Translate this page into:
Spatial patterns of Pisidium chilense (Mollusca Bivalvia) and Hyalella patagonica (Crustacea, Amphipoda) in an unpolluted stream in Navarino island (54° S, Cape Horn Biosphere Reserve)
⁎Corresponding author at: Laboratorio de Ecología Aplicada y Biodiversidad, Escuela de Ciencias Ambientales, Facultad de Recursos Naturales, Universidad Católica de Temuco, Casilla 15-D, Chile. prios@uct.cl (Patricio De Los Ríos Escalante)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The southern South American inland waters have many endemic species and some of them are considered as endangered for IUCN, that inhabits in unpolluted ecosystems, one of these ecosystems are the sub-Antarctic perennial forests located in the Cape Horn Biosphere Reserve at 54° S. The aim of the present study is to analyze the spatial patterns of Pisidium chilense Ituarte, 1999 (Mollusca Bivalvia) and Hyalella patagonica (Cunningham, 1871) (Crustacea, Amphipoda) in an unpolluted stream. Both species had aggregated spatial distribution, both have a negative binomial distribution pattern, and both are associated. The present results would agree with similar patterns in Patagonian rivers where both species coexist.
Keywords
Pisidium chilense
Hyalella patagonica
Species association
Negative binomial distribution
1 Introduction
The Southern Patagonia has unpolluted ecosystems with low human intervention due mainly to their geographic isolation and difficult access. There are many endemic species, especially in inland water bodies (Jara et al., 2006). The Cape Horn Biosphere Reserve is located in the southern island of South America with unpolluted and pristine sub-Antarctic perennial forests with unpolluted lakes, rivers and streams, the fauna is markedly endemic (Moorman et al., 2006). The benthic fauna spatial distribution can be explained in accordance to probabilistic models that can explain if the species have random, aggregated or uniform spatial distribution (Elliot, 1983; Zar, 1999). On this basis, the spatial pattern can adjust to Poisson, negative binomial or binomial distribution respectively (Elliot, 1983; Fernandes et al., 2003; De los Rios-Escalante, 2011). The aim of the present study is to analyze the spatial distribution of two species representative from Southern Patagonian inland waters, the bivalve Pisidium chilense Ituarte, 1999 and the crustacean Hyalella patagonica (Cunningham, 1871), P. chilense is considered as endangered for IUCN (IUCN, 2015). Both species are important in inland water communities in Southern Patagonia (Moorman et al., 2006).
2 Materials and methods
The studied site was a small stream close to Robalo river located close to Puerto Williams town in an protected unpolluted area, close to Cape Horn Biosphere Reserve, in Navarino island (54° 55′ 47,3′ S; 67° 33′ 43,8″ W, Fig. 1). Samples were collected from the riparian zone of this shallow stream using a ½ L plastic cube with a 1 mm mesh, 20 replicas were collected random along a transect of 50 m from the study site, and the specimens were counted in situ. The site was visited and sampled on the 10th February 2010. It was used in a 2 × 2 contingency table analysis with the species H. patagonica and P. chilense as the first and second categorical variables. The Mean abundance (and variance) was calculated for both species with the aim of determining if their spatial dispersions are aggregated, random or uniform using Variable/Mean ratio to determine if both species are associated (Zar, 1999; Gotelli and Ellison, 2004). Software Xlstat 10.0 was used when the population dispersions were described by the negative binomial distribution (Zar, 1999; Fernandes et al., 2003).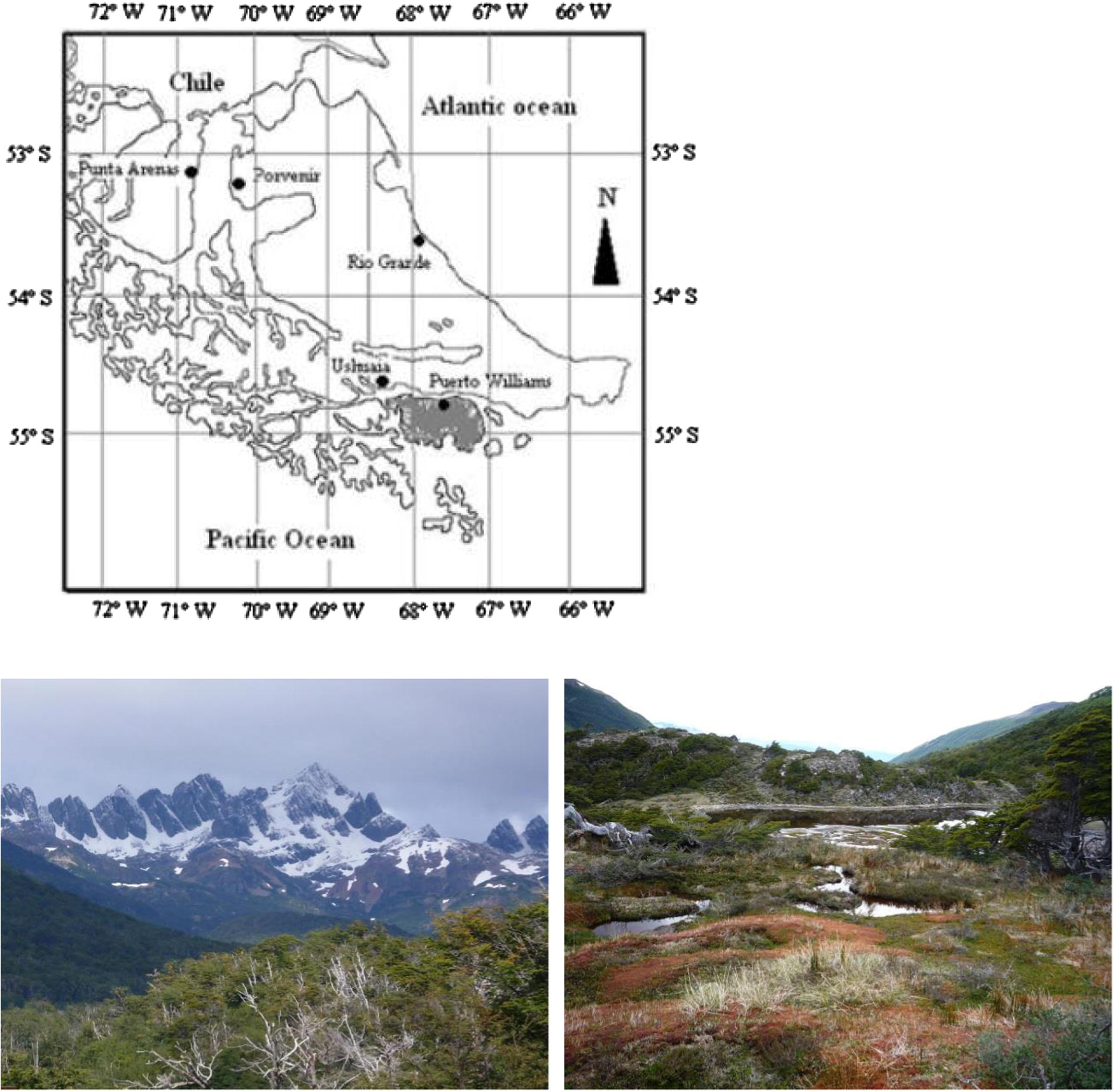
Location Navarino island (in gray), Puerto Williams town and photograph of studied site.
3 Results and discussion
The results of the 2 × 2 contingence table revealed first that both species are not independent in the sampled site, this means that both species share the habitat together (χ2 observed = 1365.03 > χ2 table, 3.841), about spatial pattern, the population has an aggregated distribution pattern (Table 1; χ2 observed = 0.656<; χ2 table, 0.05;14 = 3.841) and a negative binomial distribution pattern for H. patagonica (χ2 observed = 0.031<; χ2 table, 0.05;14 = 23.685; Fig. 1) and P. chilense (χ2 observed = 1.114<; χ2 table, 0.05;1 = 23.685; Fig. 2). The results in density would be similar with ecological observations for central Chilean rivers where both species can coexist mainly in middle zones of the rivers with high organic matter contents (Figueroa et al., 2003, 2007; Dominguez and Fernández, 2009).
Sampling unit
Hyalella patagonica
Pisidium chilense
1
3
1
2
1
0
3
7
0
4
0
0
5
2
3
6
2
0
7
1
3
8
0
0
9
3
0
10
3
6
11
1
3
12
0
3
13
1
3
14
1
3
15
2
1
16
1
6
17
5
0
18
1
5
19
6
1
20
6
2
Mean
2.30
2.00
Variance
4.54
4.11
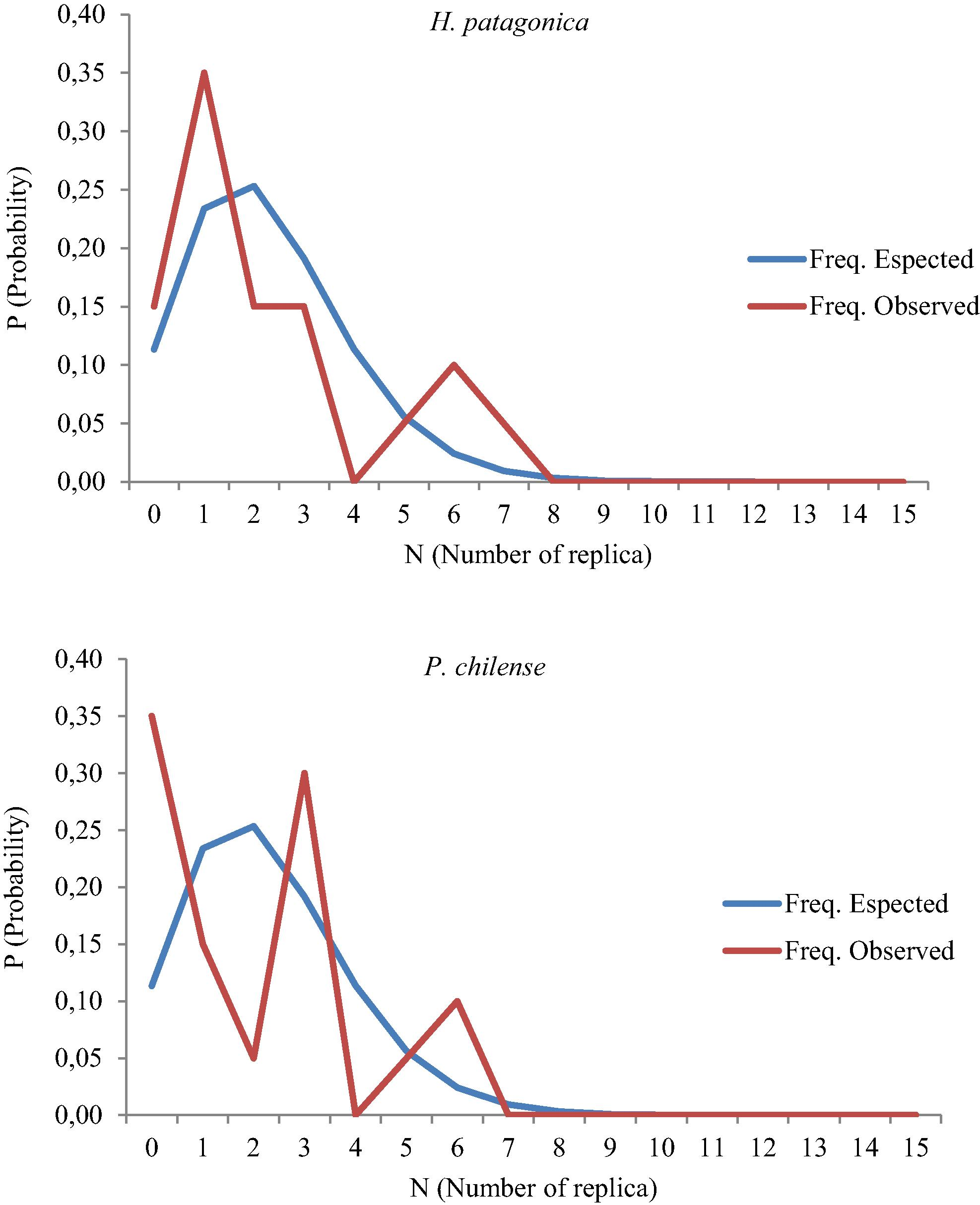
Results of negative binomial distribution observed for H. patagonica and P. chilense.
The coexistence of genus Hyalella and Pisidium was also described for small streams in Uruguay at temperate latitude (Morelli and Verdi, 2014) and in tropical wetlands in Colombia (Rivera et al., 2013). The association of both species would agree with associations of Hyalella and Pisidium genus in central Chilean Patagonia at 45° S, because both species are frequent preys of native fish populations (Belk et al., 2013). Also, both species are present in salmonid diets, these results would agree with descriptions for Iceland Salvelinus alpinus populations (Woods et al., 2013). The obtained results agree with descriptions of Anderson et al. (2014) who mentioned associations between Hyalella and Pisidium in different kinds of waterbodies in Tierra del Fuego island and Beagle Channel islands in sites with beaver (Castor canadiensis) presence and absence.
Negative binomial distribution pattern observed in both populations agree with the literature descriptions about ecological data (Hilborn and Mangel, 1997; Zar, 1999; Maruyama et al., 2002; Fernandes et al., 2003), mainly benthic data (Elliot, 1983; Gray, 2005; Noro and Buckup, 2010), for ectocommensals (De los Ríos-Escalante, 2014) and parasitological interactions (Shaw et al., 1998; Peña-Rehbein and De los Ríos-Escalante, 2012; Peña-Rehbein et al., 2013). Nevertheless, De los Rios-Escalante (2011) did not find distributions for benthic crustaceans in lakes and rivers which would be due to marked environmental heterogeneity of different studied sites that would not happen in the present study.
Acknowledgements
The present study was funded by project MECESUP UCT and valuable suggestions of M.I. for improvement of the manuscript.
References
- Engineering by an invasive species alters landscape-level ecosystem function, but does not affect biodiversity in freshwater systems. Divers. Distrib.. 2014;20:214-222.
- [Google Scholar]
- Ecology of Galaxias platei in a depauperate lake. Ecol. Freshwater Fishes. 2013;23 615-521
- [Google Scholar]
- Probabilistic model for understand the presence of Temnocephala chilensis (Moquin-Tandom 1846)(Platyhelminthes: Temnocephalidae) on adults of a population of Parastacus pugnax (Poeppig 1835)(Decapoda: Parastacidae) in southern Chile. Vol vol. 78. Gayana; 2014. p. :81-84.
- The presence of the genus Hyalella (Smith, 1875) in water bodies near to Puerto Williams (Cape Horn Biosphere Reserve, 54° S, Chile)(Crustacea, Amphipoda) Pan Am. J. Aquat. Sci.. 2011;6:273-279.
- [Google Scholar]
- Dominguez E., Fernández H.R., eds. Macroinvertebrados bentónicos sudamericanos. Sistemática y Biología: 1-654. Tucumán, Argentina: Fundación Miguel Lillo; 2009.
- Some methods for the Statistical Analysis of Benthic Invertberates. Vol 25. Freshwater Biological Association of Sciences Publications; 1983. 1–157
- Distribucao espacial de Alabama argillacea (Hubner)(Lepidoptera: Noctuidae) Neotropical Entomol.. 2003;32:107-115.
- [Google Scholar]
- Macroinvertebrados bentónicos como indicadores de calidad de agua de ríos del sur de Chile. Rev. Chil. Hist. Nat.. 2003;76:275-285.
- [Google Scholar]
- Análisis comparativo de índices bióticos utilizados en la evaluación de la calidad de las aguas en un río mediterráneo de Chile: río Chillán, VIII Región. Rev. Chil. Hist. Nat.. 2007;80:225-242.
- [Google Scholar]
- The Ecological Detective Confronting Model with Data. Monographs on Population Biology. Princeton, New Jersey: Princeton University Press, New Jersey; 1997. pp. 315
- Selecting a distributional assumption for modeling relative densities of benthic macroinvertebrates. Ecol. Model.. 2005;185:1-12.
- [Google Scholar]
- A Primer of Ecological Statistics. Sunderland, Massachusetts, U.S.A: Sinauer Associated, Inc; 2004. pp. 510
- International Union for Conservation of Nature, IUCN, 2015. <http://www.iucnredlist.org/details/189042/0> (visited 23.06.2016).
- Estados de conocimiento de los malacostracos dulceacuícolas. Gayana. 2006;70:40-49.
- [Google Scholar]
- Distribucao espacial de Dilobopterus costalimai Young (Hemiptera: Cicadellidae) em Cistros na regiao de Tamaringa SP. Neotropical Entomol.. 2002;31:034-040.
- [Google Scholar]
- Watershed conservation and aquatic benthic macroinvertebrate diversity in the Alberto D’Agostini National Park. Anales del Instituto de la Patagonia. 2006;34:41-58.
- [Google Scholar]
- Diversidad de macroinvertebrados acuáticos de agua dulce con vegetación ribereña nativa de Uruguay. Rev. Mex. Biodiversidad. 2014;85:1160-1170.
- [Google Scholar]
- The burrows of Parastacus defossus (Decapoda: Parastacidae), a fossorial freshwater crayfish from southern Brazil. Zoologia (Curitiba). 2010;27:341-346.
- [Google Scholar]
- Use of negative binomial distribution to describe the presence of Anisakis in Thyrsites atun. Rev. Bras. Parasitol. Vet.. 2012;21:78-80.
- [Google Scholar]
- Use of a negative binomial distribution to describe the presence of Sphyrion laevigatum in Genypterus blacodes. Rev. Bras. Parasitol. Vet.. 2013;22:602-604.
- [Google Scholar]
- Grupos tróficos de macroinvertebrados acuáticos en un humedal urbano andino de Colombia. Acta Biol. Colomb.. 2013;18:279-292.
- [Google Scholar]
- Patterns of macroparasite aggregation in wildlife host populations. Parasitology. 1998;117:597-610.
- [Google Scholar]
- Variability in the functional role of ArcticcharrSalvelinusalpinus as it related to lake ecosystem characteristics. Environ. Biol. Fishes. 2013;96:1361-1376.
- [Google Scholar]
- Biostatistical analysis: 1–663. New Jersey: Prentice Hall; 1999.







