Translate this page into:
Solanesol alleviates metal oxide nanoparticles generated toxicity in human placental BeWo cells
⁎Corresponding authors. ahmadisrardr@gmail.com (Israr Ahmad), mahamed@ksu.edu.sa (Maqusood Ahamed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Recent research has provided evidence that metal oxide nanoparticles (NPs) have the potential to traverse the placental barrier, potentially endangering the health of both pregnant individuals and the developing fetus. The function of oxidative stress is of utmost importance in the pathophysiology of poor pregnancy outcomes. The therapeutic potential of solanesol in mitigating pregnancy complications is attributed to its robust antioxidant activity. The objective of this work was to inspect the preventative effects of solanesol on the detrimental effects generated by CuO and NiO NPs in human placental cells (BeWo). It was noticed that the exposure of BeWo cells to solanesol at concentrations ranging from 1 to 100 µg/ml for a duration of 24 h did not result in any harmful effects. However, the presence of CuO and NiO NPs led to a dose-dependent decrease in cell viability when exposed to dosages of 5 to 200 µg/ml for 24 h. It is noteworthy that solanesol significantly reduced the cytotoxicity that CuO and NiO NPs generated in BeWo cells. The data on apoptosis also demonstrated that CuO and NiO NPs have effect on the expression of the apoptotic gene caspase-3 and the decline of mitochondrial membrane potential. However, this effect was effectively reversed when solanesol was administered in combination with NPs. The findings of the mechanistic investigation indicate that the CuO and NiO NPs induced oxidative stress was notably negated when solanesol was concurrently treated. This paper presents novel findings indicating that the toxicity generated by CuO and NiO NPs in human placental cells was significantly alleviated by solanesol. Further work is necessary to explore the pharmacological potential of solanesol against nanoparticle-generated pregnancy problems.
Keywords
Solanesol
Nanomaterials
Pregnancy complications
Protective effects
Oxidative stress
1 Introduction
Placenta performs a decisive part in the sustenance of a healthy pregnancy. Placenta facilitates the transfer of oxygen and essential nutrients to the developing baby while simultaneously eliminating carbon dioxide and waste products from the fetal circulation (Eliesen et al., 2021). The trophoblasts, which possess the capacity to differentiate into many cell types inside the placenta, including syncytiotrophoblasts and cytotrophoblasts, serve as the precursor cells of the placenta. These cells are essential in facilitating dynamic contact between the mother and fetus (Huang et al., 2015). These cells may be regarded as the initial fetal-derived cells that come into contact with any substances found in the maternal bloodstream. Furthermore, it is worth noting that the placenta exhibits the expression of many xenobiotic transporters, thereby serving as a protective barrier against detrimental medications and environmental toxins, as documented in reference (Zhao et al., 2013). There is evidence linking impaired placental function to several pregnancy problems, such as preeclampsia, gestational diabetes, premature delivery, and particular birth abnormalities (Eliesen et al., 2021).
Transition metal oxide nanoparticles (NPs), such as CuO and NiO, have garnered significant interest because of their distinctive chemical, optical, catalytic, and electrical characteristics (Diallo et al., 2018; Nayak et al., 2020). The utilization of these physicochemical features has facilitated their application in several fields, including fuel cells, solar cells, sensors, electrodes, and light-emitting diodes (Adinaveen et al., 2019; Bonomo et al., 2018). Metal oxide NPs exhibit remarkable antibacterial properties, making them very suitable for application in the medical domain (Azam et al., 2012). The extensive manufacturing and broad utilization of CuO and NiO NPs have the potential to result in increased exposure to the human body, hence generating concerns over potential health hazards, particularly during vulnerable life stages such as pregnancy, which can impact the growth of the fetus in the womb (Campagnolo et al., 2017). Pregnancy problems give rise to several disorders that have adverse consequences for mother and the fetus (Higgins et al., 2015). The potential effect of NPs exposure on the placenta's formation and functionality, leading to subsequent effects on fetal development, has been documented (Pritchard et al., 2021). A burgeoning body of data suggests that nanomaterials possess the ability to traverse the placental barrier in pregnant mice, eliciting harmful effects in both maternal organisms and developing fetuses (Hawkins et al., 2018; Zhang et al., 2019). As an illustration, pregnant mice exposed orally to ZnO NPs at a dosage above 180 mg/kg throughout the period of gestation from day 10.5 to day 17.5 may result in adverse effects such as maternal damage, restricted fetal development, and a decrease in the number of foetuses (Chen et al., 2020).
Solanesol is a compound classified as a sesquiterpenes alcohol, comprised of nine isoprene units. This compound is predominantly present in plants belonging to the Solanaceae family, including tobacco (Nicotiana tabacum), potato (Solanum tuberosum), and tomato (Solanum lycopersicum). Among these, tobacco leaves are the most prolific source of solanesol (Yan et al., 2019). Multiple research data have presented testimony supporting the presence of antioxidant, anti-inflammatory, neuroprotective, antibacterial, and anti-ulcer properties in solanesol (Liu et al., 2023). Solanesol plays a significant role as an intermediary compound in the production of pharmaceutical substances such as coenzyme Q10, vitamin K2, and anticancer agents (Yao et al., 2017). The potent antioxidant properties of solanesol make it a crucial factor in mitigating the adverse effects of metal oxide NP exposure on pregnancy problems. There is limited availability of research about the potential adverse effects of CuO and NiO NPs on pregnancy, as well as the potential mitigating effects of solanesol. This study aimed to figure out how solanesol might help protect human placental BeWo cells from the harmful effects of CuO and NiO NPs. The BeWo cell line comes from human placental choriocarcinoma epithelial cells. This cell line is being used as a research model to look at how medicines and toxins cause toxicity in the placenta (Nabekura et al., 2022).
2 Materials and methods
2.1 Nanoparticles and solanesol
Chemical techniques were used to produce CuO (43 nm) and NiO (51 nm) NPs. Previous investigations have given information on the characterization and synthesis of these NPs (Ahamed et al., 2023, 2022). Solanesol (PubChem ID: 24899813) was bought from Sigma-Aldrich (St Louis, MO, USA).
2.2 Cell culture and exposure procedure
DMEM was used to cultivate human placental BeWo cells. It was supplemented with 10 % fetal bovine serum, 2 mM glutamine, 100 g/ml streptomycin, and 100 unit/ml penicillin. Cells were kept at 37 °C in an incubator that was humidified and supplied with 5 % CO2. Grown cells were collected further cultured for additional tests at 80–85 % confluence. Before being treated to NPs and solanesol for 24 h, cells were given an overnight period to adhere to the surface of the culture flask. To prevent agglomeration before exposing cells, DMEM with varying dosages of NPs (1–200 µg/ml) and solanesol (1–200 µg/ml) was sonicated for 10 min at 40 W in a water bath sonicator.
2.3 Biochemical assays
MTT assay was conducted following the methodology previously described by Mosmann (Mosmann, 1983), with minor adjustments as outlined by Ahamed et al. (Ahamed et al., 2016). The intracellular level of ROS was measured using a microplate reader (Synergy-HT, BioTek) with the application of 2‘-7‘-dichlorodihydrofluorescein diacetate (H2DCFDA) as described by Siddiqui et al. (Siddiqui et al., 2013). The quantification of glutathione (GSH) level was performed using Elman's method (Ellman, 1959). The activity of the glutathione peroxidase (GPx) enzyme was assessed using the experimental methods described by Rotruck et al. (Rotruck et al., 1973). The examination of mitochondrial membrane potential (MMP) was conducted using a microplate reader (Synergy-HT, BioTek) in accordance with established methods (Creed and McKenzie, 2019), with slight modifications as described by Ahamed et al. (Ahamed et al., 2021). Expression of caspase-3 gene was estimated applying real-time PCR (Applied Biosystems, Foster City, CA, USA) with the application of SYBR green. Caspase-3 enzyme was assayed using a colorimetric kit provided by BioVision (Milpitas, CA, USA). The protein content estimation in this study was conducted using Bradford's method, as described by Bradford (Bradford, 1976).
2.4 Statistical assessment
The results were analyzed using a one-way analysis of variance (ANOVA) followed by Dennett's multiple comparison tests. The significance level of p < 0.05 was accredited to the observed results. Data is reported as the mean ± standard deviation (SD) of three distinct tests (n = 3).
3 Results and discussion
3.1 Nanoparticles generated cytotoxicity
Cytotoxic effects of CuO and NiO NPs in BeWo cells was tested with MTT test. The cells were exposed to various doses (ranging from 1 to 200 µg/ml) of each of these nanomaterials for a 24 h period. The MTT test is a method used to evaluate cellular metabolic activity. Data shown in Fig. 1 suggest that both types of NPs elicit a cytotoxic response in BeWo cells, with the magnitude of this response depending on the dosage administered. Nevertheless, the cytotoxicity induced by CuO NPs exhibited a somewhat greater magnitude compared to NiO NPs. The viability of cells of CuO NPs was observed to be 99 %, 96 %, 75 %, 60 %, 47 %, 34 %, 17 %, and 11 % at the corresponding doses of 1, 2, 5, 10, 25, 50, 100, and 200 µg/ml. The cell viability of NiO NPs exhibited a range of percentages, namely 99 %, 98 %, 84 %, 68 %, 52 %, 40 %, 25 %, and 19 % at the corresponding doses of 1, 2, 5, 10, 25, 50, 100, and 200 µg/ml. The cytotoxicity statistics found in this investigation align with previous publications that have indicated a correlation between the dosage of CuO and NiO nanomaterials and their cytotoxic effects in several human cell lines (De Carli et al., 2018; Fahmy et al., 2020). As an illustration, the application of CuO nanomaterials at doses ranging from 2 to 50 µg/ml for a duration of 24 h resulted in a dose-dependent manifestation of cytotoxicity in liver cells (HepG2) (Siddiqui et al., 2013). A separate investigation revealed that the presence of NiO nanostructures resulted in cytotoxicity in mouse spermatogonia cells, with the extent of harmfulness depending on the dosage administered (ranging from 10 to 320 µg/ml) over a period of 24 h (Ahamed et al., 2023).
Cytotoxic response of CuO NPs and NiO NPs in BeWo cells. * p < 0.05 vs. control.
3.2 Cytocompatibility of solanesol
In this experiment, we conducted a more comprehensive investigation of the impact of solanesol in placental cell line. Cells were subjected to various doses (1–200 µg/ml) solanesol for a duration of 24 h, and the assessment of cytotoxicity was conducted using the MTT test. According to the data presented in Fig. 2, it can be observed that solanesol did not harm the BeWo cells at doses up to 100 µg/ml. Consistent with the findings of the current study, previous research has also demonstrated the biocompatibility of solanesol.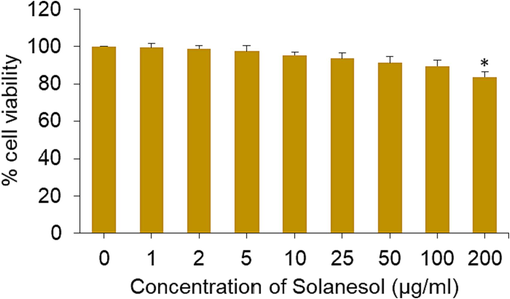
Cytotoxic response of solanesol in BeWo cells. * p < 0.05 vs. control.
3.3 Solanesol alleviates nanoparticles generated cytotoxicity
In order to decide the optimal dose of solanesol for efficiently reducing the toxicity of CuO and NiO NPs in BeWo cells, we conducted the experiments where cells were exposed to a medium concentration (25 µg/ml) of both types of NPs and simultaneously co-exposed them to various safe concentrations of solanesol (1 to 100 µg/ml) for a duration of 24 h. The resulting effects were then assessed using the MTT assay. The findings indicated that the administration of solanesol at a dose of 10 µg/ml resulted highest level of mitigation against the cytotoxicity generated by both types of nanomaterials in BeWo cells (Fig. 3). Based on the obtained findings, a concentration of 10 µg/ml of solansol has been chosen for further investigation into its mechanism of action in alleviating the toxic effects generated by CuO and NiO nanomaterials (25 µg/ml) in human placental cell line.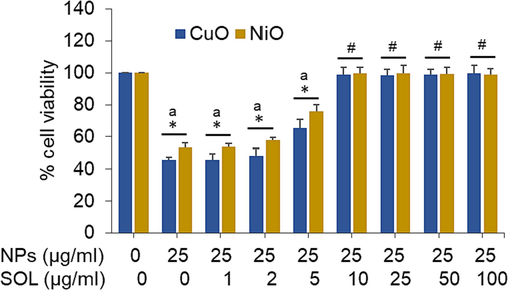
Alleviating effect of solanesol in BeWo cells following exposure to CuO NPs and NiO NPs. * p < 0.05 vs. control. # p < 0.05 vs. a (NPs treated groups).
3.4 Solanesol alleviates nanoparticles generated oxidative stress
Studies have shown that placental dysfunction and other pregnancy problems could be caused by oxidative stress in placental tissues (Onoda et al., 2017). Hence, the administration of antioxidants has the potential to safeguard placental trophoblasts against oxidative stress, potentially reducing the incidence of pregnancy problems and reproductive damage (Duhig et al., 2016). Solanesol displays excellent free radical consumption capabilities due to the existence of numerous non-conjugated double bonds (Yan et al., 2019). To illustrate the potential mechanism behind the protective properties of solanesol against the toxicity generated by NiO and CuO NPs in placental cells, various biomarkers of oxidative stress were assessed. Placental cells were exposed to metal oxide NPs (25 µg/ml) and/or solanesol (10 µg/ml) for a duration of 24 h. Data displayed in Fig. 4A suggesting the intracellular production of ROS caused by both CuO and NiO NPs was greatly reduced in the presence of solanesol. Moreover, the presence of NPs (CuO and NiO) resulted in a significant production in intracellular H2O2 levels, which was successfully counteracted by concurrent exposure to solanesol (Fig. 4B).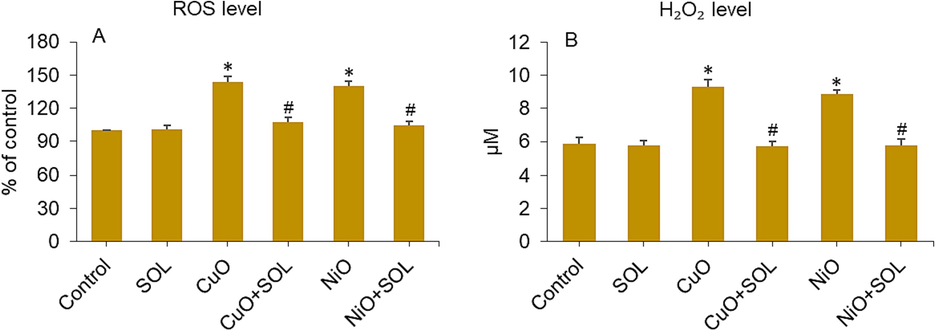
ROS (A) and H2O2 (B) levels in BeWo cells treated for 24 h to NPs (25 µg/ml) and/or solanesol (10 µg/ml). * p < 0.05 vs. control. # p < 0.05 vs. NPs treated group. SOL: solanesol.
Solanesol is helpful during pregnancy because it has antioxidant properties that stop lipid peroxidation and keep antioxidant enzymes working (Yan et al., 2019). Here, we looked at how solanesol might slow down the reduction of antioxidants in BeWo cells induced by selected metal oxide (CuO and NiO) NPs. The findings of this study indicate that the levels of GSH and the activity of the GPx enzyme were significantly reduced in placental BeWo cells treated with both NPs. However, this depletion was effectively reversed when solanesol was co-exposed to the cells, as seen in Fig. 5A and B.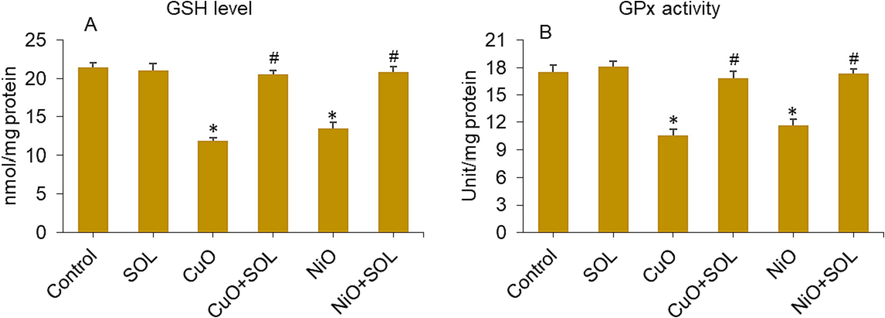
GSH level (A) and GPx enzyme activity (B) in BeWo cells treated for 24 h to NPs (25 µg/ml) and/or solanesol (10 µg/ml). * p < 0.05 vs. control. # p < 0.05 vs. NPs treated group. SOL: solanesol.
3.5 Solanesol alleviates nanoparticles generated apoptosis
The processes of apoptosis are crucial factors in the impaired development of the placenta and present significant hazards to the pregnancy (Ghaneifar et al., 2020; Teng et al., 2021). Exposure to NPs like TiO2 and polystyrene during pregnancy has been shown to cause apoptosis in trophoblasts, which can lead to problems with the placenta and poor fetal development (Ba et al., 2015; Teng et al., 2021). A study by Ebrahimzadeh Bideskan et al. (Ebrahimzadeh Bideskan et al., 2017) found that the regulation of bax and bcl-2 genes in the hippocampus of their children is affected by whether or not their mothers were exposed to TiO2 NPs while they were pregnant or nursing. This exposure leads to apoptosis and a decrease in foetal neurogenesis. The previously reported research also indicated that exposure to CuO and NiO NPs resulted in the induction of apoptosis and oxidative stress in human placental cells and mouse spermatogonia cells, respectively (Ahamed et al., 2023, 2022). Here, potential mitigating impact of solanesol on apoptosis induced by CuO and NiO NPs in human placental cells was investigated. The assessment of caspase-3 gene expression and MMP level was employed to evaluate the observed effects. The cells were treated with CuO and NiO NPs at a concentration of 25 µg/ml, along with solanesol at a dose of 10 µg/ml, for a duration of 24 h. Results presented in Fig. 6A indicate a substantial upregulation of caspase-3 gene upon exposure to CuO and NiO NPs. It is worth noting that the concurrent exposure to solanesol had a notable mitigating effect on the activation of the caspase-3 gene produced by these metal oxide NPs. To provide additional evidence for mRNA expression results, we conducted further analysis on the enzymatic activity of caspase-3 after exposure to metal oxide NPs (CuO and NiO) and/or curcumin. In a manner consistent with the findings related to mRNA, the presence of CuO NPs led to an increased level of caspase-3 enzyme activity. However, this impact was significantly reduced when curcumin was concurrently administered, as depicted in Fig. 6B. In a study conducted by Huang et al. (Huang et al., 2014), it was observed that the administration of N-acetylcysteine, an antioxidant approved by the FDA, had a preventive effect on the induction of DNA damage and fetal brain developmental defects in pregnant mice after carbon nanotubes exposure.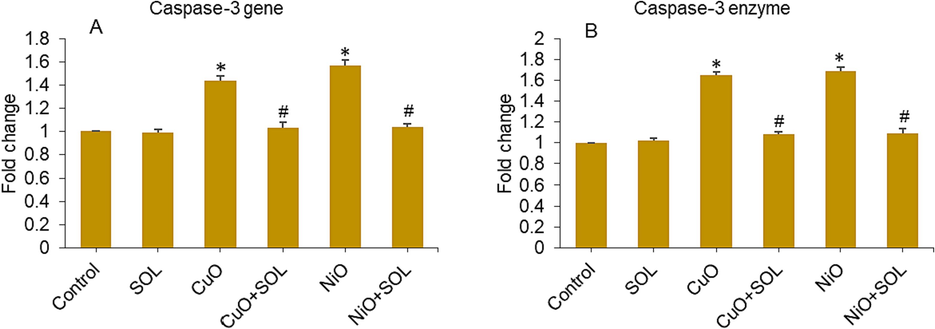
Mrna expression level (a) and enzymatic activity (b) of caspase-3 gene in bewo cells treated for 24 h to NPs (25 µg/ml) and/or solanesol (10 µg/ml). * p < 0.05 vs. control. # p < 0.05 vs. NPs treated group. SOL: solanesol.
The defect in mitochondria is likely to have a significant impact on the correlation between neurodevelopmental problems in the offspring and chronic maternal inflammation during pregnancy. This correlation is frequently observed in the presence of prevalent maternal conditions or circumstances, including obesity, stress, and exposure to environmental pollutants (Gyllenhammer et al., 2022). A recent study demonstrated that solanesol enhances antioxidant capacity and ameliorates mitochondrial deficits (Liu et al., 2023). Utilization of antioxidant compounds, such as solanesol, has the ability to mitigate MMP deficit, decreasing the likelihood of premature disruption of membranes, a significant contributing factor to preterm birth (Tossetta et al., 2021). This study also investigated the loss of MMP, which serves as an early apoptotic indication, in BeWo cells after being exposed to CuO and NiO NPs, as well as solanesol, for a duration of 24 h. The results presented in Fig. 7 demonstrate a considerable reduction in the MMP in BeWo cells following exposure to these metal oxide NPs. However, this impact was effectively reversed when curcumin was co-administered with the NPs. Mitochondrial dysfunction is a probable factor contributing to the correlation between neurodevelopmental disorders in offspring and chronic maternal inflammation during pregnancy. This association is frequently observed in the presence of prevalent maternal circumstances, including obesity, stress, and pollutants. The findings of our earlier investigation showed that the apoptotic effects induced by CuO NPS in placental cells were significantly mitigated with the administration of the dietary antioxidant curcumin (Ahamed et al., 2022).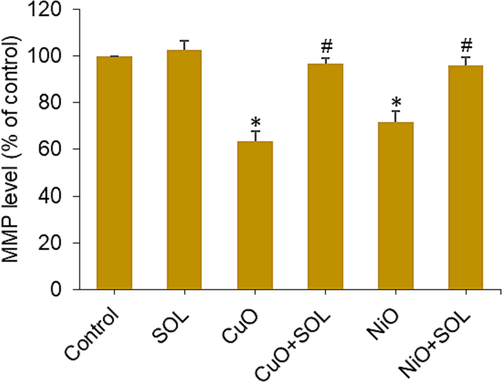
MMP level in BeWo cells treated for 24 h to NPs (25 µg/ml) and/or solanesol (10 µg/ml). * p < 0.05 vs. control. # p < 0.05 vs. NPs treated group. SOL: solanesol.
4 Conclusion
Overall, this study showed that exposing CuO and NiO NPs to human placental BeWo cells led to the start of toxicity and the death of those cells. Additionally, the presence of solanesol, which works through oxidative stress and apoptosis pathways, lessened the adversity of these NPs. This study suggests that solanesol supplementation might be useful to protect metal oxide NPs from causing problems during pregnancy. These results justify the need for more research to investigate the potential of solanesol in mitigating the toxicity of nanostructures on pregnancy outcomes, utilizing appropriate animal models.
Acknowledgment
The authors extend their sincere appreciation to the Researchers Supporting Project number (RSP2023R129), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photocatalytic and optical properties of NiO added Nephelium lappaceum L. peel extract: An attempt to convert waste to a valuable product. Heliyon. 2019;5
- [CrossRef] [Google Scholar]
- Cobalt iron oxide nanoparticles induce cytotoxicity and regulate the apoptotic genes through ROS in human liver cells (HepG2) Colloids Surf. B Biointerfaces. 2016;148:665-673.
- [CrossRef] [Google Scholar]
- SnO2-Doped ZnO/reduced graphene oxide nanocomposites: synthesis, characterization, and improved anticancer activity via oxidative stress pathway. Int. J. Nanomed.. 2021;16:89-104.
- [CrossRef] [Google Scholar]
- Dietary antioxidant curcumin mitigates CuO nanoparticle-induced cytotoxicity through the oxidative stress pathway in human placental cells. Molecules (Basel, Switzerland). 2022;27:7378.
- [CrossRef] [Google Scholar]
- Natural antioxidant curcumin attenuates NiO nanoparticle-induced cytotoxicity in mouse spermatogonia cells: A mechanistic study. J. King Saud Univ. – Sci.. 2023;35:102624
- [CrossRef] [Google Scholar]
- Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomed.. 2012;7:6003-6009.
- [CrossRef] [Google Scholar]
- Effects of benzo[a]pyrene exposure on human hepatocellular carcinoma cell angiogenesis, metastasis, and NF-κB signaling. Environ. Health Perspect.. 2015;123:246-254.
- [CrossRef] [Google Scholar]
- Electrochemical and photoelectrochemical properties of nickel oxide (NiO) with nanostructured morphology for photoconversion applications. Front. Chem. 2018
- [CrossRef] [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [CrossRef] [Google Scholar]
- Campagnolo, L., Massimiani, M., Vecchione, L., Piccirilli, D., Toschi, N., Magrini, A., Bonanno, E., Scimeca, M., Castagnozzi, L., Buonanno, G., Stabile, L., Cubadda, F., Aureli, F., Fokkens, P.H.B., Kreyling, W.G., Cassee, F.R., Pietroiusti, A., 2017. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. 11, 687–698. https://doi.org/10.1080/17435390.2017.1343875.
- Nano zinc oxide induced fetal mice growth restriction, based on oxide stress and endoplasmic reticulum stress. Nanomaterials.. 2020;10:259.
- [CrossRef] [Google Scholar]
- Measurement of mitochondrial membrane potential with the fluorescent dye tetramethylrhodamine methyl Ester (TMRM) Methods Mol. Biol.. 2019;1928:69-76.
- [CrossRef] [Google Scholar]
- Evaluation of the genotoxic properties of nickel oxide nanoparticles in vitro and in vivo. Mutation Res. - Genetic Toxicol. Environ. Mutagen.. 2018;836:47-53.
- [CrossRef] [Google Scholar]
- Structural, optical and photocatalytic applications of biosynthesized NiO nanocrystals. Green Chem. Lett. Rev.. 2018;11:166-175.
- [CrossRef] [Google Scholar]
- Oxidative stress in pregnancy and reproduction. Obstetric Med.. 2016;9:113.
- [CrossRef] [Google Scholar]
- Maternal exposure to titanium dioxide nanoparticles during pregnancy and lactation alters offspring hippocampal mRNA BAX and Bcl-2 levels, induces apoptosis and decreases neurogenesis. Exp. Toxicol. Pathol.. 2017;69:329-337.
- [CrossRef] [Google Scholar]
- Toxicity of anticancer drugs in human placental tissue explants and trophoblast cell lines. Arch. Toxicol.. 2021;95:557-571.
- [CrossRef] [Google Scholar]
- In-vitro evaluation of copper/copper oxide nanoparticles cytotoxicity and genotoxicity in normal and cancer lung cell lines. J. Trace Elem. Med Biol.. 2020;60:126481
- [CrossRef] [Google Scholar]
- The potential therapeutic effects of curcumin on pregnancy complications: Novel insights into reproductive medicine. IUBMB Life. 2020;72:2572-2583.
- [CrossRef] [Google Scholar]
- Maternal inflammation during pregnancy and offspring brain development: the role of mitochondria. Biol. Psychiatry: Cognit. Neurosci. Neuroimag.. 2022;7:498-509.
- [CrossRef] [Google Scholar]
- Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nature Nanotechnol.. 2018;13(5):427-433.
- [CrossRef] [Google Scholar]
- Placental features of late-onset adverse pregnancy outcome. PLoS One. 2015;10:e0129117.
- [Google Scholar]
- Nanoparticles can cross mouse placenta and induce trophoblast apoptosis. Placenta. 2015;36:1433-1441.
- [CrossRef] [Google Scholar]
- The genotype-dependent influence of functionalized multiwalled carbon nanotubes on fetal development. Biomaterials. 2014;35:856-865.
- [CrossRef] [Google Scholar]
- Protective effect of solanesol in glucose-induced hepatocyte injury: Mechanistic insights on oxidative stress and mitochondrial preservation. Chem. Biol. Interact.. 2023;383:110676
- [CrossRef] [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55-63.
- [CrossRef] [Google Scholar]
- Antidepressants induce toxicity in human placental BeWo cells. Curr. Res. Toxicol.. 2022;3:100073
- [CrossRef] [Google Scholar]
- Fabrication of CuO nanoparticle: An efficient catalyst utilized for sensing and degradation of phenol. J. Mater. Res. Technol.. 2020;9:11045-11059.
- [CrossRef] [Google Scholar]
- Onoda, A., Takeda, K., Umezawa, M., 2017. Pretreatment with N-acetyl cysteine suppresses chronic reactive astrogliosis following maternal nanoparticle exposure during gestational period. 11, 1012–1025. https://doi.org/10.1080/17435390.2017.1388864.
- Nanoparticles in pregnancy: the next frontier in reproductive therapeutics. Hum. Reprod. Update. 2021;27:280.
- [CrossRef] [Google Scholar]
- Selenium: Biochemical role as a component of glatathione peroxidase. Science. 1973;179:588-590.
- [CrossRef] [Google Scholar]
- Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS One. 2013;8
- [CrossRef] [Google Scholar]
- Fetotoxicity of nanoparticles: causes and mechanisms. Nanomaterials. 2021;11:1-25.
- [CrossRef] [Google Scholar]
- The multifaced actions of curcumin in pregnancy outcome. Antioxidants. 2021;10:1-20.
- [CrossRef] [Google Scholar]
- Bioactivities and medicinal value of solanesol and its accumulation, extraction technology, and determination methods. Biomolecules. 2019;9
- [CrossRef] [Google Scholar]
- Solanesol induces the expression of heme oxygenase-1 via p38 and Akt and suppresses the production of proinflammatory cytokines in RAW264.7 cells. Food Funct.. 2017;8:132-141.
- [CrossRef] [Google Scholar]
- Systematic evaluation of graphene quantum dot toxicity to male mouse sexual behaviors, reproductive and offspring health. Biomaterials. 2019;194:215-232.
- [CrossRef] [Google Scholar]
- Nanosized TiO2-induced reproductive system dysfunction and its mechanism in female mice. PLoS One. 2013;8:e59378.
- [Google Scholar]







