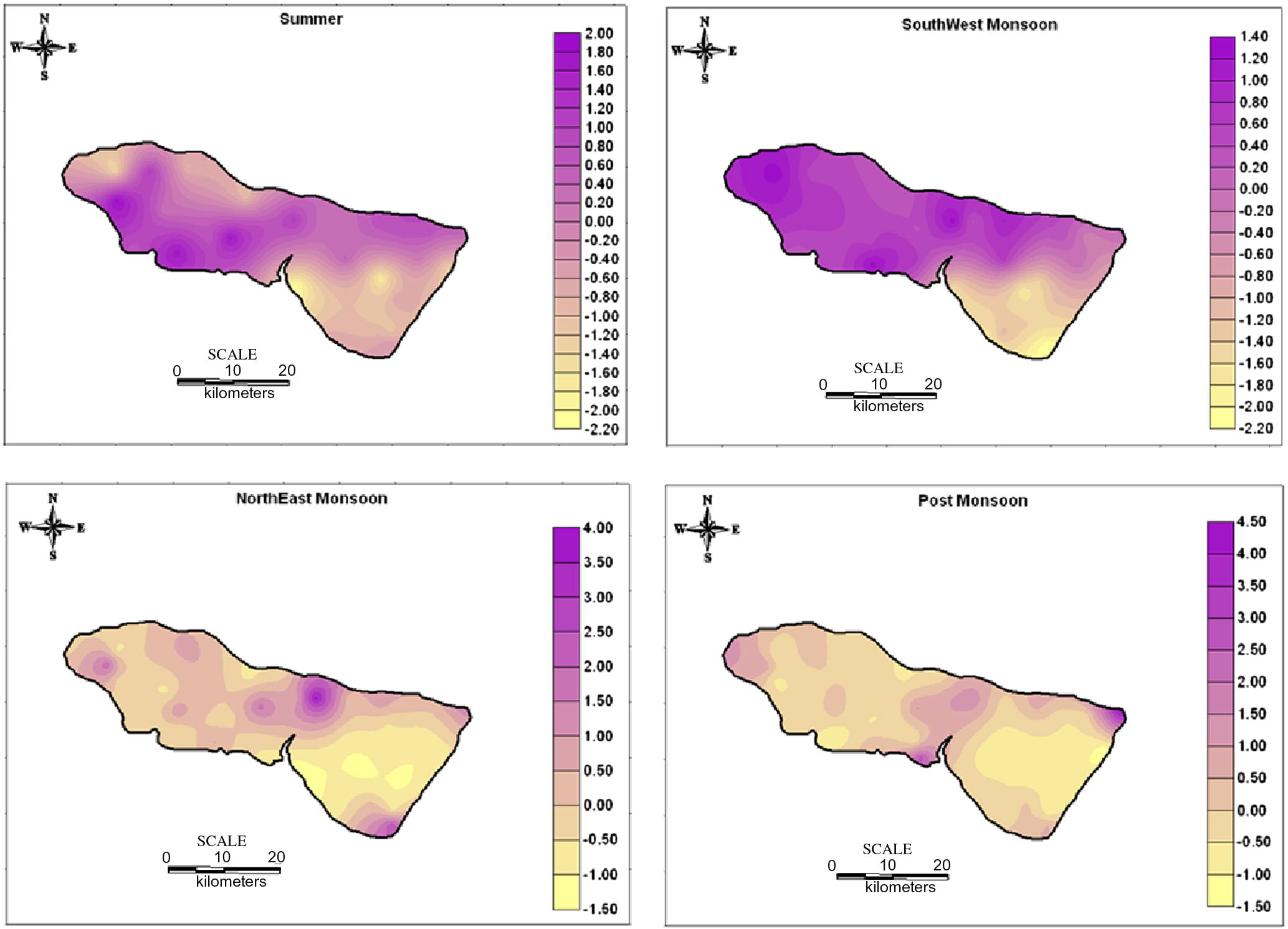
Spatial distribution of Factor 2 in groundwater.
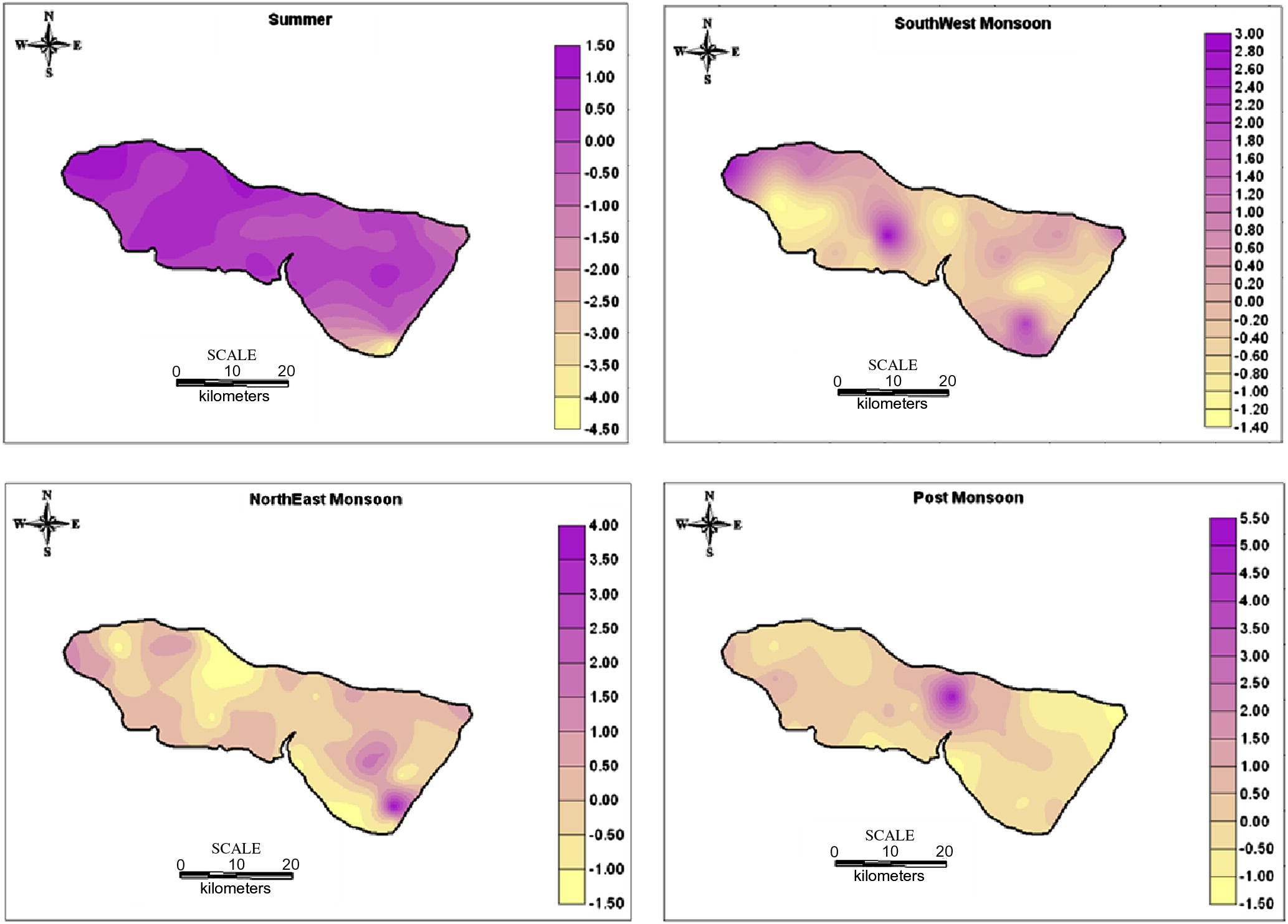
Spatial distribution of Factor 3 in groundwater.
As the hydrogeochemical system is complex and it is not possible to delineate and reason out all possible mechanism controlling the water chemistry of the region. The dominant factors responsible are extracted and their factor scores alone are used for the representations. To study the dominant hydrogeochemical regime of the study area all the factor score representation were overlaid for all seasons (Fig. 9). The overlay indicates the dominant hydrogeochemical regimes are distributed in the upstream side, contact of lithology and along the coast. In the upstream side, Archaean formation is dominantly influenced by leaching process and in the central part contacts of lithology and structural control are the dominant factors. In the NE side, saline water intrusion into the coastal aquifers, anthropogenic activities from industry and agricultural practices are the major controlling factors in the study area.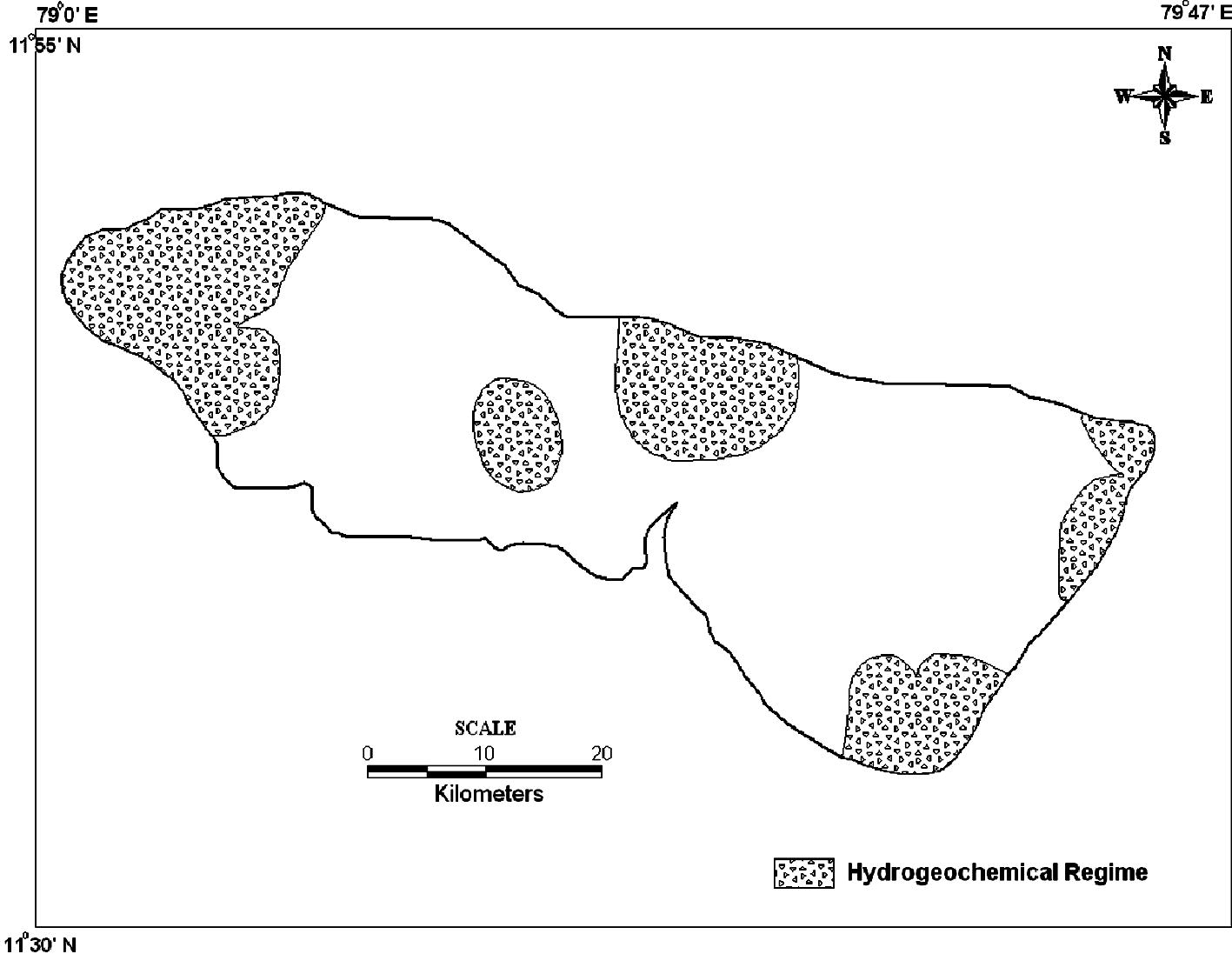
Spatial distribution of dominant hydrogeochemical regime in the study area.
4.5 Ionic ratio
Table 3 shows the variation of cations in different litho units of the study area. In Alluvium and Tertiary formations, Na/(Ca + Mg) ratios are >3 irrespective of seasons indicating the dominance of alkalies over alkali earth. In Archaean formation, ratios fall between >1 and <3 category indicating their approach towards equilibrium. Na/Cl ratio in Alluvium formation shows that the dominance of chloride indicates the saline water intrusion into the coastal aquifers (Chidambaram et al., 2005). In Tertiary, ratios fall in >1–<3, indicating their approach towards equilibrium during SUM and SWM and dominance of chloride is well noted in NEM and POM seasons. In Archaean same trend was followed as in Alluvium, indicating the dominance of leaching process. In Alluvium formation, Cl/HCO3 ratio indicates most of the samples fall below one during SWM and NEM and between >1 and <3 category during SUM and POM seasons indicates the impact of saline water intrusion (Prasanna et al., 2008b). In Tertiary formation, most of the samples irrespective of seasons fall in >1–<3 ratio indicates the mobilization of Cl ions during SUM, SWM and POM seasons. In Archaean formation, most of the samples irrespective of seasons fall in <1 indicates the dominance of bicarbonate due to intensive weathering process. In Alluvium formation, Cl/SO4 ratio indicates majority of samples irrespective of seasons ranges between in >1 and <3 category indicates the equilibrium of both ions due to the mixing of saline water composition into the groundwater. In Tertiary formation, ratios show lesser value indicating the dominance of sulphate due to the reduction process of sulphides present in the formation (Sivalingam, 2005). In Archaean formation, most of the samples irrespective of seasons fall in >3 indicating the dominance of Cl ions due to the leaching of secondary minerals and longer residence time in the aquifer matrix (Sinivasamoorthy et al., 2008). In (Ca + Mg)/HCO3 ratio samples are fall below one and range from >1 to <3 categories indicating the dominance of bicarbonate and equilibrium state of both ions i.e. (Ca + Mg) and HCO3. In Ca + Mg/TZ+ ratio of Alluvium formation, majority of samples irrespective of seasons shows lesser values indicates the dominance of total cations. In Na + K/TZ+ ratio, most of the samples irrespective of seasons show higher values indicate the dominance of alkalies. Same trend was followed by Tertiary formation. In Ca + Mg/TZ+ ratio of Archaean formation, most of the samples fall between <0.3 and >0.6 category indicating their approach towards equilibrium and in Na + K/TZ+ ratio, samples show higher values indicate the dominance of alkalies. Generally, NaCl is the dominant facies with a chief factor of rock water interaction was observed in the study area. Overall, the ionic ratios are well substantiated with the results of correlation and factor analysis.
Grade
Na/(Ca + Mg)
Na/Cl
Na/Ca
Cl/HCO3
Cl/SO4
Ca + Mg
(Ca/Mg)/HCO3
Grade
Ca + Mg/Tc
Na + K/Tc
Alluvium
SUM
<1
0
5
0
5
3
0
7
<0.3
10
0
>1–<3
4
7
2
5
6
3
5
>0.3–<0.6
2
1
>3
8
0
10
2
3
9
0
>0.6
0
11
SWM
<1
4
8
2
7
1
0
5
<0.3
5
2
>1–<3
4
4
5
5
7
1
7
>0.3–<0.6
5
4
>3
4
0
5
0
4
11
0
>0.6
2
6
NEM
<1
0
9
0
3
1
2
7
<0.3
7
0
>1–<3
5
3
2
8
3
7
4
>0.3–<0.6
5
1
>3
7
0
10
1
8
3
1
>0.6
0
11
POM
<1
0
7
0
6
1
0
5
<0.3
9
0
>1–<3
4
5
3
4
8
6
5
>0.3–<0.6
3
1
>3
8
0
9
2
3
6
2
>0.6
0
11
Tertiary
SUM
<1
0
2
0
3
6
0
3
<0.3
6
0
>1–<3
5
8
4
5
4
2
5
>0.3–<0.6
4
2
>3
5
0
6
2
0
8
2
>0.6
0
8
SWM
<1
3
4
3
4
7
0
5
<0.3
5
10
>1–<3
2
5
2
6
3
2
3
>0.3–<0.6
2
0
>3
5
1
5
0
0
8
2
>0.6
3
0
NEM
<1
4
8
3
7
5
0
5
<0.3
5
3
>1–<3
3
3
4
4
2
3
7
>0.3–<0.6
4
2
>3
5
1
5
1
5
9
0
>0.6
3
7
POM
<1
1
9
0
5
2
0
2
<0.3
5
0
>1–<3
6
3
7
6
6
3
10
>0.3–<0.6
6
5
>3
5
0
5
1
4
9
0
>0.6
1
7
Archaean
SUM
<1
2
10
1
10
2
0
8
<0.3
3
1
>1–<3
10
3
10
4
2
3
6
>0.3–<0.6
10
5
>3
2
1
3
0
10
11
0
>0.6
1
8
SWM
<1
6
12
5
8
3
0
4
<0.3
2
5
>1–<3
7
2
8
6
1
1
10
>0.3–<0.6
7
4
>3
1
0
1
0
10
13
0
>0.6
5
5
NEM
<1
1
11
0
10
4
1
10
<0.3
6
0
>1–<3
10
5
9
7
1
7
7
>0.3–<0.6
11
6
>3
6
1
8
0
12
9
0
>0.6
0
11
POM
<1
1
12
0
8
2
1
5
<0.3
8
0
>1–<3
8
4
9
9
11
7
12
>0.3–<0.6
9
4
>3
8
1
8
0
4
9
0
>0.6
0
13
5 Conclusion
The statistical application carried out by using the correlation and the factor analysis for the samples analysed indicate that Ca, Mg, Na, HCO3 and Cl in Alluvium formation; Na, K, Cl and SO4 in Tertiary and Na, K, Ca, Cl and HCO3 in the Archaean formation forms the spinal species and other are seasonal. It is interesting to note that Na is chief cation and Cl is the dominant anion in all the seasons and in the different formations studied. The Ca and HCO3 associations in the Alluvium and the Archaean formations reveal the variation in source rock composition. The factor analysis for the data generated brings out leaching of secondary salts, weathering and anthropogenic impacts are the dominant controlling factors in the study area. The spatial distribution of the chief factor scores reveals that dominant hydrogeochemical regimes are distributed along the upstream side, contact of lithologies and along the coast (downstream). This hydrogeochemical evolution includes dissolution and leaching of secondary salts in the upstream side, weathering process in the lithological contact and saline water intrusion with anthropogenic activities in the coastal aquifers along the downstream. This inference with the aid of statistical tools will enlighten the water resource managers, developers in Gadilam basin and its environs. The recent drive towards industrialization and the attendant urbanization means a greater demand for the quality and quantity of portable groundwater in the area.
Acknowledgements
The authors wish to express their thanks to Department of Science and Technology (DST), India for providing the necessary financial support to carry out this study. They are also thankful to Centre for Water Resource Development and Management (CWRDM), Kozhikode, Kerala for their cooperation.
References
- American Public Health Association (APHA), 1998. Standard methods for the examination of water and wastewater, 19th ed. APHA, Washington DC, USASS.
- Anandhan, P., 2005. Hydrogeochemical studies in and around Neyveli mining region, Tamilnadu, India. Unpublished Ph.D. Thesis, Department of Earth Sciences, Annamalai University, p. 189.
- Groundwater quality in the hard rock area of the Gadilam river basin, Tamilnadu. Journal of Geological Society of India. 2004;63:625-635.
- [Google Scholar]
- Centre for Groundwater Board (CGWB), 1997a. Groundwater resources and development prospects in South Arcot-Vallalar District, Tamilnadu. South Eastern Coastal Region, Chennai.
- Centre for Groundwater Board (CGWB), 1997b. Groundwater resources and development prospects in Villupuram Ramasamy Padayachi District, Tamilnadu. South Eastern Coastal Region, Chennai.
- WATCLAST-A Computer Program for Hydrogeochemical Studies. Recent Trends in Hydrogeochemistry (case studies from surface and subsurface waters of selected countries). New Delhi: Capital Publishing Company; 2003. (pp. 203–207)
- A comparative study on the coastal surface and ground water in and around Puduchattiram region, Tamilnadu. Special issue in International Journal of Ecology and Environment Sciences. 2005;31(3):299-306.
- [Google Scholar]
- Identification of hydrogeochemically active regimes in groundwaters of Erode district, Tamilnadu-A statistical approach. Asian Journal of Water, Environment and Pollution. 2007;5(3):93-102.
- [Google Scholar]
- Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. Journal of Hydrology. 2008;353:294-313.
- [Google Scholar]
- Geochemistry of surface and sub-surface waters of Rewalsar lake, Mandi district, Himachal Pradesh: constraints on weathering and erosion. Journal Geological Society of India. 2007;69(5):1020-1030.
- [Google Scholar]
- Statistics and Data Analysis in Geology. New York: John Wiley and Sons Inc.; 1973. (p. 550)
- Deciphering groundwater flow systems in Oasis Valley, Nevada, using trace element chemistry, multivariate statistics, and geographical information system. Mathematical Geology. 2000;32:943-968.
- [Google Scholar]
- Factor analytical approaches for evaluating groundwater trace element chemistry data. Analytical Chimica Acta. 2003;490:123-138.
- [Google Scholar]
- Groundwater. New Jersey: Prentice – Hall, Inc. Englewood cliffs; 1979. (p. 604)
- Harmann, H.H., 1960. Modern Factor Analysis. University of Chicago Press.
- The Varimax criterion for analytic rotation in factor analysis. Psychometrika. 1958;23b:187-200.
- [Google Scholar]
- Major ion chemistry and identification of hydrogeochemical processes of groundwater in a part of Kancheepuram district, Tamilnadu, India. Environmental Geosciences. 2003;10(4):157-166.
- [Google Scholar]
- The ‘principal components’ statistical method as a complementary approach to geochemical methods in water quality factor identification; application to the Coastal Plain aquifer of Israel. Journal of Hydrology. 1992;140:49-73.
- [Google Scholar]
- Muthukrishnan, N., 1993. A study of detrital minerals from the sediments of Gadilam river basin. Unpublished M.Phil. Thesis, Tamil University, Thanjavur.
- Prasanna, M.V., 2008. Hydrogeochemical studies in the Gadilam river basin, Tamilnadu. Unpublished Ph.D. Thesis, Annamalai University, India, p. 300.
- Integrated geophysical chemical study in the lower subbasin of Gadilam, Tamilnadu, India. Environmental Geosciences. 2008;15(4):145-152.
- [Google Scholar]
- Assessment of groundwater quality using geographical information system in the Gadilam river basin, Tamilnadu, India. Environmental Media, International Journal of Ecology and Environment Conservation. 2008;14(2–3):293-298.
- [Google Scholar]
- Prasanna, M.V., Chidambaram, S., Shahul Hameed, A., Srinivasamoorthy, K., 2009. Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. J. Environmental Monitoring and Assessment. 10.1007/s10661-009-1092-5.
- The utility of multivariate statistical rechniques in hydrogeochemical studies: an example from Karnataka, India. Water Research. 2002;36:2437-2442.
- [Google Scholar]
- Human impact on regional groundwater composition through intervention in natural flow patterns and changes in land use. Journal of Hydrology. 1992;134:297-313.
- [Google Scholar]
- Identification of major sources controlling groundwater chemistry from a hard rock terrain – a case study from Mettur taluk, Salem District, Tamilnadu, India. Journal of Earth System Sciences. 2008;117(1):49-58.
- [Google Scholar]
- Sivalingam, C., 2005. Geochemistry of Marcasite associated with Lignite deposits of Neyveli area, Tamil Nadu, South India. Unpublished Ph.D. Thesis, Annamalai University, India.
- Aquifer geometry, basement-topography and groundwater quality around Ken Graben, India. Journal of Spatial Hydrology. 2005;2(2):1-7.
- [Google Scholar]
- Discrimination of groundwater sources using cluster analysis, MANOVA, canonical analysis and discriminant analysis. Water Resources Research. 1985;21:1149-1156.
- [Google Scholar]
- Geochemical and statistical evidence of deep carbonate groundwater within overlying volcanic rock aquifers/aquitards of southern Neveda, USA. Journal of Hydrology. 2001;243:254-271.
- [Google Scholar]
- Multivariate analysis in hydrochemistry: an example of the use of factor and correspondence analyses. Ground Water. 1989;27:27-34.
- [Google Scholar]
- Ferri hydrite, lepidocrocite and goethite in coatings from the east texas vertic soils. Soil Science Society of America Journal. 1993;57:1381-1386.
- [Google Scholar]







