Translate this page into:
Special glass for silver-sodium ion exchanged waveguides
⁎Corresponding author. bentouila.om@univ-ouargla.dz (Omar Bentouila)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In the present paper, we report an experimental study on a special glass developed for Ag+-Na+ ion-exchange. The glass composition and the influence of each element of the composition on the diffusion parameters were studied. Some parameters of exchange are determined, as well as the diffusion coefficient, refractive index variation, propagation and coupling losses. Such parameters can determine the fabrication conditions of integrated devices. The obtained waveguides have low coupling and propagation losses. The behavior of the fabricated waveguides at low temperatures is tested. It observed that, at these temperatures the diffusion is not negligible, which can affect the life time of the components. In order to improve this parameter, a modification of the amount of some elements of the glass composition is necessary, specially the alumina.
Keywords
Integrated optical waveguides
Ion-exchange technology
Glass composition
1 Introduction
Ion exchange in glass is a suitable technique which is widely applied to modify the surface composition and properties of the glass surface without changing the bulk glass properties (Guldiren et al., 2016). This technique is usually used to fabricate glass waveguide owing to their benefits compared with other fabrication techniques (Wang et al., 2015). Waveguides made by ion-exchange on glass substrates are good candidates for passive integrated optics applications (Ramaswamy and Srivastava, 1988; Opilski et al., 2000). In addition, the integrated waveguide fabricated by this technology is a process well suited to industrial production. The quality and the performances of such waveguides depend strongly on the glass composition and on the type of the dopant ions (Ag+, K+, Tl+, Cs+, etc.), which determine the diffusion parameters of the exchange (Rehouma and Aiadi, 2008). Some conditions are required on glass to use it in this technology. It must contains enough of alkaline oxides in order to create an important variation of refractive index, low intrinsic losses, the refractive index of the material is close to that of the silicon, chemical durability, no bubbles and high degree of homogeneity, easy diffusion of the ions, simple production, the fusion temperature is higher than the diffusion temperature and finally a low Haven ratio.
In this work, we report an experimental study on a special glass developed for Ag+-Na+ ion-exchange. The study includes the glass composition, and the influence of each element of the composition on the diffusion parameters. The fabrication parameters, the characterization of the fabricated waveguides on this glass and its performances are presented. The results are discussed in order to improve this composition.
2 Experimental
2.1 Glass's composition and characteristics
The glass used in this study contains as network former oxide with quantity in weight (%) of 61–64%. This amount gives the glass strength and high transparency in the visible and near infrared ranges. The alumina ( ) is introduced as network intermediate oxide with 11–13%. The intermediate oxide, contribute to the glass strength, causes an elevation of refractive index. The existence of this quantity in the glass permits to increase the electrical conductivity, the chemical durability of glass and the diffusion efficiency in the material. As network modifier oxides, Na2O, Li2O, MgO and CaO are introduced with 12–14, 0.5–1, 3–4 and 3–5% respectively. The addition of alkaline oxides (Na2O and Li2O) permits to reduce the elaboration temperature, the viscosity and the chemical durability of glass. Moreover, the presence of alkaline oxides increases the dilatation coefficient, the electrical conductivity and the refractive index. The MgO and CaO improve also the chemical resistance of glass and reduce the electrical conductivity. The introduction of B2O3, in this case (4–6%), increases the chemical resistance and decreases the dilatation coefficient. It should be noted, that the composition do not contains FeO, As2O3 or Sb2O3 since these elements cause the reduction of silver ions leading to excessive losses in the substrate. The glass samples, with size of 6 cm, were prepared starting from high purity powders according the weight (%) composition mentioned above by melt-quenching method. This method includes a series of classical steps: mixing of starting materials, melting, fining, casting and annealing (Murugasen et al., 2015). The refractive index of this glass is nearly of 1.51 (λ = 0.8 μm) and the temperature of fusion (Tg) is in order of 560 °C.
2.2 Ion-exchanged waveguide's parameters
In order to design optical waveguides made by ion-exchange in this glass, it is important to characterize the diffusion properties of cations in the substrate. The kinetic of diffusion is obtained by analysis of the refractive index profile of planar waveguides calculated by the inverse WKB method from the effective index of multimode structures (Hocker and Burns, 1975; Kaul and Thyagarajan, 1984; Ding et al., 2004).
The diffusion coefficient and ionic mobility are deduced from this experiment by fitting numerically the index profiles by finite difference program. Such program simulates the differential equation (Fick’s law) which governs the diffusion kinetic of the two cations. The diffusion coefficient D (
) is determined experimentally for two different concentrations (2% and 10% Molar fraction) of silver in a molten bath of Sodium Nitrate (NaNO3). The measurements of D were performed for different temperatures varying from 250 to 350 °C (Table 1). The time of diffusion is a few seconds for each operation.
Temperature (°C)
250
300
350
D(
), CAg = 2%
0.037
0.15
0.6
D(
), CAg = 10%
0.08
0.35
1.2
The refractive index variation (Δn) after the thermal exchange depends also on the silver concentration in the molten bath. The experimental results are given in the table 2.
Concentration of AgNO3
(molar fraction%)0.2
0.4
0.5
1
5
10
14
at the glass surface (x10−2)
1.02
1.91
2.1
2.9
5.85
6.95
7.33
We assume that D (T) has temperature dependence given by the following law:
The Fig. 1 illustrates the variation of D as a function of temperature (°C).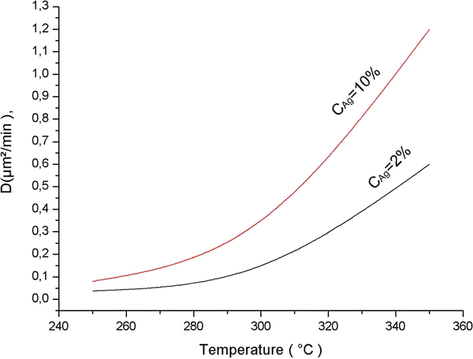
The variation of D as function of silver concentration and temperature.
A glass waveguide elaborated with a silver concentration of 10% has a diffusion coefficient which is equal to 0.00002 µm2/min at 80 °C, which is not negligible. That is to say that it takes about 9 days for Ag+ ion move over a distance of 1 µm ( ) into the glass. This aspect is confirmed by heating the fabricated planner waveguides in a furnace, at low temperature (100 °C), for several days (until 750 h). It observed that the variation of refractive index of the fundamental mode, decrease from 0.055 to 0.047.
The diffusion of K+ is also tested, by immersing the glass substrate in a molten bath of KNO3. The temperature of the exchange is more than 400 °C, for a time of diffusion more than four hours. From the obtained planner waveguides, the diffusion coefficient D and Δn are determined experimentally. D depends on the temperature of diffusion. For T varies from 390 to 460 °C, D changes from 0.012 to 0.065 µm2/min. The refractive index variation depends only on the kind of the dopant. In this case, it is about 0.0125. This glass is not well-suited for K+ exchange due to the qualities of the obtained buried waveguides (Tervonen et al., 2011).
A channel-waveguides are made by two-step exchange process (Rehouma et al., 1995). During the first step, a surface channel-waveguide is fabricated by purely thermal exchange between the Ag+ cations of the bath and the Na+ cations present in the glass, through a mask previously deposited on the glass surface. This mask is then removed; the waveguide is buried below the surface by application of an external electric field across the substrate, during the second step. The obtained waveguides, 3 cm of length, are well compatible with optical fiber. The insertion losses (input, output coupling and propagation losses) of such waveguides are less than 0.8 dB. These losses were divided as follow: 0.4 dB in input and output coupling losses (Rehouma et al., 2007), less than 0.39 dB (0.13 dB/cm) in propagation losses (at λ= 0.785 μm) (Rehouma and Aiadi, 2008). The propagation losses are measured by the Cut back method (Cai et al., 2013; Kaminow and Stulz, 1978).
3 Discussions
We can draw several remarks from the results. Firstly, the important quantity of Na2O in the studied glass is responsible of the considerable variation of the refractive index of the exchanged waveguides (Inácio et al., 2013; Rehouma, 1994). The diffusion coefficient (D) of the external cations into glass depends on the concentration of Al2O3 and Na2O of the glass in the one hand (Mendez and Morse, 2007), on the other hand, it depends on the concentration of silver in the molten bath and its temperature (Rehouma and Aiadi, 2008). D is important, in spite of the existence of B2O3 in the glass. This parameter remains important even at low temperature (60–100 °C), which affects on the lifetime of the fabricated integrated devices. It will be preferable to have a value of activation energy 23 kcal/mol in order to extend the stability of the components over 20 years. To improve this factor, a reduction of Al2O3 and replace it by an alkaline oxide like K2O is necessary (Tomozawa, 1993). A saturation of Δn is obtained for a silver concentration about 10% in the bath. It means economically, is not advised to use more than this concentration in order to obtain a maximum of Δn. The temperature of the exchange (300–400 °C) is largely less than the Tg of the glass, then the fabrication condition has no affect on the substrate. The transparency and the homogeneity of the material are demonstrated by the obtained low losses propagation. Its refractive index (1.51) is nearly close to that of optical fiber (1.46), but the addition of small quantity of fluoride (less than 4%) can reduce significantly this parameter (García-Bellés et al., 2017; Rabinovich, 1983).
4 Conclusions
This study shows that this composition of glass is well adapted for silver-sodium ion-exchange. A good qualities and performances of the fabricated waveguides were obtained in terms of propagation and coupling losses. The factor of stability of the components must be taken in consideration for a future evolution of this composition.
References
- A new fabrication method for all-PDMS waveguides. Sensors Actuators, A Phys.. 2013;204:44-47.
- [CrossRef] [Google Scholar]
- Determination of optical waveguide refractive-index profiles with the inverse analytic transfer matrix method. Opt. Quantum Electron.. 2004;36:489-497.
- [CrossRef] [Google Scholar]
- Effect of fluorine and nitrogen content on the properties of Ca-Mg-Si-Al-O-(N)-(F) glasses. Ceram. Int.. 2017;43:4197-4204.
- [CrossRef] [Google Scholar]
- Influence of silver and potassium ion exchange on physical and mechanical properties of soda lime glass. J. Non. Cryst. Solids. 2016;441:1-9.
- [CrossRef] [Google Scholar]
- Modes in diffused optical waveguides of arbitrary index profile. IEEE J. Quantum Electron.. 1975;11:270-276.
- [CrossRef] [Google Scholar]
- Silver migration at the surface of ion-exchange waveguides: a plasmonic template. Opt. Mater.. 2013;3:390.
- [CrossRef] [Google Scholar]
- Loss in cleaved Ti-diffused LiNbO3waveguides. Appl. Phys. Lett.. 1978;33:62-64.
- [CrossRef] [Google Scholar]
- Inverse WKB method for refractive index profile estimation of monomode graded index planar optical waveguides. Opt. Commun.. 1984;48:313-316.
- [CrossRef] [Google Scholar]
- Specialty Optical Fibers Handbook (1st ed.). Academic Press; 2007.
- Preparation, techniques and tools used for investigating glasses: an overview. Int. J. Chem. Sci.. 2015;13:693-713.
- [Google Scholar]
- present state and perspectives involving application of ion exchange in glass. Opto-Electronics Rev.. 2000;8:117-127.
- [Google Scholar]
- Ion-Exchanged Glass Waveguides: A Review. J. Light. Technol.. 1988;6:984-1000.
- [CrossRef] [Google Scholar]
- Study of silver ion exchange in aluminoborosilicate glass. France: Institut National Polytechnique de Grenoble; 1994. (Doctoral dissertation)
- Integrated structure for dual role: measuring the fiber – Guide coupling losses and sensing the external medium variations. J. Optoelectron. Adv. Mater.. 2007;9:2351-2353.
- [Google Scholar]
- A new fabrication method for waveguides with controlled surface-interaction length. Sensors Actuators B. Chem.. 1995;29:406-409.
- [CrossRef] [Google Scholar]
- Ion-exchanged glass waveguide technology: a review. Opt. Eng.. 2011;50:71107.
- [CrossRef] [Google Scholar]
- Alkali ionic transport in mixed alkali glasses. J. Non. Cryst. Solids. 1993;152:59-69.
- [CrossRef] [Google Scholar]
- Alkaline aluminum phosphate glasses for thermal ion-exchanged optical waveguide. Opt. Mater. (Amst). 2015;42:484-490.
- [CrossRef] [Google Scholar]







