Translate this page into:
Spectroscopic features of PHOTOGEM® in human Rhabdomyosarcoma (RD) cellular model
⁎Corresponding author at: Department of Physics, Govt. College, University, Faisalabad, Punjab, Pakistan. fakhar@gcuf.edu.pk (Muhammad Fakhar-e-Alam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Our study demonstrated the biological changes produced in the Rhabdomyosarcoma (RD) cell line triggered by the photochemical reaction between PHOTOGEM® (photosensitizing agent) and He-Ne laser light (wavelength = 632.8 nm of red light). The basic parameters of photodynamic therapy were optimized to study photosensitizer localization/uptake, PHOTOGEM® absorption spectra, cytotoxic effects, phototoxic effects, and morphological changes in the RD cell line. The experiment included three steps. First, spectrometric measurements were obtained to optimize the absorbance and optimal density of PHOTOGEM® in the experimental biological model (RD cell line). A neutral red assay was used to estimate the loss of cellular viability. Second, the RD cell line containing PHOTOGEM® was irradiated with laser light (dose up to 100 J/cm2). In addition, a non-fluorescent compound was used to determine the intracellular production of reactive oxygen species. The treated cells were examined and photographed. The optical density of the PHOTOGEM®-exposed RD cell line was insignificant after 0–2 h but increased significantly after 20 and 24 h. A photosensitizer concentration of 120 μg/ml and a red He-Ne laser dose of 100 J/cm2 having wavelength 632.8 nm produced the maximum phototoxic effect in the RD cell line. The cell viability of the PHOTOGEM®-labeled RD cells decreased to 65% in the absence of the laser dose, but there was significant cell viability loss under suitable laser exposure (100 J/cm2).
Keywords
Rhabdomyosarcoma
RD cell line
Photosensitizer
PHOTOGEM®
Photodynamic therapy
Reactive oxygen species
- RD
Rhabdomyosarcoma
- PDT
photodynamic therapy
- ROS
reactive oxygen species
Abbreviations
1 Introduction
Rhabdomyosarcoma (RD Cells) arises from human skeletal muscle precursors. Most common type of soft tissue sarcoma is found in children and adolescent less than 20 years old. Rhabdomyosarcoma type is divided into two major categories i. e embryonal and alveolar. It is commonly found in 15–20 year olds and approximately 5–8% of malignancies in children is diagnosed as RD (Baker et al., 2002; Pappo et al., 1995). Almost 350 cases of RD are diagnosed annually in the United States. A survival rate of 50–70% has been observed in patients younger than 15 years ((Gurney et al., 1999). Previous studies reported that almost 65% of all the carcinomas related to RD cells were found in children less than 6 years old. Moreover, more male patients (58.4%) were affected by muscle carcinomas than females (41.6%) (Parham, 1994; Toro et al., 2006). The tumors were extremely aggressive and resembled skeletal muscle cells that were restrained along the normal myogenic pathway to maturation (Brown et al., 2004).
There are plenty of traditional cancer treatment techniques e.g. radiotherapy, chemotherapy, surgery and proton therapy which found very invasive to human health. Photodynamic therapy (PDT) is a sophisticated treatment against cancerous cells in which a photosensitizer (PS) drug is activated by laser light. Recently, many researchers have used PDT to treat cancer especially gynecological disease and basal carcinogenic disease via social health care clinical trial. Very satisfactory PD/PDT outcome results are recorded by two famous researchers (Allison, et al., 2005; Buzzá et al., 2019). There are manifold PS/Drug administration techniques i.e. systematically and topically (form of cream). But due to prominent quantity of low density lipoprotein receptors in cancerous part, it only accumulates into targeted/selected region of abnormal tissues part and make very strong bond due to weak metabolic activity of malignant tissue stroma’s. Drug even retain after drug light interval (30 min to 4 h) in cancerous site and produces the significant ROS which leads to cell death.
PDT depends upon the nature of the PS, the localization of the PS in the targeted site, and the wavelength of the laser used (Singh et al., 1991). It is a less invasive technique and shows very promising results in a short time. In addition, there is maximum uptake of the PS into the cancerous cells while the normal cells are saved. PDT induces stress in the cells followed by recovery or cell death due to necrotic and apoptotic effects (Dougherty, 2002). In PDT, after administration of the PS drug, it is activated by visible light, resulting in a chain of multiple photoactivated reactions whereby the excited molecules of the PS result in the form of fluorescence compatible for system of photodynamic diagnostic (PDD) and in terms of intersystem crossing, PS provides energy to surrounding water molecules and molecular from of oxygen after returning back to ground state as resultant providing phosphourescence (Phospor) resulting of type 1 reaction and type II reaction e.g. reactive oxygen species (ROS) singlet 1O2 state (S1) and triplet 3O2 state (T1). This transition of intersystem ROS leads to toxicity. The T1 state has a longer lifetime than the S1 state, which provides an opportunity for the PS drug to interact with adjacent molecules (Dougherty et al., 1998). Recently, different types of cell lines have been exposed to various types of photosensitizers to treat malignant cells (Stewart et al., 1998).
In previous studies, inducing resistance in a targeted site (i.e., a tumor) was considered a productive way to study the mechanisms of action between various antineoplastic drugs and physical treatments such as hyperthermia (Jain, 2012). Recently, researchers tried to investigate the characteristics of PDT cytotoxicity using several malignant cell lines. PDT or other treatment modalities were used to treat the current biological model, i.e., RD cell line. However, PDT is the preferred technique over many traditional developed therapeutic techniques for studying resistance to the localization and uptake of the PS drug to targeted site (Melo et al., 2004). Similar kind of studies/experiments regarding drug and nanomaterials accumulation were conducted by (Akram et al., 2019; Atif et al., 2019; Fakhar-e-Alam et al., 2011a; Iqbal et al., 2019a; Munir et al., 2019). Recently new development in field of therapeutic techniques are made by introducing Vitamin D3–assisted chemo-photodynamic therapy of rhabdomyosarcoma cancer cells for effective treatment for the first time by (Mahmood et al., 2018). They claimed that therapeutic drug complex with Vitamin D3 under laser exposed to RD cells creates 80% cell viability loss which was found 50% in prior evaluation, so Vitamin D3 assisted photochemotherapy is more effective and shows convincing results as compared to single photosensitizer. For satisfactory PDT results the suitable quantity of drug towards, targeted sites, molecular oxygen and tunable light illuminations is basic factor of significant photochemical reactions and reactive oxygen species liberation. Problem of Multidrug resistance (MDR) to cancerous part are overcome by introducing many novel techniques like targeted drug delivery e.g. polymer capping to required photosensitizer, magnetic nanoparticle composite/inorganic hybrid form with recommended photosensitizers (Akram et al., 2019). In addition, the problem of superficial tissue illumination by laser light are elucidated by developing modified Hypericin (HYP)-based photosensitizers, as well as combining PDT and targeted internal radiotherapy with 131I, to produce an additive anti-tumor effect (Ocker et al., 2020).
The goal of our study was to optimize the parameters for effective PHOTOGEM®-mediated PDT in the RD cell line with respect to accumulation time, phototoxicity, cytotoxicity, and ROS test.
2 Materials and methods
2.1 Cell culture
The RD cell line was cultured in minimum essential medium (MEM) containing 10% fetal bovine serum (FBS), 100 μg/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml fungizone, and 2 mM L-glutamate. The cell line was incubated at 37 °C in humidified air. Further experimental steps were performed until 75% cell confluence was achieved (Atif et al., 2010; Fakhar-e-Alam et al., 2011b, 2011a).
2.2 Photosensitizer
Stock solution of PHOTOGEM® (TimTec LLC, Newark, DE, USA) was prepared at a concentration of 50 μg/50 ml, prepared stock solution was formed in phosphate buffer saline (PBS). Standard solutions at different concentrations were then made by diluting the stock solution within the ranges of 0–180 and 0–200 μg/ml using phosphate-buffered saline (PBS) as a solvent with serum-free medium (Atif et al., 2011; Fakhar-e-Alam et al., 2011b). PHOTOGEM® comprises a mixture of monomers, dimers, and oligomers in equilibrium between the aggregates and monomeric species (Gouterman, 1978). It has a specific monomer-to-oligomer ratio (17% monomers, 22% dimers, and 61% oligomers) compared with that of ® (14% monomers, 19% dimers, and 67% oligomers) (Ehrenberg et al., 1985).
2.3 Absorption spectrum of PHOTOGEM®
Purpose of given experimental step is to trace the favorite light wavelength region which must be compatible for PDT suitable procedure. On the basis of published literature and evidence, we chose the 632.8 -nm wavelength red laser for irradiation (He-Ne laser), which is close to matching the peak of the absorption spectrum (Atif et al., 2011; Fakhar-e-Alam et al., 2011a). There similar type of photosensitizers e.g. PHOGEM®, Photosan and Photofrin having almost very strong visible region absorption peak (Fakhar-e-Alam et al., 2011b). For this purpose, Y-shape fiber connected with second harmonic 532 nm of Nd: YAG laser with green spot-spectrophotometer were used for collecting absorption spectra of PHOTOGEM®.
2.4 Quantification of cellular uptake and incubation time
The human RD cell line was cultured in 96-well plates (1015 cells/well) and incubated for up to 24 h. The PHOTOGEM® concentration with working solution (0–200 μg/ml) was exposed to the laser. All steps were performed at room temperature. The cytotoxic and phototoxic effects of the PHOTOGEM® on the RD cell line were assessed using a microplate reader (filter wavelength 490 nm).
2.5 Cell viability
The RD cell line in varying concentrations of PHOTOGEM® in the dark and irradiated by laser light and then cell viability was examined by neutral red assay (NRA) (Atif et al., 2010; Fakhar-e-Alam et al., 2011b, 2011a). Next, the medium containing PHOTOGEM® was replaced with fresh medium containing neutral red (50 mg/ml) and incubated for 4 h. The medium was removed and the cultures were washed with a mixture of 10% CaCl2 and 40% formaldehyde (v/v ratio = 1:4). Neutral red was removed by adding a mixture of 1% acetic acid and 50% ethanol (v/v ratio = 1:1). After shaking the plate for 5 min, it was kept at room temperature for 15 min. The cells were quantified at 490 nm (absorbance) and the number was compared with the live cell number. The same protocol used in previous research studies was used here (Atif et al., 2010; Fakhar-e-Alam et al., 2011a). In parallel, control plates without PHOTOGEM® were prepared and exposed to neutral red. Cellular viability (% age) at different concentrations of PHOTOGEM® was assessed and calculated as % viability = (mean absorbance of PHOTOGEM®-treated cells ÷ mean absorbance of control cells) × 100.
2.6 Cytotoxicity and phototoxicity of PHOTOGEM®
In first step, the human RD cells were cultured and incubated with different concentrations of PHOTOGEM® (0–180 μg/ml) in MEM at 37 °C for 24 h. A control plate of RD cells but no PHOTOGEM® was prepared in parallel control cells (Atif et al., 2010; Fakhar-e-Alam et al., 2011a). A microplate reader (490 nm) was used to measure the increasing optical density and the corresponding relevant absorption peak at 1-h intervals over 24 h. All results were plotted as mean absorbance (±σ = 4) as reported in previous publications (Atif et al., 2011, 2010; Fakhar-e-Alam et al., 2011a). The experiment was performed four times to determine the optimal dose of the 632.8-nm He-Ne laser.
2.7 ROS test
Intracellular production of ROS was determined using the non-fluorescent compound CM-H2DCFDA (2,7-dichlorodihydrofluorescein diacetate acetyl ester; Invitrogen). This compound undergoes deacetylation by an esterase resulting in nonfluorescent CM-H2DCFDA after crossing the cell membrane. Cells were seeded in a 96-well plate and incubated in different concentrations of PHOTOGEM® for 12 h in humidified air containing 5% CO2 at 37 °C. The cells were then washed twice with Dulbecco’s modified Eagle’s medium (DMEM), loaded with 100 μl of 5 μM CM-H2DCFDA, and incubated for 35 min at 37 °C in the absence of light. Then, the cells were irradiated by UV light (20 J/cm2) for 2 min and assessed for ROS fluorescence using a Wallac 1420 Victor Plate Reader (PerkinElmer, Waltham, MA, USA) (λex485/λem 530 nm). Cells were also cultured in 12-well plates, excited using blue light (488 nm), and photographed under an inverted fluorescence microscope with a digital camera.
All relevant experimental steps of overall conducted work is depicted/summarized in Fig. 1.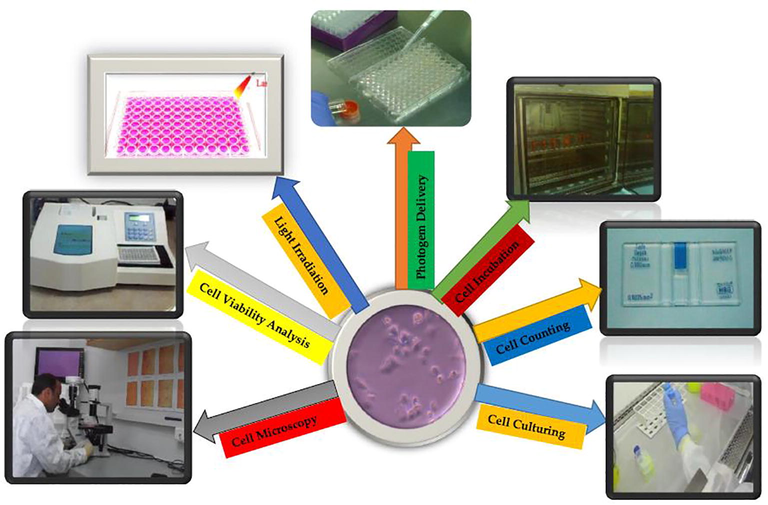
Work Flow Chart of RD-PDT Experiment.
3 Results and discussion
The photodynamic effects of PHOTOGEM® on the RD cell line were the main focus of our study. The uptake of PHOTOGEM® in the RD cell line was greater than that of α-aminolevulonic acid (ALA), which our group had studied previously (Atif et al., 2011). Many research groups have shown that the cytotoxic effects of a drug without nanocarriers are not reasonable. Nanoparticles are more efficient drug vehicles than individual photosensitizer molecules because of the high immune response to them. Before the current study, we labeled ZnO nanoparticles for comparative study of feasible photodynamic effects. The current study is a comparative study of PHOTOGEM®-mediated PDT results.
Fig. 2 shows the photochemical reaction response of the RD cells under the effect of PHOTOGEM® and He-Ne laser (632.8 nm of red wavelength) exposure. The results pointed toward the collective effect of a type I reaction with a type II reaction for cell death, which might be very effective for cancer therapy.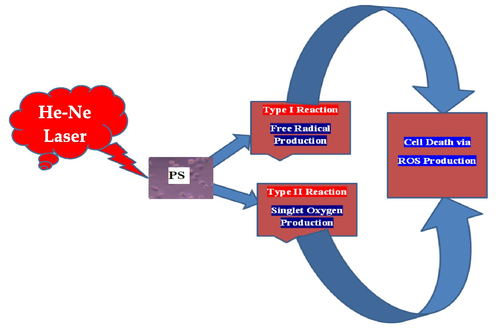
Schematic Illustration of PDT in RD cells.
The optical density of the PHOTOGEM®-exposed RD cell line was insignificant after 0–2 h of incubation but increased significantly after 20 and 24 h as depicted in Fig. 3. Therefore, 24 h was selected as the optimal incubation time. In addition, the consistency of the optical density vs. PHOTOGEM® concentration was calculated. The most suitable threshold level concentration of the photosensitizer was 120 μg/ml; above that level, the drug became toxic and initiated necrosis in normal cells. Our results agree with our previous (Atif et al., 2011; Fakhar-e-Alam et al., 2011a) whereby increasing the drug concentration in an abnormal or cancerous model usually increased the optical density. In those studies, the results were verified by treating different malignant cellular models with 5-ALA and a 632.8-nm He-Ne laser and by using various concentrations of PHOTOGEM® for up to 25 h of incubation. In this study, we repeated the experiments three times for each concentration and collected the data. Moreover, after 5 h of incubation, the optical density of PHOTOGEM® decreased, which was the result of secretion of the photosensitizer from the interior of the cell organism. After 24 h, the consistency of absorbance by the cells was observed. Loss in cell viability also depends on photosensitizer uptake, which can stimulate ROS and result in cell death. Previously reported data showed that a significant quantity of ROS and free radical production was the key to the success of PDT (Atif et al., 2011; Fakhar-e-Alam et al., 2011a).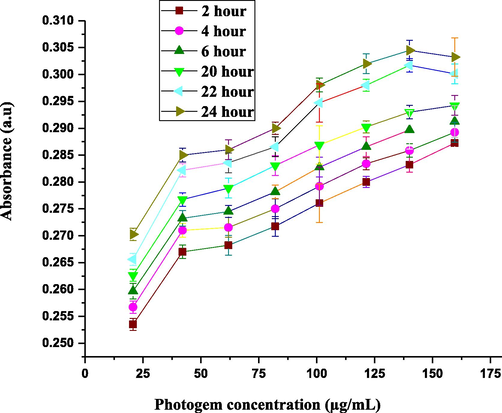
PHOTOGEM® concentration vs. absorbance for different incubation times (0–24 h). Each data point corresponds to mean absorbance (±σ = 4).
Fig. 4 shows that as the concentration of PHOTOGEM® increased from 0 to 160 μg/ml, cellular viability decreased to about 67% in the absence of laser light.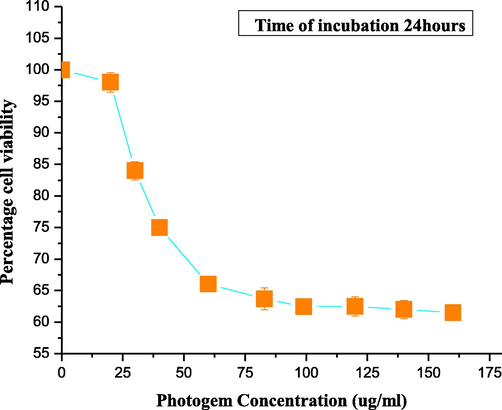
Cellular viability for individual PHOTOGEM®-treated cells. Each data point corresponds to mean absorbance (±σ = 4).
Low concentrations of PHOTOGEM® are preferable in PDT to decrease necrosis caused by high concentrations of the photosensitizer. Therefore, for PHOTOGEM®-mediated PDT for human muscle carcinoma (RD cell line), a concentration of 120 μg/ml was selected as the optimal dose and 5 h was the suitable incubation time. Once the optimal drug concentration was selected and used, no significant toxic effects were noted, and the results were consistent with those of published data (Atif et al., 2011; Fakhar-e-Alam et al., 2011a). Compared with the results of previous studies, PHOTOGEM® indicated promising cytotoxic effects against muscle carcinoma. Published data has shown that the optical density of the photosensitizer is structure dependent (Yow et al., 2007). Two important factors for PDT are the concentration of the drug and a suitable light dose. This study illustrated that PHOTOGEM®-induced cytotoxicity to RD cell line was dose dependent. In Fig. 4, the % cell viability minimally decreased from 100% to 98% for PHOTOGEM® concentration up to 20 μg/ml. Again, toxic effects instead of photochemical reactions were seen when the drug concentration was >120 μg/ml, which can damage the normal cell lines.
Fig. 5 presents the results when RD cells were exposed to a laser light dose of 100 J/cm2 after they were labeled with 0–200 μg/ml PHOTOGEM® and 120 μg/ml was selected as the optimal dose. Fig. 4 clearly shows that a drug dose of 120 μg/ml and a red laser dose of 100 J/cm2 produce the maximum phototoxic effects on muscle carcinoma. Results were verified via ROS detection and staining for mitochondria.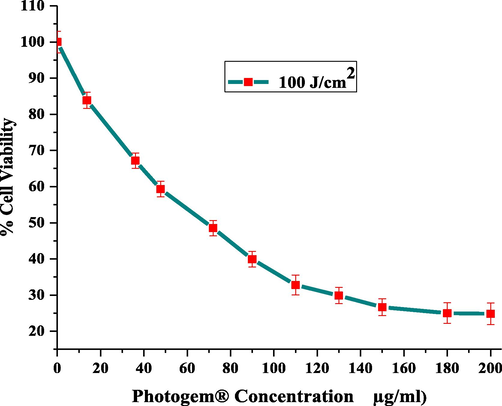
Cellular viability of irradiated (100 J/cm2) cells for different drug doses (0–200 μg/ml). Each data point corresponds to mean absorbance (±σ = 4).
Fig. 6 compares the effects of PDT on RD cells between when no laser was used and a low laser dose of 100 J/cm2 was used with an effective dose concentration of 120 μg/ml. The current study shows strong evidence of cell death enhancement in the PHOTOGEM®-loaded cells with the use of laser treatment and a suitable drug concentration. There are a number of bases for proposing the enhancement of photodynamic reaction by the activation of photosensitizers under feasible circumstances. The production of ROS results in the process of cell death mentioned by many researchers (Kolářová et al., 2007; Nevřelová et al., 2005).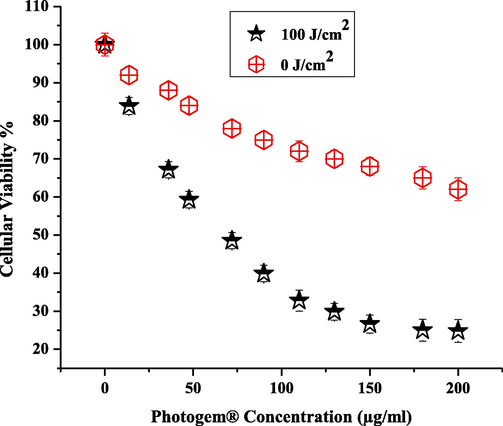
Comparison of irradiated (100 J/cm2) and non-irradiated (0 J/cm2) cells for different drug doses (0–200 μg/ml). Each data point corresponds to mean absorbance (±σ = 4).
We investigated whether loss of cell viability showed a consistent relationship with the fluorescence of ROS. As the ROS increased due to excess photochemical reaction, the loss in cell viability increased, as verified in Fig. 7. The data in Fig. 7 show that the % cell viability of PHOTOGEM®-labeled RD cells decreased to 65% in the absence of laser irradiation, but the decrease was significant for the current biological model when the cells were counted after exposure to the suitable laser dose of 100 J/cm2. When laser irradiation was included, only 25% of viable cells were recorded. In our previous work, we tried to optimize the different PDT parameters such as laser light dose, drug concentration, drug uptake, and incubation time. Results were obtained using many techniques (Dougherty et al., 1998; Iqbal et al., 2019b). In this study, RD cells were labeled with120 µg/ml PHOTOGEM® by incubating the cells and irradiating them with a 100 J/cm2 red laser light for 5 h and then were studied with microscopy for morphological analysis.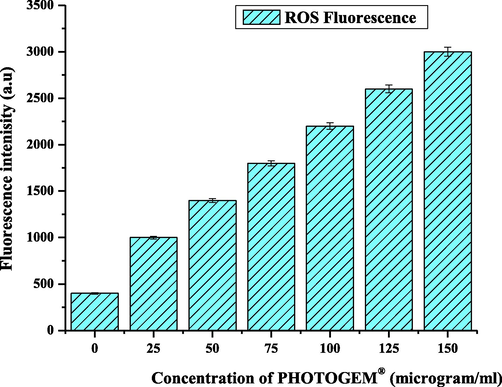
Production of ROS Fluorescence Intensity (a. u).
Fig. 7 revealed the liberation of reactive oxygen species (ROS) via type I and type II mechanism, which reflected the best agreement with loss in cell viability factor. As long as the concentration of drug and light approached to optimum value but also ROS fluorescence increased to maximal value which ultimately enhanced the cell killing effect. (Iqbal et al., 2019b) already reported in published data. This ROS production had in good agreement with drug concentration and light dose.
Fig. 8 depicts morphological analysis of RD cells under various experimental condition. It is easy to visualize the control RD cells with the 50–60% confluence (shown in Fig. 8 (a) in addition, only laser is effective for cell apoptosis mechanism but not completely treated the muscle carcinogenic cells as shown the abnormal size form of cell morphology (shown in Fig. 8 (b)). Luckily 100 µg/ml of PHOTOGEM® liberates enough quantity of reactive oxygen species which leads to necrosis as necrosis form of RD Cells can be seen in Fig. 8 (c). But the most favorable results were obtained when optimal dose of PHOTOGEM® accumulated to RD Cells were treated with 100 J/cm2 of laser light. As the cell cluster and totally ill define structure of rhabdomyosarcoma were seen in Fig. 8 (d). Similar pattern results with various cells line under various experimental conditions in same manner were already published by Iqbal et al. (2019b). It is expected that current study will contribute significant specially to treat muscle cancer, which off course will be great success of biomedical researcher. Photodynamic reaction produced due to PHOTOGEM® under 632.8 nm of He-Ne laser irradiation were seen in Fig. 9.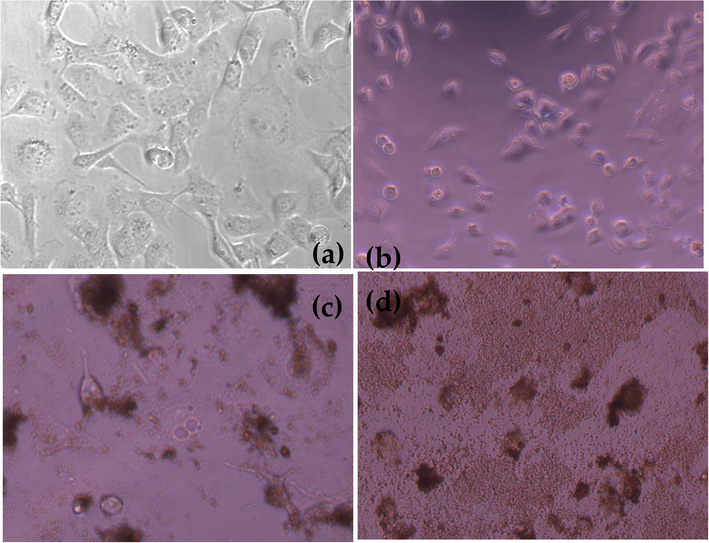
(a) RD Cell Morphology of Control RD Cells (b) Treated with Laser Dose of 100 J/cm2 (c) RD Cells Treated with PHOTOGEM® 100 µg/ml (d) RD Cells Treated with PHOTOGEM® 100 µg/ml exposed under 100 J/cm2 illumination dose of laser.
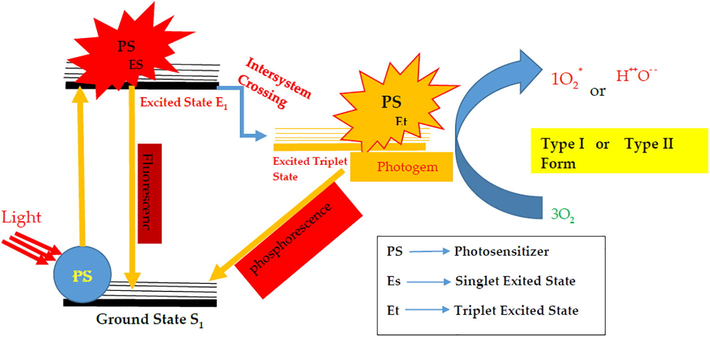
Schematic of Photodynamic Effect produced due to PHOTOGEM® localization in RD cells.
PHOTOGEM® localized into muscle carcinoma under irradiation of red light (He-Ne Laser) response produce synergistic response which decreases the cell viability up to 25% via ROS production as shown in Fig. 9.
4 Conclusion
Recent studies have raised the point that neither PHOTOGEM® nor an optimal radiation dose (100 J/cm2) can produce a cytotoxic reaction in cancer cells perfectly that leads to cell death. An optimized combination of photosensitizer and light was the basic requirement for an effective tumoricidal PDT that was a direct, efficient, sophisticated, painless, noninvasive, rapidly healing, and effective technique for cancer cell death. This tumor-selective technique is becoming a more popular treatment modality for muscle cancer. The current study showed PHOTOGEM® as the unique chemical agent that not only induces cytotoxicity in RD cells but also can cause a synergistic effect with suitable laser exposure (He Neon Laser). The viability of human muscle carcinoma (RD) cells was 25% when the cells were incubated with PHOTOGEM® at an effective dose of 120 μg/ml and irradiated with a 100-J/cm2 632.8 nm of He-Ne laser irradiation. In summary, cell viability loss found was 75%.
Acknowledgments
The Higher Education Commission (HEC), Islamabad, Pakistan, is acknowledged for its help under NRPU Research Grant number:8056/Punjab/NRPU/R&D/HEC/2017. The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group number RGP-293.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tailoring of Au-TiO2 nanoparticles conjugated with doxorubicin for their synergistic response and photodynamic therapy applications. J. Photochem. Photobiol., A 2019
- [CrossRef] [Google Scholar]
- PD/PDT for gynecological disease: a clinical review. Photodiagnosis Photodyn. Ther.. 2005;2:51-63.
- [Google Scholar]
- Study of the efficacy of 5-ALA mediated photodynamic therapy on human rhabdomyosarcoma cell line (RD) Laser Phys. Lett. 2010
- [Google Scholar]
- Analysis of the combined effect of lasers of different wavelengths for PDT outcome using 600, 630, and 660 nm. Laser Phys. Lett. 2011
- [Google Scholar]
- Manganese-doped cerium oxide nanocomposite induced photodynamic therapy in MCF-7 cancer cells and antibacterial activity. BioMed Res. Int. 2019
- [Google Scholar]
- Children from ethnic minorities have benefited equally as other children from contemporary therapy for rhabdomyosarcoma: a report from the intergroup Rhabdomyosarcoma study group. J. Clin. Oncol. 2002
- [Google Scholar]
- The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004
- [Google Scholar]
- Overall results for a national program of photodynamic therapy for basal cell carcinoma: a multicenter clinical study to bring new techniques to social health care. Cancer Control 2019
- [CrossRef] [Google Scholar]
- fluorescence spectral changes of hematoporphyrin derivative upon binding to lipid vesicles, staphylococcus aureus and Escherichia coli Cells. Photochem. Photobiol. 1985
- [Google Scholar]
- Role of ALA sensitivity in HepG2 cell in the presence of diode laser. Laser Phys. 2011
- [Google Scholar]
- The role of sensitivity of ALA (PpIX)-based PDT on Human embryonic kidney cell line (HEK293T) Laser Phys. 2011
- [Google Scholar]
- Gouterman, M., 1978. Optical spectra and electronic structure of porphyrins and related rings. In: The Porphyrins. https://doi.org/10.1016/b978-0-12-220103-5.50008-8.
- Gurney JC, Smith MA, Bunin GR, et al, 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program. In: National Cancer Institute SEER Program.
- Application of silver oxide nanoparticles for the treatment of cancer. J. Mol. Struct. 2019
- [Google Scholar]
- Structural, morphological, antimicrobial, and in vitro photodynamic therapeutic assessments of novel Zn+2-substituted cobalt ferrite nanoparticles. Results Phys. 2019
- [CrossRef] [Google Scholar]
- Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2012
- [Google Scholar]
- In vitro study of reactive oxygen species production during photodynamic therapy in ultrasound-pretreated cancer cells. Physiol. Res. 2007
- [Google Scholar]
- Vitamin D3–assisted chemo-photodynamic therapy of rhabdomyosarcoma cancer cells for effective treatment. Laser Phys. Lett.. 2018;15:125602.
- [CrossRef] [Google Scholar]
- Pharmacokinetics of Photogem using fluorescence monitoring in Wistar rats. J. Photochem. Photobiol., B 2004
- [Google Scholar]
- Treatment of breast cancer with capped magnetic-NPs induced hyperthermia therapy. J. Mol. Struct. 2019
- [Google Scholar]
- Nevřelová, P., Kolářová, H., Bajgar, R., Maceček, J., Tomečka, M., Tománková, K., Strnad, M., 2005. Measurement of reactive oxygen species after photodynamic therapy in vitro. Scr. Medica Fac. Medicae Univ. Brun. Masaryk.
- Hypericin and its radio iodinated derivatives – A novel combined approach for the treatment of pediatric alveolar rhabdomyosarcoma cells in vitro. Photodiagnosis Photodyn. Ther. 2020
- [CrossRef] [Google Scholar]
- Parham, D.M., 1994. The molecular biology of childhood rhabdomyosarcoma. Semin. Diagn. Pathol.
- Resistance to photodynamic therapy in radiation induced fibrosarcoma-1 and Chinese hamster ovary-multi-drug resistant cells in vitro. Photochem. Photobiol. 1991
- [Google Scholar]
- What does photodynamic therapy have to offer radiation oncologists (or their cancer patients)? Radiother. Oncol. 1998
- [Google Scholar]
- Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: an analysis of 26,758 cases. Int. J. Cancer 2006
- [Google Scholar]
- Study of the efficacy and mechanism of ALA-mediated photodynamic therapy on human hepatocellular carcinoma cell. LiverInt 2007
- [Google Scholar]







