Translate this page into:
Solvent extraction of Pb(II) and Zn(II) from a Nigerian galena ore leach liquor by tributylphosphate and bis(2,4,4-trimethylpentyl)phosphinic acid
*Corresponding author. Tel.: +234 8035010302 alafara@unilorin.edu.ng (Alafara A. Baba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 17 July 2013
Abstract
Physico-chemical conditions for the optimal separation of Pb(II) and Zn(II) from a galena ore leachate using a combination of tributylphosphate (TBP) and bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex®272) have been investigated. The results of fundamental studies using synthetic solution of Pb(II) showed that the extraction efficiency increased with increasing pH, extractant concentration and temperature. An extraction yield of 92.1 ± 0.2% for Pb(II) at pH 5.0 by 1 mol/L TBP in 100% MIBK and 94.6 ± 0.2% for Zn(II) at pH 3.0 by 0.032 mol/L Cyanex®272 in kerosene was obtained from an initial galena leach liquor containing 1705.1 mg/L Pb, 98.7 mg/L Zn and 130.2 mg/L total iron as major constituents at 25 ± 2 °C at a stirring time of 30 and 25 min, respectively. Iron was also effectively removed by precipitation using 1 mol/L ammoniacal solution at pH 3.5. The stoichiometry of the lead-extractable species was found to be HPbCl3·TBP. Values of 52.7 ± 0.01 kJ mol−1, −5.05 kJ mol−1, 159.6 ± 0.01 J K−1 mol−1 and 0.127 ± 0.003 M−2 were calculated for the apparent standard molar enthalpy (ΔH°), Gibb’s free energy (ΔG°), molar entropy (ΔS°) and extraction constant, respectively. About 94.5 ± 0.2% Pb(II) and 94.6 ± 0.2% Zn(II) were stripped from the TBP and Cyanex®272, respectively using 0.1 mol/L HCl solution. Finally, a compact extraction scheme has been provided.
Keywords
Galena ore
Lead
Zinc
Solvent extraction
TBP
Cyanex®272
1 Introduction
The galena ore (PbS) is the most important sulfide mineral and is generally treated pyrometallurgically. However, lead smelting faces difficulties with respect to environmental regulations. Therefore, the hydrometallurgical recovery of lead from galena may be a promising process with environmentally inert elemental sulfur being formed instead of sulfur dioxide (Baba and Adekola, 2011; Adebayo et al., 2003). As a result of drastic decrease in high grade ore reserves globally and because of high metal demand, hydrometallurgical route has been an alternative to pyrometallurgical processes for sulfidic ores and concentrates, particularly for small scale production and for low grade ores (Sarangi et al., 2007; Kesari et al., 2009; Rotuska and Chmielewski, 2008).
The intrinsic nature of chloride leach liquors resulting from the hydrometallurgical treatments of sulfide ores is rather complex; these solutions usually contain relatively high concentrations of basic metals, as well as small contents of other rare metals or frequently precious metals (Dutrizac et al., 1993). Therefore, for the separation and/or purification of these valuable elements, the application of solvent extraction procedures has been extensively considered (Quinn et al., 2013; Paiva and Abrantes, 2001; Cote and Jakubiak, 1996; Rydberg et al., 1992).
Consequently, solvent extraction is one of the efficient hydrometallurgical techniques used for purification, separation or concentration of metals from aqueous media. It has found extensive application in mineral processing (Facon et al., 2007). Hence, hydrometallurgical solvent extraction processes have become major purification operations in practice with special emphasis on Pb(II) and Zn(II) separation from their coexisting species such as Co(II), Ni(II) and Cu(II). Presence of impurities such as Cd, Ni, Fe and Co in dissolved low grade sulfide ores and the need for concentrated zinc production, for example, made solvent extraction a very attractive pretreatment sequence for electrowinning process (Tsakiridis et al., 2010; Sayar et al., 2007; Yadav and Khopkar, 1971). The process train consists of three sections: leaching, leachate purification and electrowinning. The leachate purification train includes precipitation and cementation in which effective metals are removed from ore and made suitable for electrowinning. The purification of the leachate is a basic requirement for good quality of final product and current efficiency in electrowinning (Casaroli et al., 2005).
In general, several studies have been carried out on Zn(II) by solvent extraction (Baba and Adekola, 2011; Hosseini et al., 2011; Fu et al., 2010; Baba et al., 2009; Facon et al., 2007; Sayar et al., 2007; Ali et al., 2006; Alguacil and Martinez, 2001); while there are limited data on the Pb(II) by solvent extraction (Shilimkar and Anuse, 2002; Mewoyo et al., 1996; Holdich and Lawson, 1985; Zaborska and Leszko, 1980; Yadav and Khopkar, 1971). Recently, the use of Cyanex®272 in the metal ion separation has been reported (Banda et al., 2012; Wang et al., 2012; Yu et al., 2012). In all of these, the efforts of investigators were geared toward the establishment of the extraction conditions in terms of the metal extractability in aqueous and organic phases at different pH and solvent concentrations, while other workers focused on the development of separation route, stripping as well as determining the number of stages for a quantitative extraction via McCabe–Thiele diagrams.
There is practically no reported work on the hydrometallurgical recovery of Pb(II) and Zn(II) from the galena ore of Nigerian origin. In recent years, the recovery of these metals from aqueous chloride solutions has attracted much attention. This is due to the high efficiency of the chloride leaching process which is now recognized as a logical choice for treating complex sulfide ore concentrates (Cote and Jakubiak, 1996). Hence, the present study was focused on Pb(II) extraction and separation from other impurities such as zinc, iron, silver, etc. from a Nigerian galena ore leach liquor and the development of a simple route for the hydrometallurgical recovery of these metals. This is the first in-depth study on the hydrometallurgical recovery of Pb(II) and Zn(II) from a Nigerian galena ore by tributylphosphate and bis(2,4,4-trimethylpentyl)phosphinic acid. The ease and high rate of separation, regeneration, complexation and stability are proven reasons for selecting the Cyanex®272 extractant in this study. Experimental data on the characterization and dissolution kinetics of this ore had earlier been discussed (Baba and Adekola, 2012).
2 Materials and methods
2.1 Reagents
For this study, three phosphorus based extractants were employed. These are:
-
Tributylphosphate TBP (Merck, purity 99%)
-
Triphenylphosphite, TPP (BDH Chemicals, Poole England, purity 97%); and
-
Bis(2,4,4-trimethylpentyl)phosphoric acid: Cyanex®272, (Cytec Industries, Rungis Cedex, France, purity 85–90%.
Distilled kerosene and methyl isobutyl ketone (MIBK) were used as organic diluents throughout this work. In the absence of TBP, the metal ion extraction such as lead by MIBK in aqueous chloride media is not quantitative (Yadav and Khopkar, 1971), but its high aqueous solubility, solvency characteristics with high solvation for complexation are reasons for choosing MIBK as diluent (Wasewar and Pangarkar, 2006). Zinc Chloride, lead nitrate, and hydrochloric acid were obtained from BDH reagent grades and were used for preparing the aqueous solutions.
2.2 Extraction procedures
The experimental procedure adopted comprised of a preliminary study aimed at establishing optimal conditions for the extraction of Pb(II) and Zn(II) from synthetic lead and zinc solutions by TBP and Cyanex®272, respectively and with subsequent applications to the recovery of Pb(II) and Zn(II) from the galena leachate (Baba and Adekola, 2011; Baba, 2008).
2.2.1 Fundamental studies on Zn(II) and Pb(II) synthetic salts
The aqueous solutions of Zn(II) and Pb(II) with metal concentration of 1 g/L were prepared using ZnCl2 and Pb(NO3)2, respectively by the addition of predetermined amount of concentrated HCl solution into doubly distilled water. The extractants for the Zn(II) and Pb(II) extraction were dissolved in distilled kerosene and 100% methyl-isobutylketone, respectively to the required concentration.
Equal volumes of aqueous and organic phases were shaken at room temperature (25 ± 2 °C) using a Gallemkamp Orbital Shaker, AMPS, for 45 min which was sufficient to reach equilibrium. After the phases had been allowed to separate, Zn(II) and Pb(II) were completely stripped from the organic phase by 0.1 mol/L HCl solution. The concentrations of these metal ions in the raffinates and the stripping aqueous phases were spectrophotometrically measured (Gomez et al., 1992; Yadav and Khopkar, 1971) using an AQUAMATE ThermoElectron Corporation UV–visible spectrophotometer together with an EPSON LQ 2070 recorder or by an ALPHA-4 Atomic Absorption Spectrophotometer. The λmax for Zn(II) and Pb(II) analysis were 491.0 nm and 254.5 nm, respectively.
The concentration of the metal ion in the organic phase was calculated from the difference between its concentration in the aqueous phase before and after extraction. Concentration of iron content in the mixture was also determined spectrophotometrically at λmax 396.0 nm using o-phenanthroline (Malati, 1999). The influence of initial metal concentration, pH and extractant concentration on the rate of Zn(II) and Pb(II) was investigated. The percent of metal extraction (% E) and the distribution ratio, D were calculated as the ratio of Zn(II) or Pb(II) in the organic to the aqueous phase according to the following relations:
2.2.2 Systematic study of the extraction of Pb(II) and Zn(II) from aqueous liquor of galena ore
The composition of the galena leach liquor used for this study at pH 1.0 was Pb: 1705.1 mg/L, Zn: 98.7 mg/L and Fe: 130.2 mg/L. Other metal ions such as Al, Cu, Sn, Mn whose concentrations were <5 mg/L were detected in traces and they were firstly separated by cementation. Total iron in the mixture was then removed by precipitation via pH adjustment of the mixture to 3.5 with 1 mol/L ammoniacal solution or HCl 37% solution prior to the extraction of zinc and lead (Baba and Adekola, 2011). Lead, which constituted by far the major metal in the leachate, was extracted by 1 mol/L TBP in 100% MIBK at pH 5.0. Pb was later stripped from the extractant with 0.1 mol/L HCl to obtain pure Pb(II) solution. Finally, the separated aqueous phase from the former was re-adjusted to a pH 3.0 in order to favor Zn(II) extraction at the expense of lead. The resulting solution at pH 3.0 was contacted with 0.032 mol/L Cyanex®272 in kerosene for 25 min. After equilibration and phase separation, the organic phase containing Zn(II) was stripped with 0.1 M HCl to obtain a pure Zn(II) solution (Baba et al., 2009).
3 Results and discussion
3.1 Fundamental studies with synthetic Zn(II) and Pb(II) salts
3.1.1 Synthetic Zn(II) salt
Recently, our findings on the extraction of Zn(II) from aqueous chloride sphalerite leach liquor by Cyanex®272 in kerosene showed that the stoichiometry of the metal species with extractant was 1:1 by ratio. The extraction equilibrium has been evaluated and consistent with the following relation:
3.1.2 Synthetic Pb(II) salt
3.1.2.1 Selectivity of the extraction of Pb(II)
The result of the preliminary studies in search of selective extractant for Pb(II) is summarized in Table 1.
Extractant concentration
% Extracted into organic phase
Pb(II)
Zn(II)
0.5 mol/L Cyanex®272 in kerosene
10.2
82.4
0.5 mol/L TBP in 100% MIBK
89.4
5.04
0.5 mol/L TPP in kerosene
47.9
33.5
From Table 1, it is clear that TBP is the best extracting agent for Pb(II) at the expense of Zn(II). Therefore, TBP was chosen for further investigations in this work. Hence, the performance of the extracting agents follows the order: TBP > TPP > Cyanex®272.
3.1.2.2 Effect of TBP concentration on Pb(II) extraction
The effect of concentration of TBP in 100% MIBK on the extraction of Pb(II) was studied using aqueous leach liquor containing 100 mg/L Pb(II) in 0.1 mol/L HCl with TBP concentration ranging between 0.1 mol/L and 3.0 mol/L at 1:1 aqueous:organic ratio during extraction time of 30 min (Yadav and Khopkar, 1971). The percentage of Pb(II) extracted increased rapidly from 46.5% to 87.5 ± 0.1% with increasing TBP concentration as shown in Fig. 1. In the absence of TBP, however, about 20% Pb(II) was extracted by MIBK. This means that extraction can only be possible as a result of solvation of Pb2+ ions by MIBK (Keshav et al., 2009; Kumar et al., 2008; Pinajian, 1966). Hence, TBP has provided a kind of enhancement from 20% to ≈90% by 1 mol/L TBP.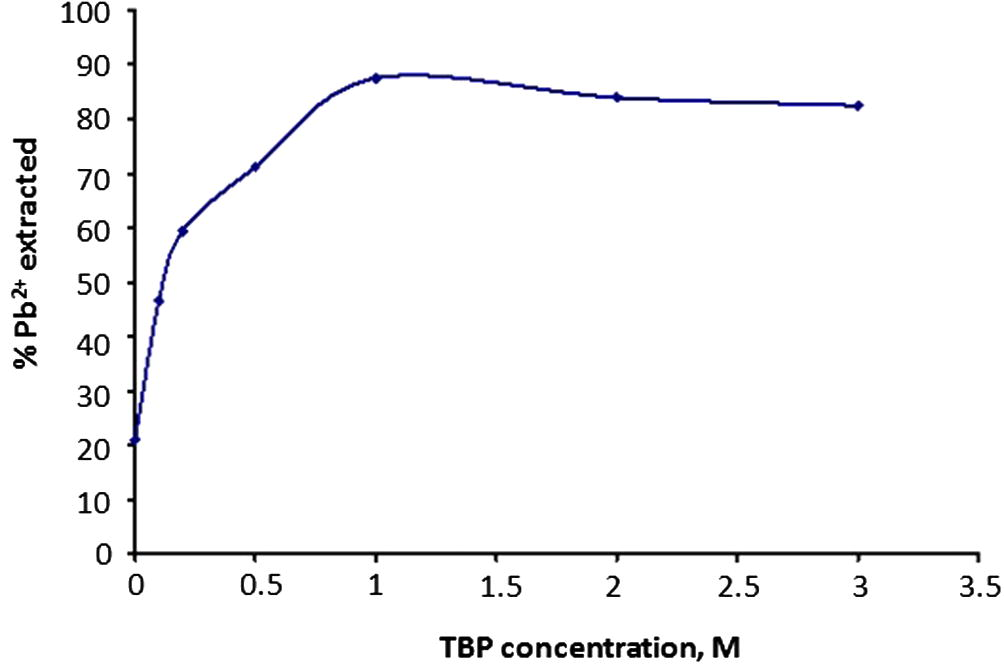
Effect of TBP concentration on Pb(II) extraction.
From Fig. 1, it is evident that above 1 mol/L TBP, there was no further increase in the Pb(II) extraction, but a slight drop to a constant value. Hence, 1 mol/L TBP in 100% MIBK was adjudged to be the optimum concentration for the Pb(II) extraction and this was subsequently kept for further use.
The composition of the extractable species was analyzed by plotting log D vs., log [TBP] at a fixed pH of 1 (Fig. 2).![log D vs. log [TBP].](/content/185/2013/25/4/img/10.1016_j.jksus.2013.07.003-fig2.png)
log D vs. log [TBP].
The slope from Fig. 2 gave 0.73 ≈ 1, suggesting that the association of one mole of extractant with extraction of a mole of metal ion. Therefore, the most probable composition of the extractable species may be PbCl2·TBP. It is worthy to note that further work is in progress on the composition of the extraction species. Hence, this contrasted with an extractable species: PbCl2·2TBP, containing 2 mol TBP per lead proposed by Yadav and Khopkar (1971) on the liquid–liquid extraction of Pb(II) in 1 mol/L HCl with tributylphosphate. These authors obtained a slope of 1.73 at a fixed acidity of 1 mol/L HCl. The difference could be attributed to the higher concentration used by these authors, which was 10-fold higher.
3.1.2.3 Effect of pH on Pb(II) extraction by TBP
The pH of the aqueous solution containing 100 mg/L Pb(II) in 0.1 mol/L HCl was varied from pH 1.0–6.0 over extraction time of 30 min at an ambient temperature of 25 ± 2 °C. The result is shown in Fig. 3a.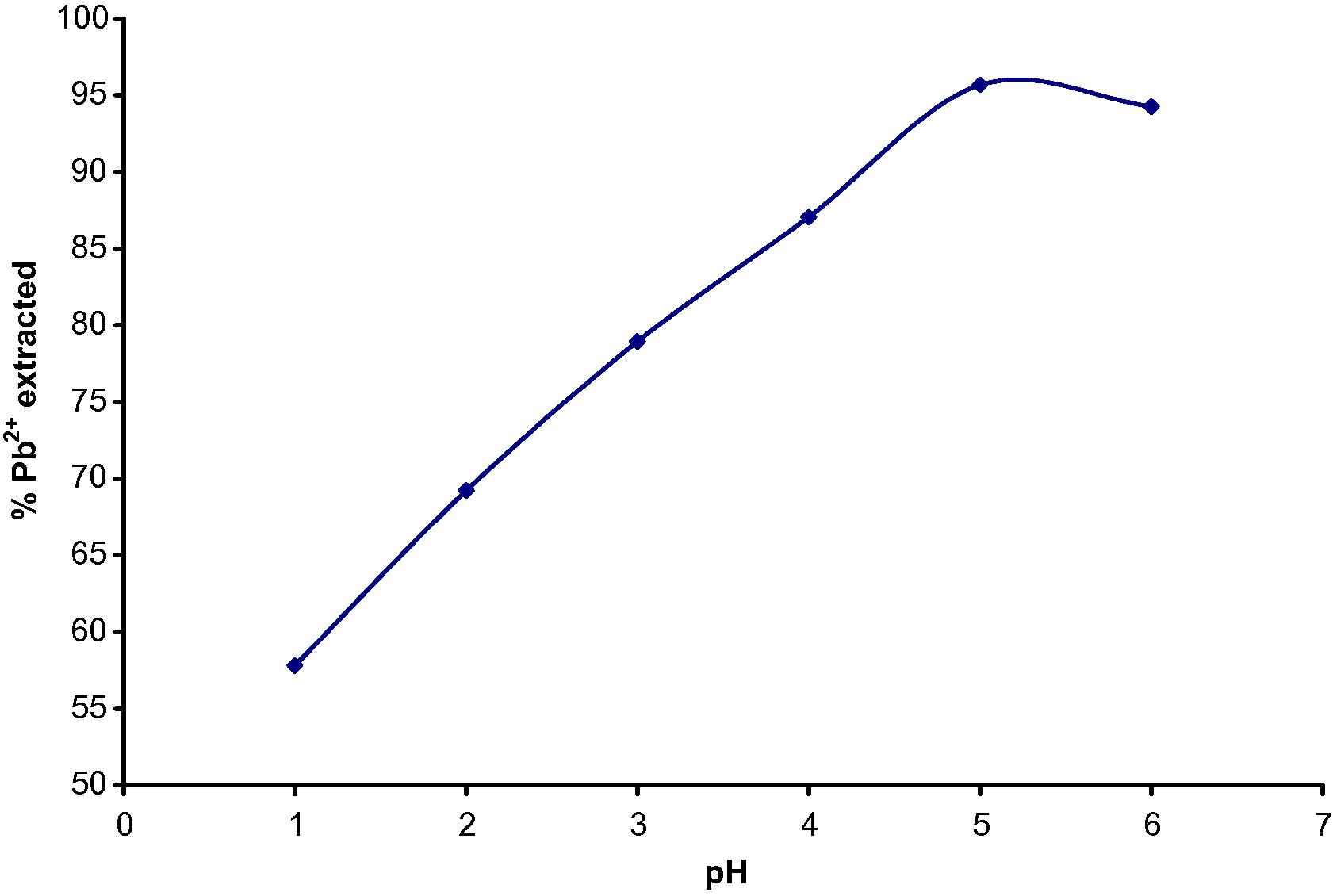
(a) Effect of pH on Pb(II) extraction by TBP.
From Fig. 3a, the extraction of Pb(II) increased progressively with increasing pH from 1.0 to 5.0, after which there was a slight decline in the percent of Pb(II) extracted. This could be attributed to the formation of higher lead hydroxide complexes and thus could apparently explain the loss in Pb(II) extraction at higher pHs. Hence, at pH < 5, the PbCl2 will be ionized to give free Pb2+ (free cation). At pH > 5, however, part of the Pb2+ ionic species may be converted for example, to hydroxide of the form: Pb(OH+) and thus may not really making lead to be free radical (Pourbiax, 1996) (Fig. 3b).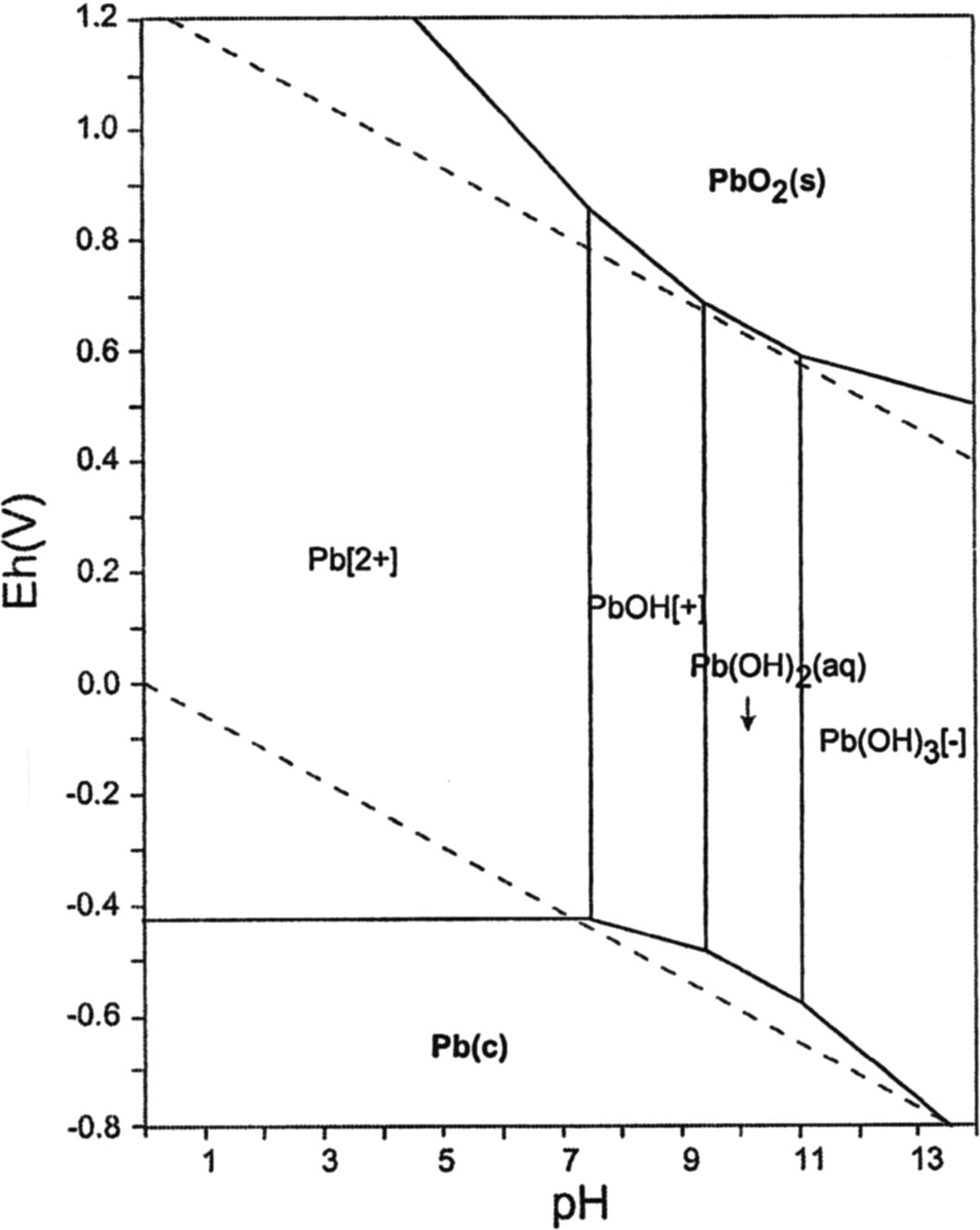
(b) Pourbiaux distribution diagram of Pb in chloride solution.
Therefore, pH 5.0 was noted as the optimum pH for the Pb(II) extraction where about 95.7 ± 0.2% extraction yield was attained. This result showed that there is a gradual release of H+ ion with increase in the amount of Pb(II) extracted.
The plot of log D vs. log [H+] as shown in Fig. 4 was linear with the slope 1.43 ≈ 1, indicating the exchange of a mole of H+ ion per extraction of a mole of metal ion in the organic phase.![log D vs. log [H+] for Pb(II) extraction by TBP.](/content/185/2013/25/4/img/10.1016_j.jksus.2013.07.003-fig5.png)
log D vs. log [H+] for Pb(II) extraction by TBP.
El-Dessouky et al. (2008) and Bartkowska et al. (2002) reported that the majority of divalent metal ions including lead species found in 2–5 mol/L chloride solution is PbCl42− together with decreasing mole fraction of PbCl3−, Pb2+, PbCl+ and PbCl2. For example, the existence of a small fraction of PbCl3− in the medium at low metal concentration as reported by Bartkowska et al. (2002) may probably explain the limited extraction of Pb(II) by TBP in the investigated system, where about 57.2 ± 0.2% was obtained (Fig. 3).
Therefore, the extraction of Pb(II) by 1 mol/L TBP in 100% MIBK at 25 ± 2 °C can be represented by:
3.1.2.4 Study on the successive extraction of Pb(II) by TBP
A study on the successive extraction of lead(II) by 1 mol/L TBP in 100% MIBK has been examined to establish the extraction efficiency in the extraction yield. The results obtained are summarized in Fig. 5.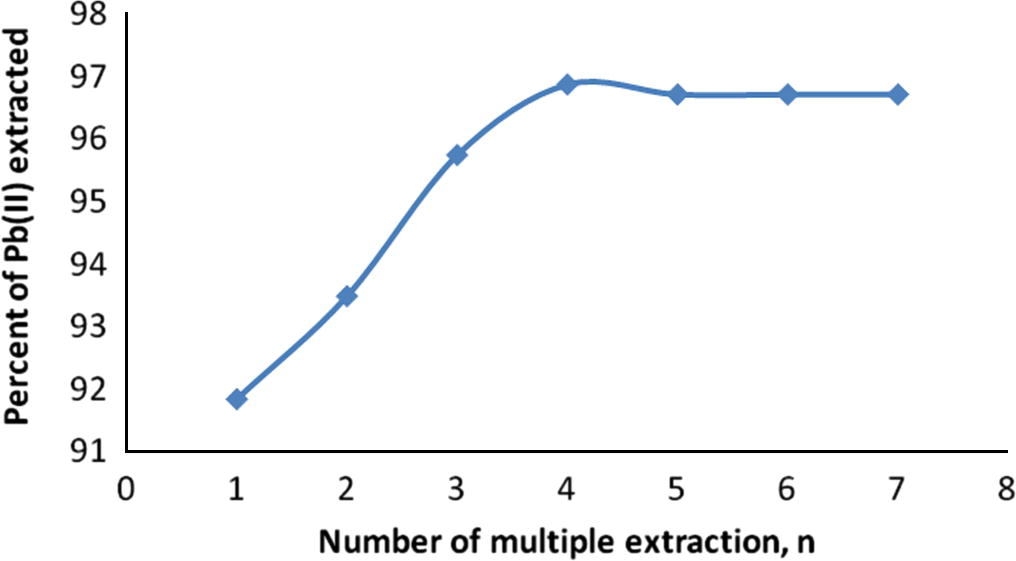
Percent of Pb(II) extracted into organic phase as a function of multiple extraction, n, by TBP in 100% MIBK.
From Fig. 5 and at n ⩾ 4, the extraction becomes practically constant as about 96.7 ± 0.2% of Pb(II) would be quantitatively extracted into the organic phase by 1 mol/L TBP in MIBK at pH 5.0. Likewise, the number of the theoretical stages for this process was calculated by the McCabe–Thiele diagram (Sarangi et al., 2007; Ali et al., 2002), and this is shown in Fig. 6.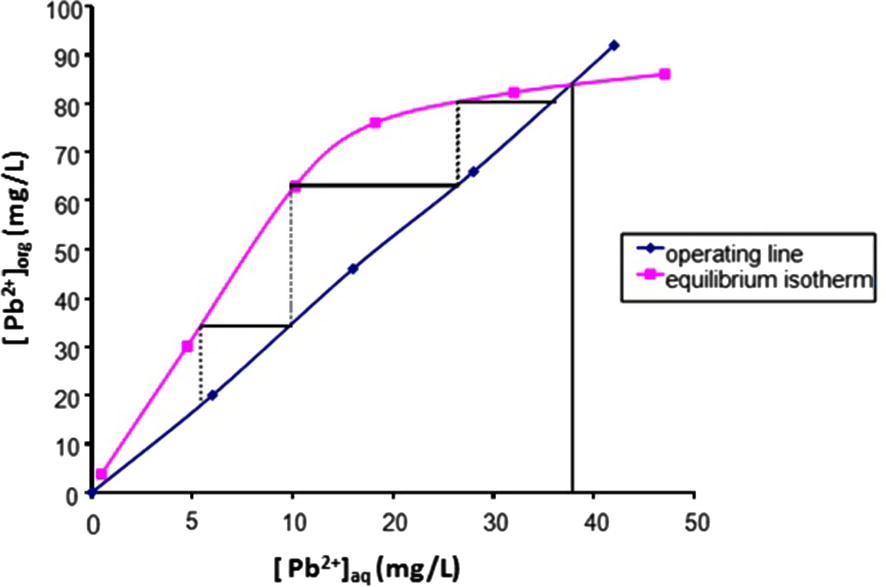
McCabe–Thiele diagram for the extraction of Pb(II) solution by 1 mol/L TBP in 100% MIBK at aqueous: organic ratio of 1:1.
From Fig. 6, it is seen that six extraction stages should be sufficient for the lead(II) extraction at the optimal conditions stated.
3.1.2.5 Influence of temperature on Pb(II) extraction by TBP
The influence of temperature on the Pb(II) extraction by TBP was investigated at different temperatures from 27 to 50 °C at fixed aqueous and extractant concentrations. The distribution ratio, D of the Pb(II) extraction by TBP were calculated and the plot of log D vs. 1/T is shown in Fig. 7, giving a slope of −2752.5.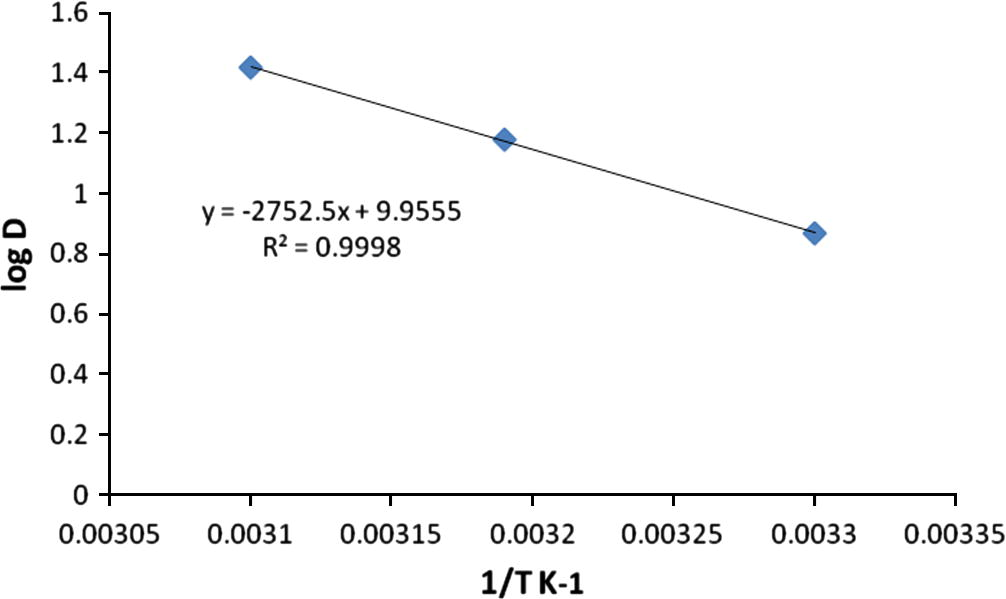
Relationship between distribution ratio, D and temperature, T.
The enthalpy change of the reaction, ΔH has been determined using the equation 6:
3.2 Pb(II) and Zn(II) extraction from aqueous liquor of galena ore
3.2.1 Iron precipitation from galena leachate
As the starting galena leach liquor contained mainly iron, lead and zinc, the leachate (from Section 2.2.2) with initial pH 1.0 was adjusted to a pH 3.5 by 1 mol/L ammoniacal solution to selectively precipitate total iron without affecting Pb(II) and Zn(II) in the leachate (ALBION PROCESS, 2010; Rotuska and Chmielewski, 2008; Loan et al., 2006; Jones and Moore, 2001). At this pH, the total iron precipitation, the total iron removal from the leach liquor was maximum (Baba, 2008). Therefore, the result obtained after total iron precipitation at pH 3.5 is summarized in Table 2. Other metal ions in solution including Al, Ca, Cu, Sn and Mn have been cemented and their final concentrations were less than 2 mg/L. Experimental conditions: pH = 3.5, Temperature = 25 ± 2 °C, Contact time = 30 min by open aeration.
Pb(II)(aq)
Zn(II)(aq)
Total iron(aq)
Conc. before total iron(aq) removal (mg/L)
Conc. after total iron(aq) removal (mg/L)
Conc. before total iron(aq) removal (mg/L)
Conc. after total iron(aq) removal (mg/L)
Conc. before total iron(aq) removal (mg/L)
Conc. after total iron(aq) removal (mg/L)
1705.1
1674.3
98.7
92.6
130.2
5.2
The result from Table 2 showed that about 96 ± 0.2% of the total iron has been precipitated and was then removed from the leachate by filtration. This result indicated more accuracy as compared to the proposed Albion process, which affirmed that iron levels in the solution following iron removal are typically maintained in the range 8–10 mg/L (Baba and Adekola, 2011; Rotuska and Chmielewski, 2008).
3.2.2 Pb(II) extraction from galena leachate by 1 mol/L TBP in MIBK
The resulting solution from Section 3.2.1 after iron removal containing Pb(II) and Zn(II) was extracted by 1 mol/L TBP in MIBK at pH 5 within 30 min contact time. The result of the extraction process is summarized in Table 3.
[Pb(II)]initial = 1674.3 mg/L
[Zn(II)]initial = 92.6 mg/L
Concentration of Pb(II) left unextracted in aqueous phase (mg/L)
Concentration of Pb(II) extracted into organic phase (mg/L)
Percent of Pb(II) extracted
Concentration of Zn(II) left unextracted in aqueous phase (mg/L)
Concentration of Zn(II) extracted into organic phase (mg/L)
Percent of Zn(II) extracted
132.9
1541.4
92.1 ± 0.2
85.7
6.9
7.4
The results from Table 3 showed that at pH 5.0, about 92 ± 0.2% Pb(II) was extracted into the organic phase by 1 mol/L TBP in 100% MIBK.
3.2.3 Stripping of Pb(II) from the TBP-extract by 0.1 mol/L HCl
The result of total lead stripping from the TBP loaded organic phase by 0.1 mol/L HCl is presented in Table 4. Analysis of other metal ions in solution including Al, Ca, Cu, Sn with values <2 mg/L; and total Fe <1 mg/L.
Concentration of Pb(II) in organic phase before stripping (mg/L)
Concentration of Pb(II) in aqueous phase after stripping (mg/L)
Percent of Pb(II) stripped from the organic phase
1541.4
1456.2
94.5 ± 0.2
The result from Table 4 showed that about 94.5 ± 0.2% of Pb(II) has been recovered.
3.2.4 Zn(II) extraction from galena leachate by Cyanex®272
The resulting aqueous solution following the Pb(II) extraction by 1 mol/L TBP in 100% MIBK at pH 5.0 was re-adjusted to a pH 3.0 in order to extract Zn(II) at the expense of Pb(II). The solution at pH 3.0 was contacted with 0.032 mol/L Cyanex®272 in kerosene for 25 min. The result of the extraction is summarized in Table 5. (The initial [Zn(II)] before extraction being 85.7 mg/L). [Zn(II)]initial before extraction = 85.7 mg/L.
Zn(II) concentration left unextracted in aqueous phase (mg/L)
Zn(II) concentration extracted into organic phase (mg/L)
Percent Zn(II) extracted
pH before extraction
pH after extraction
4.7
81.0
94.6 ± 0.2
3.0
2.2
3.2.5 Stripping of Zn(II) from Cyanex®272-extract by 0.1 mol/L HCl
The result obtained from the stripping of Zn(II) from Cyanex®272 organic phase by 0.1 mol/L HCl is summarized in Table 6. Analysis of other metal ions in solution including Al, Ca, Cu, Sn and Mn with values < 2 mg/L; and total iron < 1 mg/L.
Concentration of Zn(II) in organic phase before stripping (mg/L)
Concentration of Zn(II) in aqueous phase after stripping (mg/L)
Percent of Zn(II) stripped from the organic phase
81.1
78.2
96.6 ± 0.2
From Table 6, the result of the stripping investigation by 0.1 mol/L HCl showed that about 97 ± 0.2% of Zn(II) was stripped from 81.1 mg/L Zn(II) loaded organic phase by 0.032 mol/L Cyanex®272. The use of hydrochloric acid for the recovery of zinc is usually preferred due to the ease of electrowinning (Ali et al., 2006). Finally, the solutions obtained after the removal of total Fe and Pb in Sections 3.2.3 and 3.2.5 were re-analyzed by an ALPHA-4 Atomic Absorption Spectrophotometer (AAS) and these results are apparently the same as reported by the UV–visible spectrophotometer.
4 Discussion
4.1 Hydrometallurgical scheme for Pb(II) and Zn(II) extraction from galena leachate
On the basis of the experimental results discussed so far, a hydrometallurgical scheme summarizing details of all analytical procedures required for the recovery of Pb(II) and Zn(II) from a Nigerian galena ore is shown in Fig. 8.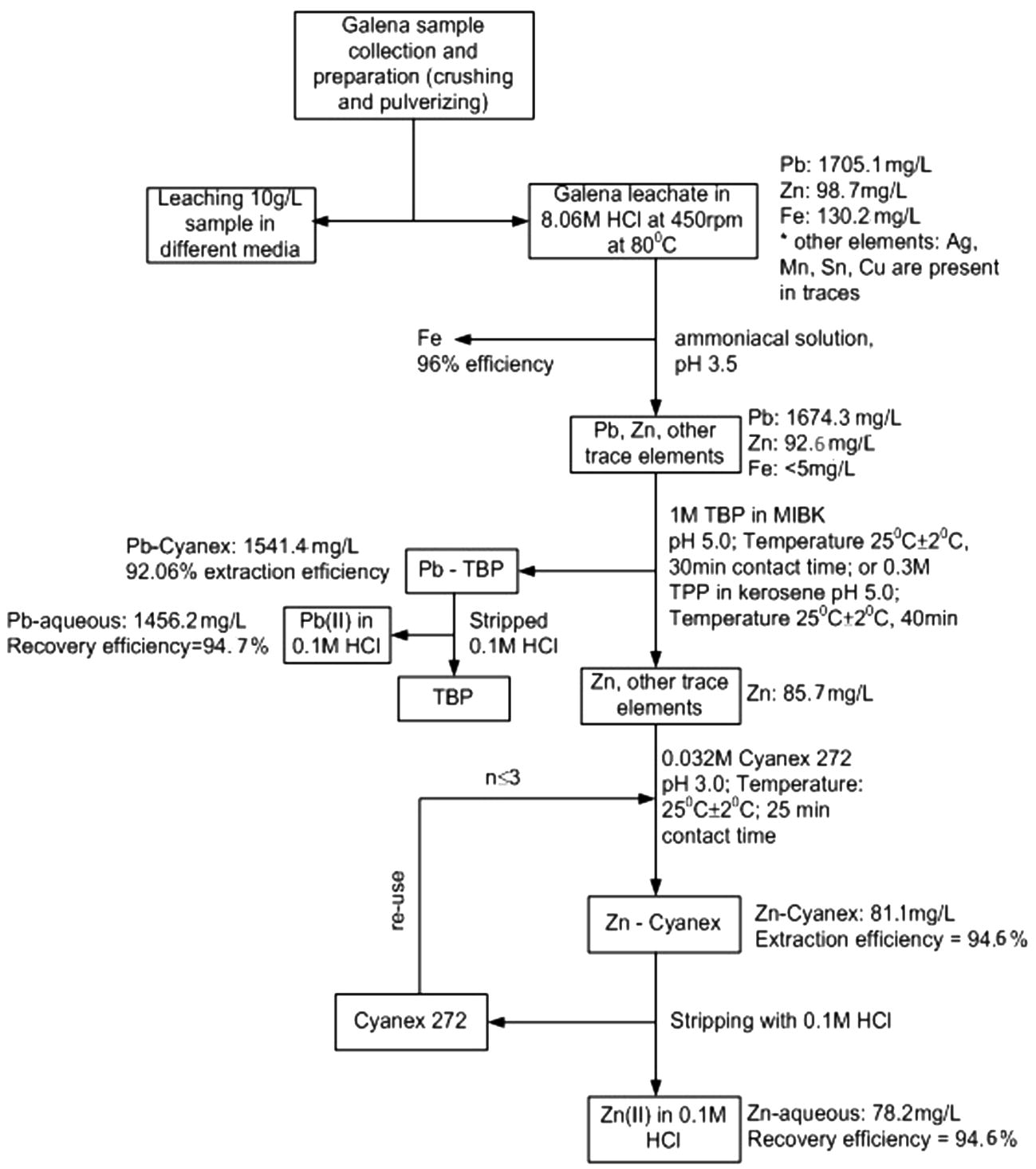
Hydrometallurgical scheme for Pb(II) and Zn(II) recovery from galena ore in hydrochloric acid medium.
5 Conclusions
The results of fundamental studies on the solvent extraction of Pb(II) from chloride solution by TBP in 100% MIBK showed that the extraction of metal ions increased with increasing pH, extractant concentration and temperature. The probable composition of the extractable species may be HPbCl3.TBP with a mole of H+ ion participating in the extracted metal complex. Values of 52.7 ± 0.01 kJ mol−1, −5.05 ± 0.01 kJ mol−1, 159.6 ± 0.01 JK−1 mol−1 and 0.127 ± 0.003 mol−2 were calculated as the apparent standard molar enthalpy (ΔH°), Gibb’s free energy (ΔG°), molar entropy (ΔS°) and extraction constant for the process, respectively. These values showed that the Pb(II) extraction by TBP is entropy driven and thus thermodynamically favorable.
Furthermore, the separation of Zn(II), total iron and other metals constituting impurities to Pb(II) was carried out from an initial galena leach liquor containing 1705.1 mg/L Pb, 98.7 mg/L Zn and 130.3 mg/L total Fe in 8.06 mol/L HCl solution by a combination of precipitation, cementation and solvent extraction techniques.
Total iron was initially precipitated from the leachate by 1 mol/L ammoniacal solution at pH 3.5 and this process recorded 96 ± 0.2% efficiency. The stripping of about 94.5 ± 0.2% Pb(II) by 0.1 mol/L HCl was achieved from the Pb-TBP complex. In addition, separation of Zn(II) from galena leachate was done by 0.032 mol/L Cyanex®272 at pH 3.0 where extraction of about 94.6 ± 0.2% was attained and 94.6 ± 0.2% of Zn(II) was stripped from Zn–Cyanex organic phase by 0.1 mol/L HCl. Finally, a simple hydrometallurgical scheme summarizing all analytical procedures employed in the separation process has been proposed for the Nigerian Galena mineral.
Acknowledgements
The authors are grateful to Cytec Industries, Rungis Cedex, France, for supplying Cyanex®272 used for this study. A.A. Baba also thanks the University of Ilorin, Ilorin-Nigeria for the 2005/2006 Staff Development Award for Ph.D research in Chemistry; Mr. J.O. Falaja (NNPC, Abuja-Nigeria); Mr. S. Ibrahim (Julius Berger Plc.) and Alhaji R.A. Lawal (RALAWAL Constructions, Nigeria Limited) are also applauded for their financial assistance towards Ph.D research.
References
- Leaching of sphalerite with hydrogen peroxide and nitric solution. J. Min. Mater. Charact. Eng.. 2003;5(2):167-177.
- [Google Scholar]
- ALBION PROCESS, 2010. The Albion process for mixed zinc/copper concentration. Simplicity in leaching. Available from: <http://www.albionprocess.com>. Retrieved on 29/05/2010.
- Solvent extraction equilibrium of Zinc (II) from ammonium chloride medium by CYANEX 923 in Solvesso 100. J. Chem. Eng. Jpn.. 2001;43(2):1439-1442.
- [Google Scholar]
- Counter–current extraction process for recovery of U(IV) from phosphoric acid using octylphenyl acid phosphate (OPAP) extractant. Radioanal. Nucl. Chem.. 2002;254(2):263.
- [Google Scholar]
- CYANEX 272 for the extraction and recovery of zinc form aqueous waste solution using a mixer-settler unit. Sep. Purif. Technol.. 2006;47:135-140.
- [Google Scholar]
- Baba, A.A., 2008. Recovery of zinc and lead from sphalerite, galena and waste materials by hydrometallurgical treatments (Ph.D Thesis). Department of Chemistry, University of Ilorin, Ilorin-Nigeria, p. 675 (544 refs).
- Beneficiation of a Nigerian sphalerite mineral: solvent extraction of zinc by Cyanex 272 in hydrochloric acid. Hydrometallurgy. 2011;109:187-193.
- [Google Scholar]
- A study of dissolution kinetics of a Nigerian galena ore in hydrochloric acid. J. Saudi Chem. Soc.. 2012;16:377-386.
- [Google Scholar]
- Solvent extraction of zinc with triphenylphosphite (TPP) from a Nigerian sphalerite in hydrochloric acid. J. Sci. Isl. Rep. Iran. 2004;15(1):33-37.
- [Google Scholar]
- Development of a combined pyro- and hydro-metallurgical route to treat zinc–carbon batteries. J. Hazard. Mater.. 2009;171:838-844.
- [Google Scholar]
- Solvent extraction separation of La from chloride solution containing Pr and Nd with Cyanex 272. Hydrometallurgy. 2012;121–124:74-80.
- [Google Scholar]
- Extraction of zinc(II), iron(III) and iron(II) with binary mixtures containing tributyl phosphate and di(2-ethylhexyl) phosphoric acid or Cyanex 302. Physicochem. Prob. Miner. Process.. 2002;36:217.
- [Google Scholar]
- Cementation for metal removal in zinc electrowinnning circuits. Miner. Eng.. 2005;18:1282-1288.
- [Google Scholar]
- Modelling of extraction equilibrium for zinc(II) by a bibenzimidazole type reagent (ACORGA ZNX50) from chloride solutions. Hydrometallurgy. 1996;43:277-286.
- [Google Scholar]
- Hydrometallurgy-Fundamentals, Technology and Innovation Society for Mining, Metallurgy and Exploration. Colorado, USA: Littleton; 1993. (908–929, In: Paiva and Abrantes, 2001 ed., Fresenius J .Analy. Chem., 370, 883–886)
- Solvent extraction separation of Zn(II), Fe(II), Fe(III) and Cd(II) using tri-butylphosphate and Cyanex 921 in kerosene from chloride medium. Chem. Eng.. 2008;47:177-183.
- [Google Scholar]
- Stripping of copper from Cyanex®301 extract with thiourea hydrazine–sodium hydroxide solution. Hydrometallurgy. 2007;89:297-304.
- [Google Scholar]
- Solvent extraction of zinc from ammoniacal/ammonium chloride solutions by a sterically hindered β-ketone and its mixture with tri-n-octylphophine oxide. Hydrometallurgy. 2010;100(3–4):116-121.
- [Google Scholar]
- Simultaneous spectrophotometric determination of metal ions with 4-(Pyridyl-2-azo)resorcinol, (PAR) Frensenius J. Anal. Chem.. 1992;342:318-321.
- [Google Scholar]
- The solvent extraction of lead from chloride solutions using di(2-ethylhexyl)phosphonic acid. Hydrometallurgy. 1985;14(3):387-393.
- [Google Scholar]
- Modeling and optimization of synergistic effect of Cyanex 302 and D2EHPA on separation of zinc and manganese. Hydrometallurgy. 2011;105:277-283.
- [Google Scholar]
- Synergistic extraction of zinc(II) by mixtures of primary amine N1923 and Cyanex 272. Solvent Extr. Ion Exch.. 2002;20(6):751-764.
- [Google Scholar]
- Jones, D., Moore, R., 2001. The application of the CESL nickel process to laterites. In: Paper Presented at ALTA 2001 Nickel/Cobalt-7, May 15–18, 2001, Perth, Australia, p. 11.
- Biomining: a useful approach towards metal extraction. Am. Eurasian J. Agron.. 2009;2(2):84-88.
- [Google Scholar]
- Reactive extraction of propionic acid using Aliquat 336 in MIBK: linear solvation energy relationship (LSEK) modeling and kinetics study. J. Sci. Ind. Res.. 2009;68:708-713.
- [Google Scholar]
- Intensification of nicotinic acid separation using organophosphorus solvating extractants: reactive extraction. Chem. Eng. Technol.. 2008;31:1584-1590.
- [Google Scholar]
- Defining the paragoethite process for iron removal in zinc hydrometallurgy. Hydrometallurgy. 2006;81:104-129.
- [Google Scholar]
- Experimental Inorganic/Physical Chemistry. Chichester, England: Horwood Publishing; 1999.
- Extraction of lead(II) by Cyanex 302 in Toluene: effect of Cyanex 301 on the extraction by Cyanex 302. Solvent Extr. Ion Exch.. 1996;14(1):69-88.
- [Google Scholar]
- Towards an understanding of solvent extraction-electroanalytical characterization of chloride-leaching solutions. Fresenius J. Anal. Chem.. 2001;370:883-886.
- [Google Scholar]
- Pinajian, J. J., 1966. The methyl isobutyl ketone (4-methyl-2-pentanone) extraction of Trivalent iron from hydrochloric acid solutions and effect of an inert diluent on the distribution coefficient, Bulletin, Oak Ridge National Library, Isotopes Development Center, US Atomic Energy Commission, No. W-7405-eng-26, 49–63.
- Atlas of Electrochemical Equilibria in Aqueous Solutions. Pergamon: Oxford; 1996. (187–189)
- Solvent extraction of uranium from saline leach liquors using DEHPA/Alamine-336 mixed reagent. Hydrometallurgy 2013
- [CrossRef] [Google Scholar]
- Growing role of solvent extraction in copper ores processing. Physicochem. Prob. Miner. Process.. 2008;42:29-36.
- [Google Scholar]
- Principles and Practices of Solvent Extraction. New York: Dekker; 1992.
- Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate chloride solution using TBP, LIX 84I and Cyanex 923. Sep. Purif. Technol.. 2007;55:44-49.
- [Google Scholar]
- Extraction of Zn(II) from aqueous hydrochloric acid solutions into Alamine 336-m-xylene systems. Modeling considerations to predict optimum operational conditions. Hydrometallurgy. 2007;86:27-36.
- [Google Scholar]
- Rapid extraction of lead(II) from succinate media with n-octylaniline in toluene. Sep. Purif. Technol.. 2002;26(2–3):185-193.
- [Google Scholar]
- Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD). Part II: downstream processing and zinc recovery by electrowinning. J. Hazard. Mater.. 2010;179(1-3):8-14.
- [Google Scholar]
- Extraction and separation of cobalt(II), copper(II) and manganese(II) by Cyanex 272, PC-88A and their mixtures. Sep. Purif. Tech.. 2012;93:8-14.
- [Google Scholar]
- Intensification of propionic acid production by reactive extraction: effect of diluents on equilibrium. Chem. Biochem. Eng. Q.. 2006;20(3):325-331.
- [Google Scholar]
- Liquid–liquid extraction of lead(II) with tributylphosphate. Talanta. 1971;18:833-837.
- [Google Scholar]
- Redox process during cobalt extraction with bis(2,4,4-trimethylpentyl)dithiophosphinic acid. Hydrometallurgy. 2012;129–130:43-49.
- [Google Scholar]
- The extraction of Zn(II), Cd(II) and Pb(II) from hydrochloric acid media by amberlite LA-2 hydrochloride dissolved in 1,2-dichloroethane. Talanta. 1980;33(9):769-774.
- [Google Scholar]







