Translate this page into:
Smoking and P53 polymorphism association with chromosomal aberration in lung cancer
⁎Corresponding author.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Tobacco exposure is the primary leading cause for cancer of the oral cavity, the airways and lung. The carcinogens in cigarette smoke have been linked as an environmental risk factor for causing genetic mutations in tumor suppressor, including TP53 (p53) which has been linked as a causative for occurrence and progression in lung cancer. The aim of the current case-control study is to assess the presence and frequency of chromosomal aberrations between smokers and non-smokers in case and control group. Further, analysis was also extended to include the p53 codon 72 Arg/Pro mutation analysis between the groups of study cohort. In methods, the case-control epidemiological study included a total of 100 cases and controls who were further categorized as smokers and non-smokers for analysis. Chromosomal aberration assay was done on peripheral blood lymphocytes and studied using Geimsa stain, while analysis of the p53 codon 72 Arg/Pro mutation was done by allele-specific polymerase chain reaction assay. The chromosomal aberration assessed include structural abnormalities like breaks, fragment, dysenteric and gaps, as well as numerical aneuploidies. Results showing the number of chromosomal aberrations was higher among smoker cases as compared to controls, and numerical aneuploidy was also detected only among this cohort. The mean of chromosomal aberrations observed between cases and controls and smokers as well as non-smokers was significant at p < 0.0001. In case of the p53 genotyping result, the frequency of the Arg/Arg allele was higher among controls as compared to cases and the difference significant at p < 0.005. Among female participants, the difference between Arg/Arg and Pro/Arg was also found to be significant between cases and controls. Combined analysis of the p53 codon 72 alleles and mean of chromosomal aberrations between cases and controls was found to be significant at p < 0.005. Our study found an association between risk of lung cancer as well as occurrence of chromosomal aberrations. In case of the p53 polymorphism, the significance was detected only among females. In Conclusion, the studied p53 variant and chromosomal aberration along with smoking has shown significant association with increased risk of lung cancer.
Keywords
Aneuploidy
Chromosome
Geimsa
Lung cancer
P53
Smoking
1 Introduction
Lung cancer is the second most common cancer among both men and women after cancer of the prostate and that of the breast, accounting for up to 25% of all cancer-related deaths. For the United States, the American Cancer Society’s estimates around lung cancer for the year 2021 include around 235,760 new cases, and about 131,880 deaths. Initial incidence studies did record a gender difference in susceptibility to lung cancer, which in today’s era is fast disappearing, and one of the primary attributed cause is smoking (American Cancer Society, 2021). Death due to use of tobacco has been projected to rise to 10 million by the year 2025, and it has been reported as the major cause of lung cancer among 90% males and 79% females (Jha et al., 2006). Further, the disease risk has been found to be 20–40 times higher among lifelong smokers, as compared to non-smokers (Ozlu and Bulbul, 2005). The correlation between smoking and increased incidence of lung cancer was published as early as in the year 1954 (Doll, 1954). In Indian context, researchers in the year 1958 reported lung cancer to be 1% of all malignancies reported in Tata Memorial Hospital, accounting for an incidence rate of 27.4 million in the 1950 s, which gradually increased to 78.6 per million in 1959, among hospital population (Sirsat, 1958; Viswanatan et al., 1962). The strong epidemiological evidence linking smoking to lung cancer led to the adoption of the Federal Cigarette Labeling and Advertising Act of 1965 and the Public Health Cigarette Smoking Act of 1969, which further banned cigarette advertisements, and enforced the need to issue public health warning on packaging (Gibbons et al., 2014). Cigarette smoke contains a minimum of 60 classified carcinogens, chronic exposure to which increases cancer risk in lung epithelium due to formation of DNA adducts that cause cancer-predisposing mutations (Pfeifer et al., 2002). Carcinogens in cigarette which will induce genotoxicity by interacting with DNA causing cytotoxicity can be estimates the effect of exposure to chromosome-damaging agents in cigarette smoke (Uma et al., 2011).
Carcinogenesis has a complicated pathophysiology involving inactivation of tumor suppressors, and activation of oncogenes. The TP53 (p53) tumor suppressor gene has been studied in relation to many cancer types, and mutation is the same is a common event in carcinogenesis. Over 50% of the human tumors have been linked to carry p53 mutation, while structural aberrations are the most common among them. The interaction between tobacco and p53 mutation has not been clearly elucidated, while transversion of G:C to T:A in lung cancer has been related to cigarette smoke exposure (Wang et al., 1995). Analysis of both small-cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) tumors identified regions in the short arm of chromosome 17 containing some regions of p53 to often be deleted along with presence of point mutations in the remaining p53 allele (Chiba et al., 1990; Takahashi et al., 1989). Mutations in p53 are common in tobacco-related cancers, wherein the prevalence of the G to T transversion mutation has been found in 30% of lung cancer cases from smokers, and 12% in non-smokers. Two main classes of carcinogens in tobacco smoke are nicotine-derived nitrosamines and polycyclic aromatic hydrocarbons (PAH), of which in lung cancer the hotspots for G to T transversion are preferential for formation of PAH-adducts in p53 gene [Pfeifer et al., 2002]. The p53 is the master regulator of intracellular functions and important in cancer, and hence is titled as “the guardian of the genome” (Alvarado-Ortiz et al., 2021).
The p53 gene inactivation has also been linked to breast cancer. The most common in primary breast carcinomas has been the loss-of-heterozygosity (LOH) in p53 gene, and among individuals with Li-Fraumeni syndrome, high proportion of germline p53 mutation occurrence has been noted, that increases risk of breast cancer (Davidoff et al., 1991; Malkin et al., 1990). Studies have also evaluated mutant p53 gene as a target for cancer treatment (Duffy et al., 2014, 2018). Mutated p53 is common in 80% of samples from difficult-to-treat cancers, and hence it is also investigated as an important target to develop novel anticancer treatments. Many reactivation agents including p53 reactivation and induction of massive apoptosis-1 (PRIMA-1), zinc metallochaperone-1 (ZMC1), and a third generation thiosemicarbazone (COTI-2) are in clinical trials after preclinical models exhibited anticancer activity (Duffy et al., 2017). A meta-analysis study associating smoking and p53 in lung cancer, analyzed data from 1770 lung cancer patients, including 69.6% smokers, and 30.4% non-smokers. The risk of mutation among lung cancer cases with smoking was found to be 2.70-fold higher, and p53 mutation risk was higher among smokers with NSCLC (OR = 2.38, 95% CI) (Xiu et al., 2014).
The p53 codon 72 Arg/Pro involves a transversion from G to C i.e arginine to proline and is a germ line mutation in exon 4. Studies have linked the Pro/Pro genotype with increased risk of lung cancer, wherein the susceptibility with Pro/Pro and Arg/Pro genotype was associated with 1.45 -fold higher risk of adenocarcinoma, and with tobacco-exposure status (Fan et al., 2000). Another evidence study involving 27,958 subjects, the p53 codon 72 mutation was linked to risk of lung cancer among patients with adenocarcinoma at OR = 1.10, 95% CI for Arg/Pro genotype (Zhou et al., 2013). Studies have also assessed the profile of chromosomal imbalances among adenocarcinoma from non-smokers using comparative genomic hybridization (CGH). Majority of the tumors were found to harbor ≤ 10 aberrations. DNA gains were linked consistently to regions 1p, 1q, 5p, 6p, 7, 8q, 12q, 16p, 17q, 19, 20q, and 22q, while loss was linked to regions 3p, 6q, 8p, 9, 13q, and 18q. Further, most over-represented chromosome arm was found to be 16p with DNA gains which is not a common observation in classic cytogenetic studies of lung cancer (Wong et al., 2003).
The aim of the current epidemiological case-control study is to assess the presence and spectrum of chromosomal aberrations among smokers and non-smokers as well as alleles of the p53 codon 72 Arg/Pro mutation with risk.
2 Materials & methods
2.1 Study cohort
The study cohort included one hundred lung cancer cases confirmed by either cytology or histology evaluation, and matched one hundred controls. All participants verbally agreed for the study, and confidentiality of all information was assured. All the participants were categorized into two groups; cases and controls, and further sub-divided into category A and B; including smokers and non-smokers. A structured questionnaire was also recorded for each participant detailing family history of cancer, personal medical history, diet, smoking, as well as alcohol intake; present and past. Demographic factors pertaining to age, gender, marital status, and socioeconomic factors, and occupation was also recorded.
2.2 Chromosomal aberration assay
Chromosomal aberration assay was done on peripheral blood samples collected from all study participants in sodium-heparin tubes. From a part of the collected 5.0 mL peripheral blood, the lymphocytes were further stimulated for culture using phytohemagglutinin in 5% CO2 atmosphere (Moorhead et al., 1960). Post culturing for 48 h, the lymphocytes were further cultured for 24 h at 37 °C. The cultures were harvested after addition of colchicine, and further the slides were stained with 4% Geimsa solution and screened for chromosomal aberrations.
2.3 TP53 codon 72 Arg/Pro mutation analysis
Genotyping for the p53 codon 72 Arg/Pro mutation was done from a part of the collected 5.0 mL peripheral blood after informed consent from all study participants. DNA extraction from the peripheral blood mononuclear cells (PBMNCs) was done by the classic salting out method which briefly involved lysis of the buffy coat nucleated cells with a lysis buffer (10 mM Tris-HCl, 400 mM NaCl and 2 mM Na2EDTA, pH 8.2), followed by overnight digestion of the cell lysates with 0.2 mL of 10% SDS and 0.5 mL of a protease K solution at 37 °C. Protein precipitation of the digested lysate was done using saturated NaCl, followed by DNA precipitation using room temperature absolute ethanol. The extracted DNA strands were dissolved in TE buffer (10 mM Tris-HCl, 0.2 mM Na2EDTA, pH 7.5) and allowed to stay at 37 °C for two hours before quantitation (Syed et al., 2011). The quantitation of the extracted DNA was done using NanoDrop™ spectrophotometer and the OD 260/280 ratios were assessed for all samples (Miller et al., 1988).
Further genotyping for the p53 codon 72 Arg/Pro mutation was done by allele-specific polymerase chain reaction (PCR) assay using primer pairs as previously described. The primer pair for p53 Pro was forward –5′– GCCAGAGGCTGCTCCCCC– 3′ and reverse - R –5′- CGTGCAAGTCACAGACTT – 3 leading to a 178-bp product. For the p53 Arg, the primer pairs used were; forward –5′–TCCCCCTTGCCGTCCCAA– 3′ and reverse –5′- CTGGTGCAGGGGCCACGC– 3′ yielding a final product of 136 bp (Mogi et al., 2001).
2.4 Statistical analysis
The collected demographic data of the study participants was assessed by mean, standard deviation (SD), and percentage for age and gender. Further, the data was also subjected to chi-square statistics, RR and 95% CI analysis to identify significance levels and establish direction of correlation by p-value, obtained using OpenEpi Version 2 software. The Cochran-Armitage test was applied on the independent data, and the independent samples t-test was applied to compare the mean of two independent samples.
3 Results
The present study included 81 males and 19 females among cases and 71 males and 29 females among controls. The type and frequency of chromosomal aberration was studied among cases and controls, as well as smokers and non-smokers. The mean of the number of aberrations was found to be higher among cases who care smokers at 137 ± 7.5. Further the mean of all types of chromosomal aberrations was higher among cases who are smokers, and aneuploidy was also detected in this cohort only in our analysis. The summary of the findings has been highlighted in Table 1. Further, the mean of chromosomal aberrations between cases and controls was also found to be significant at p < 0.0001. The findings have been highlighted in Table 2. Microscopic evaluation of the chromosomal aberrations has been highlighted in Fig. 1A and B. Our study analysis detected occurrence of chromosomal aberrations even among controls and non-smokers, the significance of which remains unknown at the time of writing this manuscript.
Type
No of Metaphase Scored
No. of aberrations (Except gaps) (Mean ± SD)
Structural abnormalities /Type of aberrations (%)
Numerical
Breaks (Mean ± SD)
Fragment (Mean ± SD)
Dicentric (Mean ± SD)
Gap (Mean ± SD)
Aneuploid
Controls
Smokers
900
97 ± 10.511
34 ± 1.41
42 ± 3.0
21 ± 1.57
26 ± 4.61
0
Non -smokers
700
62 ± 13.0
8 ± 1.0
34 ± 2.51
20 ± 1.5
19 ± 2.51
0
Cases
Smokers
600
137 ± 7.5
46 ± 3.114
53 ± 3.114
38 ± 4.16
45 ± 3.51
1
Non -smokers
500
87 ± 16.05
24 ± 0.91
47 ± 3.21
16 ± 2
20 ± 1.8
0
Mean chromosomal aberrations observed in Controls
Mean chromosomal aberrations observed in Case
Standard Error (SE)
P value
Smokers
97 ± 10.511
179 ± 17.6
0.83
0.0001*
Non-Smokers
62 ± 13.0
137 ± 7.5
0.4966
0.0001*
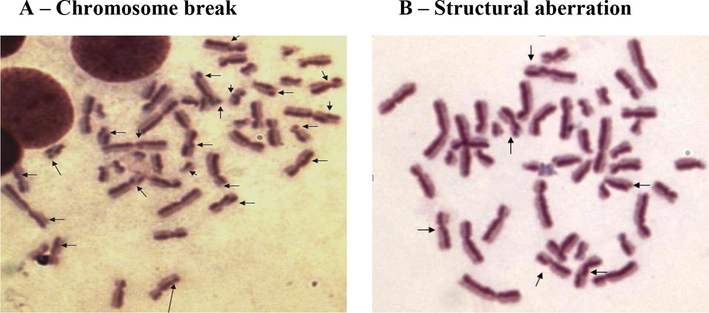
Microscopic analysis of chromosomal aberrations. A – Chromosome breakB – Structural aberration.
Allele-specific PCR was used for genotyping of the p53 codon 72 Arg/Pro mutation both among cases and controls. The frequency of the Arg/Arg homozygous allele was 76% and 87% among cases and controls respectively. Further, among females, the difference in frequency between Arg/Arg and Pro/Arg between cases and controls was found to be significant at p < 0.005. Further analysis was extended to assess distribution of p53 codon 72 Arg/Pro genotypes and chromosomal aberrations between cases and controls. No significant difference was found between p53 number of alleles and mean of chromosomal aberrations either among cases or controls. The findings have been highlighted in Table 3.
P53 Genotype
Controls
X2
CC
P value
Cases
X2
CC
P value
No of allele
Chromosomal aberration (Mean ± SD)
No of allele
Chromosomal aberration (Mean ± SD)
Arg/Arg
48
2.4 ± 1.5
2.5
0.2
0.6
45
2.8 ± 1.4
1.6
0.16
0.8
Pro/Pro
2
2.1 ± 1.4
3
2.3 ± 1.56
Arg/Pro
5
2.8 ± 1.5
07
2.8 ± 1.2
Further combined analysis comparing mean of p53 alleles and chromosomal aberration between cases and controls was done by F-test and T-Test. The difference was found to be significant at p < 0.005. The findings have been highlighted in Table 4.
F-test for equal variances (p53)
T-test (assuming equal variances)
Controls p53 genotyping & chromosomal aberration
Cases p53 genotyping & chromosomal aberration
P value
Difference
−0.6
P value
Mean
1.2
1.8
0.01*
0.02*
95% CI
0.9 – 1.4
1.2 – 2.3
95% CI
−1.10 – 0.09
SE for Mean
0.11
0.26
SE
0.25
SD
0.5
1.0
4 Discussion
The focus of this epidemiological case-control study was to assess presence and types of chromosomal aberrations and the p53 codon 72 Arg/Pro mutation in lung cancer between smokers and non-smokers. The overall survival rate for lung cancer all stages combined is lower at 21% compared to cancer of the prostate (98%), breast (90%), and melanoma of the skin (93%) (American Cancer Society, 2021). Until the first epidemiological connection between smoking and lung cancer was given in the year 1957, experimental data summarizing tobacco as a cancerous agent, as well as the biochemical aspect was missing, which made the topic of impact of smoking on lung cancer, highly debatable (Gibbons et al., 2014). In the year 1959, the Surgeon General of the United States classified smoking as the principle etiologic factor for increased incidence of lung cancer (Burney, 1959).
Mutations in the p53 gene have been found to be higher among tobacco-related cancers and in lung cancer, the pattern was been found to be different between smokers and non-smokers. The mutational hotspots in p53 have been found between codons 248 and 273, wherein the G to A transitions is mostly present in non-tobacco-related cancer types (Pfeifer et al., 2002). Studies have concluded somatic mutations in p53 to be crucial in pathogenesis of early-stage NSCLC, and not associated with tumor stage, nodal status, or gender. Further, presence of p53 mutations have also been linked to younger age and squamous histology (Chiba et al., 1990). Our study was focused at assessing presence and allele distribution of the codon 72 Arg/Pro mutation between cases and controls as well as smokers and non-smokers. The prevalence of the Arg/Arg genotype was higher among controls at 87%, compared to cases at 76% and the difference significant at p < 0.005. Further, only among female participants, the difference in allele distribution of Arg/Arg and Pro/Arg was found to be significant at p < 0.005 between cases and controls. Meta-analysis studies have linked the p53 codon 72 Arg/Pro with lung cancer risk among Asians wherein the dominant genetic model correlated with lung cancer risk at OR = 1.14, 95% CI (Wang et al., 2013). Gender-based differences in lung cancer and the studied p53 mutation have been linked to survival with platinum-based chemotherapy. A study from North India involving 420 each of cases and controls identified the Pro/Pro genotype to exhibit better median survival of 10 months (HR = 0.65, 95% CI), and among female patients, the Arg/Pro carriers (HR = 0.08, 95% CI) and Pro/Pro carriers (HR = 0.21, 95% CI) exhibited better survival and prognosis as compared to males (Kumari et al., 2016).
Chromosomal aberration in lung cancer have been associated with long arm of chromosome 6, wherein the susceptibility locus has been found to be 6q23-25, and promoter methylation of genes around this region have been studied. These studies identified frequent inactivation of multiple tumor suppressor genes within chromosome 6q to be linked with development of sporadic cases of lung cancer (Tessema et al., 2008). Studies have also found deletion between regions 6q14 and 6q24 in 60% of the primary lung tumors (Goeze et al., 2002). Studies have also investigated chromosomal alterations associated with clonal evolution to metastatic stage among lung squamous cell carcinoma. The metastatic phenotype was found to be characterized by deletions at regions 3p12-p14, 3p21, 4p15-p16, 6q24-qter, 8p22-p23, 10q21-qter and 21q22, and over-representation at 1q21-q25, 8q, 9q34, 14q12 and 15q12-q15; indicating that primary tumor among cases can be evaluated for presence of chromosomal aberration signatures associated with metastasis to identify risk (Petersen et al., 2000). Our study was focused at identifying spectrum of chromosomal aberrations between cases and controls using Geimsa stain technique. More than 500 metaphases were scored for both cases and controls including smokers and non-smokers and the difference in the mean was found to be significant at p < 0.0001. Further, numerical aneuploidy as well as all four categories of imbalances including breaks, fragment, dicentric, and gap was higher among smoker cases as compared to other categories. Analysis of p53 alleles and mean of chromosomal aberrations between cases and controls was not found to be significant. However, combined analysis between mean of p53 genotyping and chromosomal aberration was found to be significant at p < 0.005.
A dose-dependent relation between smoking and risk of lung cancer has been highlighted by many studies which recommend “smoking cessation” to be one of the prime cornerstones to prevent smoking-related cancers (Song et al., 2008). Our study assessed the relation between chromosome aberration as well as the p53 codon 72 Arg/Pro mutation with lung cancer and found the combined to be significant between cases and controls.
5 Conclusion
Mutations in tumor suppressor p53 have been linked to lung cancer, and the risk of mutations are higher among smokers. Chromosomal aberrations become a noteworthy diagnostic tool in lung cancer to identify mutation accruement signature at different disease stages, and also changes in the same during treatment. Multiple mutations have also been studied in relation to disease progression and treatment outcome, with p53 codon 72 Arg/Pro being common in many cancer types. Our study did detect a gender difference in prevalence and significance of the Arg/Arg and Arg/Pro allele between cases and controls, highlighting the need to study gender-based risk influence in a large cohort.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1441-465.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mutant p53 gain-of-function: Role in cancer development, progression, and therapeutic approaches. Front. Cell Dev. Biol.. 2021;2021
- [CrossRef] [Google Scholar]
- American Cancer Society. Record drop in cancer mortality for second straight year due to improved lung cancer treatment; COVID-19 impact still unknown. Jan 21, 2021. Accessed on: 17th Feb, 2021. Available online: Record Drop in Cancer Mortality For Second Straight Year Due to Improved Lung Cancer Treatment; COVID-19 Impact Still Unknown.
- Burney, L.E., 1959. Smoking and lung cancer: a statement of the Public Health Service. J Am Med Assoc 171, 1829-1837. doi: 10.1001/jama.1959.73010310005016.
- Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung cancer study group. Oncogene. 1990;5(10):1603-1610.
- [Google Scholar]
- Genetic basis for p53 overexpression in human breast cancer. Proc. Natl. Acad. Sci. U.S.A.. 1991;88(11):5006-5010.
- [CrossRef] [Google Scholar]
- Doll, R., 1954. Review of Cancer of the Lung (Endemiology). Edited by J Clemmeson. Br Med J 2:1402.
- Duffy, M.J., Synnott, N.C., McGowan, P.M., Crown, J., O’Connor, D., Gallagher, W.M., 2014. p53 as a target for the treatment of cancer. Cancer Treat Rev. 2014, 40(10), 1153-1160. doi: 10.1016/j.ctrv.2014.10.004.
- Mutant p53 as a target for cancer treatment. Eur. J. Cancer. 2017;83:258-265.
- [CrossRef] [Google Scholar]
- Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res. Treat.. 2018;170(2):213-219.
- [CrossRef] [Google Scholar]
- The p53 codon 72 polymorphism and lung cancer risk. Cancer Epidemiol. Biomark. Prev.. 2000;9(10):1037-1042.
- [Google Scholar]
- Smoking, p53 mutation, and lung cancer. Mol. Can. Res.. 2014;12(1):3-13.
- [CrossRef] [Google Scholar]
- Chromosomal imbalances of primary and metastatic lung adenocarcinomas. J. Pathol.. 2002;196(1):8-16.
- [CrossRef] [Google Scholar]
- Jha, P., Chaloupka, F.J., Moore, J., Gajalakshmi, V., Gupta, P.C., Peck, R., Asma, S. and Zatonski, W., 2006. Tobacco addiction. Disease Control Priorities in Developing Countries. 2nd edition.
- Association of p53 codon 72 polymorphism and survival of North Indian lung cancer patients treated with platinum-based chemotherapy. Mol. Biol. Rep.. 2016;43(12):1383-1394.
- [CrossRef] [Google Scholar]
- Germline p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233-1238.
- [CrossRef] [Google Scholar]
- Miller, S.A., Dykes, D.D., Polesky, H.F., 1988. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16(3), 1215. doi: 10.1093/nar/16.3.1215.
- Chromosome preparations of leukocytes cultured from human peripheral blood. Exp. Cell Res.. 1960;20(3):613-616.
- [CrossRef] [Google Scholar]
- Chromosomal alterations in the clonal evolution to the metastatic stage of squamous cell carcinomas of the lung. Br. J. Cancer. 2000;82(1):65-73.
- [CrossRef] [Google Scholar]
- Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435-7451.
- [CrossRef] [Google Scholar]
- Some aspects of the pathology of primary carcinoma of the lung. J Postgrad Med.. 1958;4:6-14.
- [Google Scholar]
- Reduction and cessation of cigarette smoking and risk of cancer: a cohort study of Korean men. J. Clin. Oncol.. 2008;26(31):5101-5106.
- [CrossRef] [Google Scholar]
- Evidence of association of a common variant of the endothelial nitric oxide synthase gene (Glu298 Asp polymorphism) to coronary artery disease in South Indian population. J. Med. Genet. Genomics. 2011;3(1):13-18.
- [Google Scholar]
- P53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246(4929):491-494.
- [CrossRef] [Google Scholar]
- Promoter methylation of genes in and around the candidate lung cancer susceptibility locus 6q23-25. Cancer Res.. 2008;68(6):1707-1714.
- [CrossRef] [Google Scholar]
- Smoking-induced satellite associations in a rural population of south India: an in vitro study. Int. J. Appl. Basic Med. Res.. 2011;1(2):75.
- [Google Scholar]
- Mutations in the p53 gene in lung cancer are associated with cigarette smoking and asbestos exposure. Cancer Epidemiol. Biomark. Prev.. 1995;4:543-548.
- [Google Scholar]
- P53 codon 72 Arg/Pro polymorphism and lung cancer risk in Asians: an updated meta-analysis. Tumour Biol.. 2013;34(5):2511-2520.
- [CrossRef] [Google Scholar]
- Chromosomal aberrations of primary lung adenocarcinomas in nonsmokers. Cancer. 2003;97(5):1263-1270.
- [CrossRef] [Google Scholar]
- Association between smoking and p53 mutation in lung cancer: a meta-analysis. Clin. Oncol.. 2014;26(1):18-24.
- [CrossRef] [Google Scholar]
- Zhou, C., Chen, H., Wang, A., 2013. P53 codon 72 polymorphism and lung cancer risk: evidence from 27,958 subjects. Tumour Biol. 2013; 34(5): 2961-2969. doi: 10.1007/s13277-013-0859.







