Translate this page into:
Simultaneous removal of NH4+ and SO42− in Sulfate-reducing anammox scale reactor using FDAARGOS_798 strain/Anammox integration
⁎Corresponding author at: Department of Chemical & Environmental Engineering, School of Science, Shenyang University of Technology, Shenyang 110870, China. liangjiyan2007@126.com (Jiyan Liang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
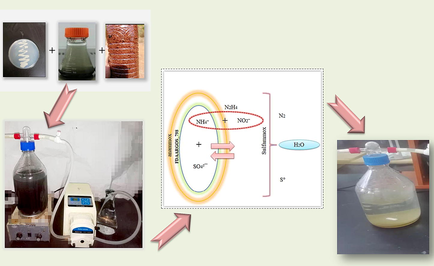
Abstract
The effect of absence and appearance of isolated Bacillus cereus on sulfammox was studied in semi-continuous stirred reactor. The highest removal efficiency of NH4+ 77 ± 0.2 % and SO42- 50 ± 1.0 % were obtained in the SCS-2 reactor with Bacillus cereus existence. Reactors with Bacillus ceruse addition reduced the startup time of the sulfammox process. The collapse of Bacillus ceruse could be caused by the high concentration release of toxic components. NH4+ and SO42− rich wastewater can be treated in a sustainable way by Bacillus cereus granules.
Abstract
In this study, an exploratory experiment conducted to assess the efficiency of time reducing Bacillus cereus FDAARGOS_798 strain in the Sulfate-reducing anaerobic ammonium oxidation –Sulfammox- process at lab-scale reactor. The sulfammox process was examine in four different semi-continuous stirred (SCS) reactors receiving a treated synthetic wastewaters by 1 % of FDAARGOS_798 strain acclimated and carried with 2 % anaerobic granular sludge (sludge) in the presence and absence of the 1 % anammox consortia granular sludge (Anammox). Subsequently, the previous work outcomes of optimized anaerobic acclimation method isolating FDAARGOS_798 strain. The SCS-2 reactor with sulfammox integration (FDAARGOS_798/Anammox) has shown significant performance in anoxic simultaneous removal of NH4 + and SO4 2-, 77 ± 0.2 % and 50 ± 1.0 %, respectively, with the sole substrate (NH4)2SO4 0.1 g/L. The NH4+ and SO42- average decrease of 40 ± 0.2 % and 46 ± 0.17 % in 26 days, respectively, in SCS-4 reactor treated by sulfammox (FDAARGOS_798/Anammox) in (NH4)2SO4 0.5 g/L. Subsequently, the N/S ratio is calculated at around 1.8 ± 0.2 as evidence of reaching sulfammox process. The sulfammox process could not detect at SCS-1and SCS-3 reactors which are in the absence of sulfammox integration. Meanwhile the exact sulfammox process pathway was not detectable due to complicated scenarios. In conclusion, the addition of Bacillus ceruse FDAARGOS_798 carried by Anammox consortium is a well indicate to an effective and economical way that accelerate the start-up of the sulfate-reducing anammox process.
Keywords
Ammonium
Bacillus cereus
N/S ratio
Sulfate
Sulfate-reducing anaerobic
Semi-continuous stirred reactor
- Anammox process
-
Anaerobic Ammonium Oxidation
- AnAOB
-
Anammox Ammonium Oxidation Bacteria
- SRB
-
Sulfate Reducing Bacteria
- SOB
-
Sulfur Oxidizing Bacteria
- Sulfammox
-
Sulfate-reducing anammox
- anammox
-
anammox granular sludge
- sludge
-
anaerobic granular sludge
- SUD-1
-
Bacillus cereus
- SCS reactor
-
semi-continuous stirred
Abbreviations
1 Introduction
Currently, wastewater treatment is one of the hot topics that need to study in deep. The industrial contamination components release rapidly into the environment with dangerous effects. Raw wastewater contains significant concentrations of ammonium, sulfate, and heavy metals that are not degraded by the conventional process of wastewater treatment. The degradation of ammonium is demanded due to the easy chemical exchange of ammonium shapes, especially in water, and there harmful (Al-Hazmi et al., 2023) (See Fig. 1).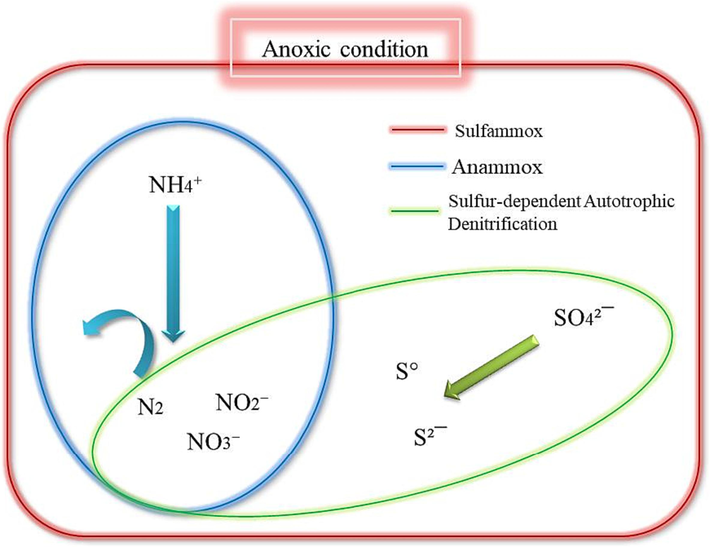
Overlap between sulfammox, anammox and sulfur-dependent autotrophic denitrification interactions.
However, an anaerobic ammonium oxidation (Anammox) reaction is known as a new process treating ammonium by oxidation with electron acceptor nitrite (NO2–) by anammox bacteria Candidatus Brocadia. This technique is widely applicable, low cost, and simple chain reducing energy and time more than conventional nitrification–denitrification. Besides, the low C/N ratio and the ammonia concentration are 50–100 times higher than that in municipal wastewater and also high concentrations of organic matter. All these contributed to the difficulty of conventional biological treatment of high NH4+ wastewaters (Madani, 2021;Wang et al., 2022). (N2H4) NH4 ++NO23 -→N2 + 2H2OΔG0 = − 360 kJ/mol
In the Netherlands, a full-scale reactor designed to use anammox bacteria was constructed in 2002.Anammox activity is unintentionally seen in other wastewater treatment facilities, such as the one in Germany (Hattingen), despite the fact that they weren't intended to do so. As of 2006, the Netherlands had three large-scale processes: one on industrial effluent and two in a municipal wastewater treatment facility (in Rotterdam). One is a tannery, while the other is a facility that processes potatoes (Derwis et al., 2023). Compared to the conventional nitrification–denitrification process, anammox saves resources by requiring less aeration, using no sources of organic carbon, and producing less sludge than the conventional systems, which in turn lowers operating costs and greenhouse gas emissions. The oxidation of NH4+ with NO3 has also been used to explain the anoxic disappearance of NH4+ in marine sediments. Additionally, calculations of the G values for the oxidation of NH4+ with NO3 have shown that the process is energetically favorable, which is another reason to expect it might occur in nature (Rios-Del Toro et al., 2018). Anammox is therefore regarded as an economical, efficient, and environmentally friendly process (Chai et al., 2021).
An important operational factor that affects the kinetics of the anammox process is temperature. In instance, the specific anammox activity significantly declines below 15 °C. This work attempted to explain the anammox process kinetics' temperature dependence in the region of 10 to 55 °C. The anammox bacteria prefer a temperature between 35 and 40 °C for growth and activity. The optimal temperature for anammox bacteria growth was set at 35 ± 1 °C (Grubba et al., 2022).
The biological treatment for nitrogen removal is carried out in two steps: in the first step (nitrification, Equation), ammonium is converted into nitrite by ammonium oxidizing bacteria (AOB, e.g. Nitrosomonas sp.), followed by nitrate by nitrite oxidizing bacteria (NOB, e.g. Nitrobacter sp.), and finally into dinitrogen gas by heterotrophic bacteria. Equation 3 describes the entire reaction (Wang et al., 2021). NH4++2O2 + 2HCO3–→NO3– +3H2O + 2CO2 5C + 4NO3-+2H2O → CO2 + 4HCO3-+2N2 4NH4++8O2 + 5C + 4HCO3 → 2 N2 + 10H2O + 9CO3
The NH4+ molecule remains in solution where it is oxidized by bacterial catalysts in the presence of oxygen to produce the oxidation products NO2– and NO3, as well as the NOx gases, which are necessary intermediates in the conversion of ammonia to dinitrogen gas. NH4+ → NO3– ↔ NO2– → NO ↔ N2O → N2
The integration of anammox and heterotrophic denitrification in a single reactor unit improves the efficiency of partial nitrification/anammox. However, heterotrophic nitrifying bacteria can worsen the anammox process, as they exhibit higher growth rates and can outcompete anammox bacteria by using nitrite as an electron acceptor. In such conditions, heterotrophic denitrifying bacteria are able to breakdown the extracellular polymeric substances (EPS) made by anammox bacteria and utilize them as an organic carbon source while encoding nitrite and/or nitrate respiration (Al-Hazmi et al., 2023). Anammox populations are dominated by the genus Candidatus Brocadia while the genus Thauera is dominant among heterotrophic denitrifies (Grubba et al., 2022). Moreover, the high concentration of different sulfate, sulfuric acid, sulfide, or thiosulfate in wastewater caused water mineralization, metal corrosion, and laxative effects in mammals and fishes. Generally, ammonium and sulfate are important compounds and necessary for living organisms but at acceptable levels of 250 mg/L (Zhang, 2022).
The high concentration of SO42- discharging from many industries like petroleum refining, pharmaceutical companies, palm oil production, seafood processing and sulfuric acid titanium dioxide enterprises (Huo et al., 2022).The sulfate-reducing bacteria (SRB) play the main role in sulfate reduction under an anoxic environment. However, the potential of traditional treatment using SRB, which might succeed harmful secondary component as hydrogen sulfide gas (H2S) (Madani, 2021). Meanwhile the Thiobacillus denitrificans with a little NO2– have a positively effect on N and S reduction. Also NO2 could be utilize at the anammox process by the sulfur-oxidizing bacteria (SOB) (Lin et al., 2022).
Due to critical environmental condition of chemolithoautotrophic AnAOB and there slow doubling time nature, the cultivation and enrichment process are too difficult. As (Mohammed Madani, 2022) mentioned that the sulfammox maximum growth rate was observed at 24–72 hrs and, calculated to be 7.3 × 109 CFU/mL/day. The modified anaerobic acclimation procedure reduces time in sulfammox reaction.
Most ammonium and sulfate traditional treatments methods are separated until find an unusual anaerobic route reduced up to 50 % of ammonium and 80 % sulfate simultaneously by Fernando Fdz-Polanco, since 2001,(Madani, 2021) which presented at equation below, as a result of granular activated carbon (GAC) anaerobic fluidized-bed reactor treating vinasse from an ethanol distillery of sugar beet molasses. The Sulfate-reducing ammonium oxidation (sulfammox) process has slight bioreactors studies due to the low doubling time of responsible bacteria and operation challenges (Dominika et al., 2021).
(Sulfate. Anammox bacteria) 2NH4++ SO42−→ N2 + S0 + 4H2O
The possibility of sulfammox appearance might found at several suggested mechanisms like the one by it is nature, as shown the SO42− could be used as an additional electron acceptor in the anaerobic oxidation of NH4 +. On another hand the sulfammox could involve the anammox process using the intermediate NO2– as an electron acceptor during the whole process. Also there is ability to an interaction between autotrophic S-dependent denitrification process due to NO2 and NO3 production during sulfammox process. Subsequently the Sulfammox, Anammox and S-dependent autotrophic denitrification could work together at some times in hidden scenarios that might lead to reduce NO2 by AAOB and autotrophic denitrifies (Grubba et al., 2022). The NH4+ reduction rate and SO42- reduction rate are enhanced by addition of SO42- high concentration as a reflection of N-S biological pathway. A good sign have given in SO42- reduction rate after 160 days at the BR-SR regular reactor without NO2.The Nitrosomonas and Thauera are playing an essential role in N and S metabolism for each on respectively. The NO2 have a sound role in simultaneous S removal and anammox process. Due to the variety of N, S and C removal processes, the research interests have been shifting to the use of single- and multi-stage systems based on the combination of several processes, such as heterotrophic sulfate reduction, S-dependent autotrophic denitrification, nitrification, denitrification, anaerobic ammonia oxidation (anammox) and sulfammox (Al-Hazmi et al., 2023).
Despite this phenomenon has begun applied under organic conditions, (Liu et al., 2008) was reported Anammoxoglobus sulfate reached a complete autotrophic sulfammox process with an intermediate appearance of nitrite as following equations. SO42- + NH4+ → NO2– + S + 2H2O NH4+ +NO2−→ N2 + 2H2O NH4+ +1/2SO42− →1/2N2 + 1/2S + 2H2O
It has been demonstrated that efficient sulfammox process access at high concentrations of ammonium and sulfate under autotrophic anoxic conditions. When the N/S ratio equals 2:1 it can be defined as an essential indicator of the sulfammox reaction successes (Liu et al., 2008).
Although the removal of NH4+ at sulfammox reactor was lowest than the conventional Anammox reactor using SO42− as electron acceptor. However, hydrazine and hydroxyl Amine injection and temperature raise were promoted enhancing the sulfammox reaction performance,(Madani, 2021).
Lower operating costs are obtained by the utilization of shared reactors and the use of some products as substrates in one procedure. Additionally, S-dependent autotrophic denitrification is environmentally beneficial, produces less sludge, and uses no carbon. The wastewater treatment, especially for NH4+ and SO42- rich industrial wastewater, to lower sludge generation and energy use (Hudaib, 2021).
One of the major process drawbacks is a long time anammox bacteria division, so few researchers have addressed the Sulfate-reducing ammonium oxidation microorganism. The first isolation of sulfate-reducing ammonium oxidation bacteria was carried out by (Cai, Jiang, and Zheng, 2010). In general (Wang et al., 2020), was explained the effect of adding bacteria to enhance the Anammox process and other co-materials. These showed the potential of sulfammox treatment of some industrial wastewater with high concentrations of ammonium and sulfate, such as those derived from effluents from factories that produce seafood, chemicals, textiles, paper, fermented foods, and sugar. In our previous work the Bacillus ceruse has shown a successful remarkable indicator in simultaneous ammonium and sulfate removal (Mohammed Madani, 2022). Meanwhile with more specific experiments, the full scale application with Bacillus ceruse will save rich ammonium and sulfate wastewater treatment costs. To achieve the benefits of NH4+ and SO42- removal shortcut and low energy.
The anammox bacteria can thrive and become more enriched in the porous carriers, which increases their biomass. This in turn presents a practical technique for anammox activation in WWTPs. A high density of anammox bacterial aggregates found in sludge can be used to inoculate full-scale WWTPs. It has also been established that the anammox reaction is essential to the overall metabolism of nitrogen. Based on this, we hypothesized that the Bacillus ceruse could provide a further possible pathway for WWTP applications by simultaneously treating NH4+ and SO42- in one of the most efficient saving routes (Huo et al., 2022).
The goal of this work is to reduce the startup time of sulfate-reducing ammonium oxidation process by applying the consortium of an isolated Bacillus ceruse as sulfammox bacteria with anammox culture in a bioreactor using different concentrations of (NH4)2SO4 as main substrate of ammonium and sulfate together. This is the first time to apply a time-reducer fresh isolated sulfammox bacteria in lab-scale reactor to detect the sulfammox process.Also to unified The N/S ratio N/S [n (NH4+ -N)/n (SO4 2– -S)] through the experiment. Using the lab-scale bioreactors, the processes which were optimized in experiments can be scaled up with more studies to a pilot level due to close observation.
2 Material and methods
2.1 Experimental setup
2.1.1 Description of substrate inoculums preparation
In order to prepare rector solutions, the inoculum consisting of anaerobic granular sludge (sludge) was collected from the municipal wastewater Shenyang Southern Sewage Treatment Plant-China. The volatile suspended solid (VSS) of the seed sludge was 3.0 g·L-1, MLSS 0.329 mg/L. Besides the consortia of Anammox granular sludge (anammox) is about 470 mm which has strong absorption from JY environment company, Jiangsu, China. The samples were stored at 4° C for further use in sterile plastic bottles and brought to the lab aseptically. So far nine species of anammox bacteria have been belong to the anammox granules (Candidatus Brocadia anammoxidans,Candidatus Brocadia fulgida, Candidatus Kuenenia stuttgartiensis, Candidatus Scalindua brodae, Candidatus Scalindua wagneri, Candidatus Anammoxoglobus propionicus, Candidatus Jettenia asiatica, Candidatus Anammoxoglobus, Candidatus Scalindua sorokinii).
Subsequently, the preparation and activation of identified Bacillus ceruse FDAARGOS_798 strain by (Mohammed Madani, 2022) named (SUD-1) achieved the unique sulfate-reducing anammox process successfully.After activation, inoculating one loop-full colony for 24hr into a 250 ml flask of the activation broth medium 0.1 % peptone water with pH 7.2–7.4, 121 °C under steam sterilization for 20 min in anaerobic flasks under followed anaerobic conditions. The flasks were incubated for 48hr at 30 ± 2 °C on a rotary shaker at 120 rpm. All previous inoculums mixed in sole substrate (NH4)2SO4 0.1 g/L with modified supported co-media per one liter contains: 0.5g NaHCO3, 0.025g KH2PO4, 0.3g CaCl2, 0.025g FeSO4·7H2O, 0.1 MgCl2 and 0.00625 EDTA (Mohammed Madani, 2022; Zhang et al., 2019b).
2.1.2 The reactor and experimental conditions
The Experimental setup was carried out at lab scale semi-continuous stirred SCS reactors. The 2L cylindrical glass container is 33 cm in height * 13 cm diameter, equipped with magnetic stirring. There are four SCS reactors with different tracks illustrated in Table. 1.
(NH4)2SO4 g/L
SCS-reactor /Treatment
SCS-reactor/Treatment
0.1
(1)/ Sludge + Anammox
(2)/ Sludge + Anammox/ Bacillus ceruse
0.5
(3)/ Sludge + Anammox
(4)/ Sludge + Anammox/ Bacillus ceruse
2.1.2.1 SCS-1 and SCS-2 reactors in 0.1 g/L (NH4)2SO4 substrate
SCS-1reactor consist 0.1 g/L (NH4)2SO4 dissolved in 1000 ml, 100 ml anammox, 200 ml sludge. In SCS-2 reactor add 200 ml of sludge to 100 ml of cultivated Bacillus cereus stirrers for 3 days at 120 rpm while incubated at 30 ± 2 °C for acclimation and adhesion with anammox and sludge considering as Bacillus.
cereus carrier before the reactor work start-up. Subsequently the SCS-2 reactor consist 0.1 g/L (NH4)2SO4, 100 ml anammox, 300 ml sludge and Bacillus cereus (SUD-1) strain with initial CFU/ml 1.43*106.
2.1.2.2 SCS-3 and SCS-4 reactors in 0.5 g/L (NH4)2SO4 substrate
SCS-3 reactor consist 0.5 g/L (NH4)2SO4 dissolved in 1000 ml, 100 ml anammox, 200 ml sludge. In SCS-4 add 200 ml of sludge to 100 ml of Bacillus cereus stirrers for 3 days at 120 rpm while incubated at 30 ± 2 °C for acclimation before the reactor work start-up. Subsequently the SCS-2 reactor consist 0.1 g/L (NH4)2SO4, 100 ml anammox, 300 ml sludge and Bacillus cereus (SUD-1) with initial CFU/ml 1.43*106.
2.2 SCS reactors work strategy
These four reactors' work strategy is in terms of certain steps, the first step is to insert one liter of the (NH4)2SO4 substrate with inoculums divided to: (sludge + anammox) on SCS-1 and SCS-3, and (sludge + anammox + Bacillus cereus), as in previous descriptions for two days with nitrogen gas flushing for 5 min. The second step discharges the outlet water and influences the water entrance (substrate) using a switch pump. This exchange between influence and effluence of synthetic wastewater has been within 24hr hydrodynamic retention time (HRT), under followed anaerobic conditions each HRT. The SCS reactors initial pH of 7.5 is incubated at 30° C ± 2 on a rotary shaker at 120 rpm. The schematic diagram of the experimental setup is shown in Fig. 2.The removal efficiency of ammonium and sulfate was calculated using the equation below:
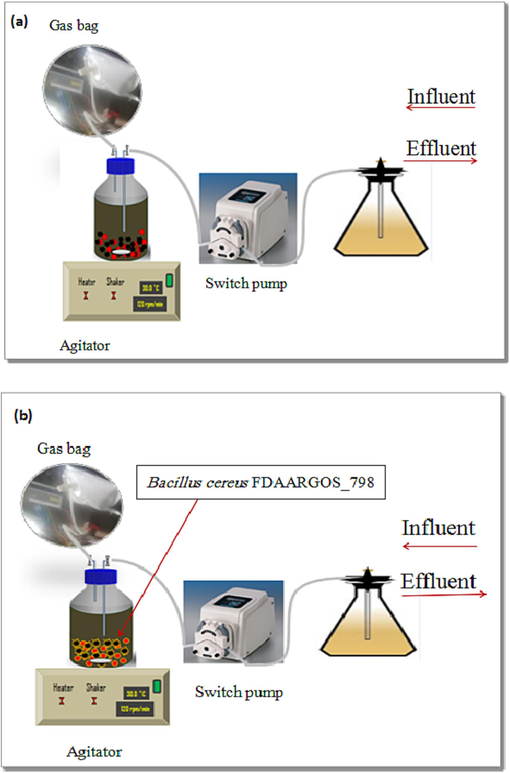
a. Scheme of the a.SCS-1 and 3 reactors (anammox) and b. The SCS-2 and 4 reactors (anammox/SUD-1strain).
All the SCS reactors were under observation and work parallel during the same time.
2.3 Chemical analysis
Each NH4+, SO42-, NO2–, and NO-3 were measured using UV- spectrophotometer (Hach DR5000) and were measured according to the standard method test (Bureau, 2002). The NH4+ sample tested by Nesslerization method, buffered and distilled. The ammonium in the distillate or in the sample is treated with Nessler’s reagent and the color developed is matched with that of a series of standard ammonium solutions and measured at 400 to 425 nm.Using cadmium reduction method for determination of Nitrate. This method is suitable for concentration below 0.1 mg per liter of NO3– and measure near 543 nm. By mixing diazotized sulphanalic acid with N- (1 napthyl)- ethylene diamine dihydrochloride (NED dihydrochloride), a reddish-purple azo dye that is formed at pH 2.0 to 2.5 can be used to measure nitrite. At 543 nm, the NO2 is measured. Subsequently, the SO42- is precipitated in hydrochloric acid medium with barium chloride in such a manner as to form barium sulfate crystals of uniform size. The absorbance of barium sulfate suspension is measured by spectrophotometer for use at 420 nm.The pH of the culture media was measured by FE28, METTLER pH meter. All liquid samples were filtrated by a 0.45 μm membrane before analysis.
2.4 Gas chromatography – Mass spectrum analysis (GC–MS)
GC–MS analyses were performed using Shimadzu (岛津) QP2010 Plus model. The gas is directly packaged in an air bag, directly sampled, injected and tested with a 10uL gas sampling needle.
2.5 Statistical analysis
Each experiment was performed in triplicate.All the data was evaluated, and the average of three distinct numbers of individual observations was calculated. After 24 h of hydrodynamic retention time was reordered as a mean percentage of ammonium and sulfate removal efficiency. The mean and standard deviation are used to express the results.
3 Results and discussion
3.1 Simultaneous anaerobic ammonium and sulfate removal detection
3.1.1 SCS-1 reactor (anammox) performance in 0.1 g/L of (NH4)2SO4
The operation of SCS-1 reactor was carried out by inoculating (anammox) and (sludge) in 0.1 g/L of (NH4)2SO4 (NH4+∼50–60 and SO42- ∼140–160 mg/L). The Hydraulic retention time (HRT) is specified as 24hrs. After chemical analysis a light appearance of ammonium oxidized with sulfate such a probable electron acceptor in the SCS-1 reactor as a decrease of ammonium with little sulfate conversion.
During around 30 days of SCS-1 reactor operation, the NH4+ concentrations of the fed were 46.2 ± 3.6 mg/L/d, and the influent SO4 2– loading rate was maintained at 137 ± 0.1 mg/L/d. The removal efficiency of ammonium and sulfate were reach 77.5 ± 2 % and 18.5 ± 1 %, respectively and the concentrations achieved in the effluent were 10.35 and 110.84 mg/L, respectively, as shown in Fig. 3. Also, it’s noted that the nitrate was detected after a run in a quite low level of 0.8–1.7 mg/L with hidden nitrite production. The pH was 7.4–6.1 during the tests therefore not matching with pH behavior during the sulfammox process. As seen the simultaneous removal efficiency of NH4+ and SO42- were not significant which may be considered as separation routes involved heterotrophic sulfate reduction coupled with sulfide-utilizing denitrification indicating the tiny quantity of sulfate reduction in the SCS-1 reactor system. Subsequently, the organic appearance could be due to microbes’ blackout. Otherwise, the ammonium reduction seems as classical Ammonium oxidation by AAOB followed by an anammox process (Zhang et al., 2020).
5SO + 6NO3-+2H2O → 5SO42- + 3 N2 + 4H+
8CH1.8O0.5N0.2 + 4.2SO2-+1.6 N+→4.2S2-+8CO2 + 1.6NH4++4.8H2
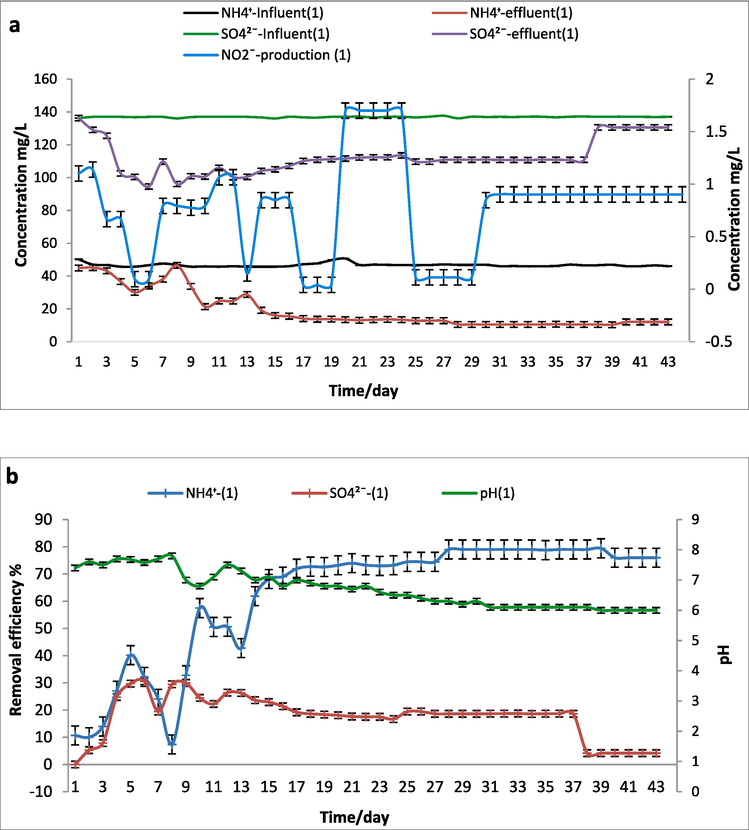
SCS-1 reactor (0.1 g/L of (NH4)2SO4) a. Influent and effluent NH4+, SO42– concentrations and NO2– production. b. The removal efficiency of NH4+ and SO42– and pH.
The most noticeable result here is the substantial decrease of ammonium with little sulfate conversion during the whole process. Thus the anammox consortium has no proof or marks to achieve the Sulfate-reducing anammox reaction. The fresh anammox biomass might be reason for undetectable sulfate conversation as (Bi et al., 2020).
3.1.2 SCS-2 reactor (anammox/SUD-1strain) performance in 0.1 g/L of (NH4)2SO4
The performance of SCS-2 reactor was directed by anammox, sludge, and Bacillus cereus (SUD-1) with initial CFU/ml 1.43*106 in 0.1 g/L of (NH4)2SO4. The SCS-2 reactor was under observation and work parallel with SCS-1 reactor during the same time. The sulfammox process was detected clearly at the average effluent concentrations of NH4+ and SO42− were 40 and 80 mg/L respectively, after approximately one month. The influence loading rate of NH4+ and SO42- were 46.2 ± 3.6 and 137 ± 0.1 mg/L/d, respectively. The effluent concentrations were 11.2 and 72.6 mg/L. The removal efficiency of ammonium and sulfate at SCS-2 reactor were 77 % and 50 % ±1.0, respectively. One anther hand the maximum growth of anammox was at 21st days shown at Fig. 4. This process revealed a little production of nitrite (NO2–) around 1.2 mg/L in the SCS-2 reactor effluent without nitrate (NO3–) an occurrence supported by. Peaks of NO2– in the influent (10 mg /L) caused considerable disruptions to the sulfammox process, which led to a notable reduction in sulfate. Through a sulfur-using denitrification process, a greater NO3-concentration also encourages sulfate resynthesize (Liu et al., 2008; Zhang et al., 2020).
SO42- + NH4+ → NO2– + S + 2H2O
NH4+ +NO2−→ N2 + 2H2O
NH4+ +1\2SO42− →1/2N2 + 1/2S + 2H2O
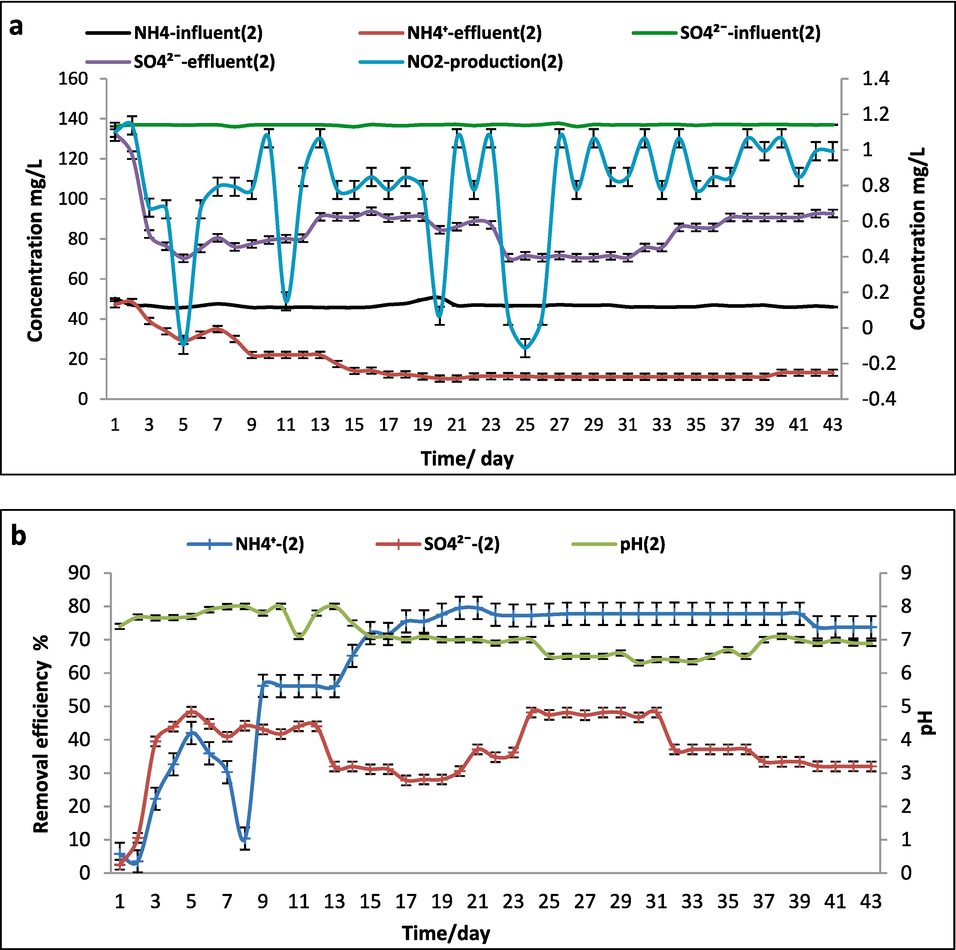
SCS-2 reactor (0.1 g/L of (NH4)2SO4) a.Influent and effluent NH4+, SO42– concentrations and NO2– production. b. The removal efficiency of NH4+ and SO42– and pH.
In the previous studies researchers are united in the opinion of sulfammox process successes by observing NH4+ and NO2– high reduction rate in the effluent (Zhang et al., 2020). The pH was 7.4 at the beginning of the reaction. Meanwhile, the sulfammox process pH has been constantly increased at 8–7.8, and then decrease silently by the end of the reaction up to 6.8. This pH transformation during the whole process in SCS-2 reactor was fitting with others who investigated the sulfammox process (Wang et al., 2022). We proposed that the addition of the Bacillus ceruse (SUD-1) strain has a clear advantage over the sulfammox process. The (SUD-1) strain is a facultative anaerobic, spore former, motile, and opportunistic bacterium capable of producing resistant endospores in the presence of oxygen. Accordingly, the (SUD-1).
assists to reach sulfammox due to work under the normal anaerobic condition with a high initial amount1.43*106 CFU/ml, which was confirmed by (Bi et al., 2020) findings. Otherwise NO2– could be converted to NO3– due to the little O2 that might be released in the SCS-2 reactor (Zhang et al., 2020). On the other hand, the bacterial strain might be considered mixotrophic due to little amount of peptone water in the isolate activation step which was added to (NH4)2SO4 as a reaction substrate. This is in agreement with the study by (Schrum et al., 2009), who proposed a mechanism referred to as the organotrophic step with NO3– appearance using the following equations. NH4++ SO42-→NO3–+HS-+H2O + H+ 5″CH O“+ 4NO3-+4H+→5CO2 + 2 N2 + 7H2O 4NH4++4SO42-+5″CH O“→5CO2 + 2 N2 + 11H2O + 4HS
Also, the nitrogen might convert by the nitrification process, the denitrification process, and the traditional anammox reaction simultaneously with the sulfammox process (Zhang et al., 2020).
By the end of the reaction, the clear yellow sludge granules were changed from the black color which refers to S0 that not easily soluble as investigated which is supported by (Lin et al., 2022). Subsequently S0 granules could be dissolving in the organic solvent following (Wu et al., 2015) method illustrated in Fig. 8.
The sulfammox bacteria can enhance nitrogen removal efficiency and sulfur – nitrogen cycle (Dominika et al., 2021; Huo et al., 2022).
3.1.3 SCS-3 reactor (anammox) performance in 0.5 g/L of (NH4)2SO4
Meanwhile, the removal amount of ammonium was increased, but the conversion efficiency of both substrates was reduced in SCS-3 reactor that working by anammox and anaerobic granular sludge (sludge) in 0.5 g/L of (NH4)2SO4 (NH4+∼100–150 and SO42-∼ 400 mg/L). According to Fig. 5, 31 % of ammonium converted without sulfate conversation might refer to ammonium consumption that led to accumulated more NO2– in the SCS-3 reactor. Thus the influence fed of NH4+ and SO42- were the same as in SCS-1 reactor. Some studies reported partial re-oxidation of sulfur or sulfide into sulfate via sulfur-utilizing denitrification/denitritation and explained how less sulfate reduction occurred than assumed (Bi et al., 2020; Zhang et al., 2019a).
5SO + 6NO–3 + 2H2O ¼ → 5SO2 –4 + 3 N2 + 4H
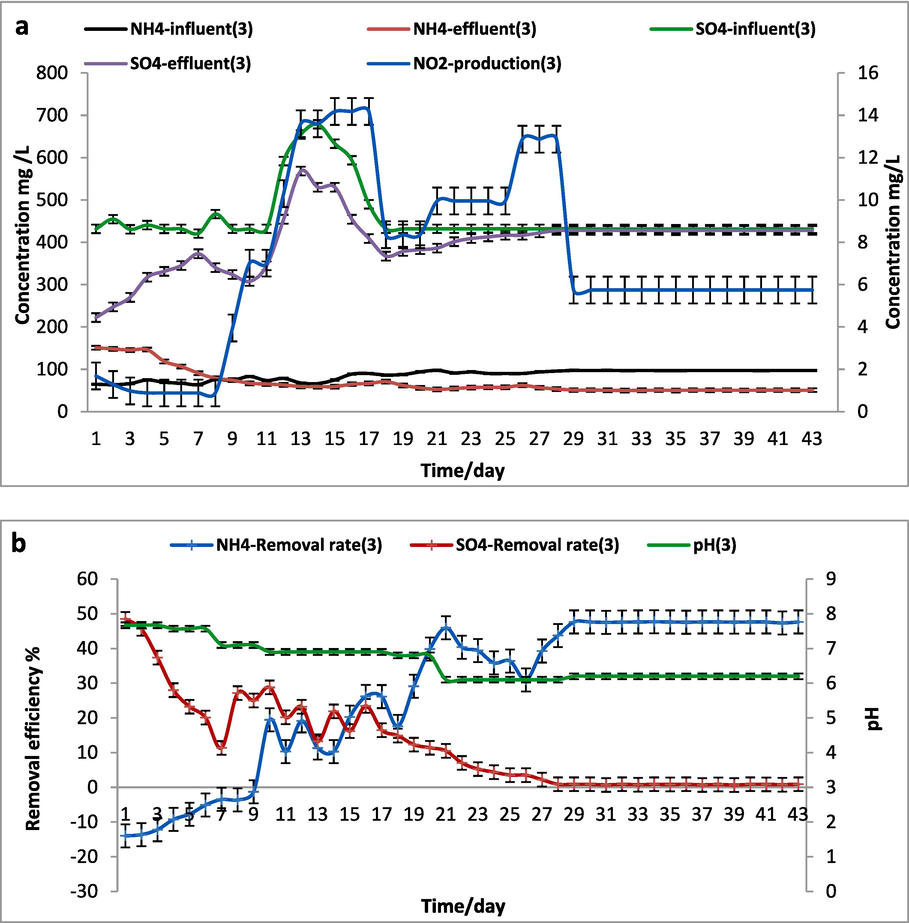
SCS-3 reactor (0.5 g\L of (NH4)2SO4) a. Influent and effluent NH4+, SO42– concentrations and NO2– production, b.The removal efficiency of NH4+ and SO42– and pH.
It could be that sulfate was less or rarely converted in experiments. Therefore, it is speculated that sulfate conversion involves heterotrophic sulfate reduction coupled with sulfide-utilizing denitrification. Also it is probably due to thiosulfate-driven denitrification and Anammox (TDDA) process that produce sulfide which restrain the Anammox bacteria activity (Zhu, 2022) as following equations: S2O3 2- + 3.1NO3 + 0·.73H2O + 0.45HCO3- + 0.09NH4+→ 0.09C5H7O2N + 3.1NO2-+1.64H++ 2SO42- NH4++1.32NO2– + 0.066HCO3- + 0.13H+ → 1.02 N2 + 0.26NO3- + 0.066CH2O0.5 N0.15 + 2·.03H2O
On another hand (Wei et al., 2022) noted that the addition of sulfate in different concentrations (100, 200, 300, and 400 mg/L) have negative effect on Anammox process. The obvious result might tick to the role of sulfammox bacteria alongside the anammox consortium promoting the sulfammox process.
The heterotrophic bacteria may be able to partially denitrify (convert nitrate back to nitrite) the nitrate produced during the synthesis of anammox biomass, and the resulting nitrite can subsequently be reduced by anammox. The interactions, however, can sometimes be detrimental. For example, competition for substrates can lead to changes in the quantity of anammox bacteria and a reduction in the process' ability to effectively remove nitrogen from the environment. Subsequently, interaction could limit the growth of anammox bacteria because of interference between some heterotrophies consume donors for anammox (Lin et al., 2022).
3.1.4 SCS-4 reactor (anammox/SUD-1 strain) performance in 0.5 g/L of (NH4)2SO4
The performance of SCSTR-4 was directed by anammox, sludge, and Bacillus cereus (SUD-1) with initial CFU/ml 1.43*106 in 0.5 g/L of (NH4)2SO4. On another hand, the SCS-4 reactor ammonium removal efficiency was constantly increased on average all over the process. The influence loading rate of NH4+ and SO4 2- were 90 ± 2 and 431 ± 0.1 mg/L/d, respectively. Subsequently, the effluent concentrations were 52.9 and 272.6 mg/L for NH4+ and SO4 2-. The conversion efficiency of both ammonium and sulfate were worked simultaneously with an average decrease of 41.2 % and 37 % in 26 days, with maximum growth of anammox at 21–23 days.
Fig. 6 was shown that the addition of the Bacillus ceruse (SUD-1) strain has a clear advantage over the sulfammox process unless is less apparent in the SCS-4 reactor than in the SCS-2 reactor due to the possibility of the high concentration toxic components release through the process in the SCS-4 reactor that might lead to decay of bacteria like free ammonia production from high NH4+ concentration (Zhu et al., 2022). The pH during the whole process in SCS-4 reactor was 7.5 till be constant at 6.5. (See Fig. 7)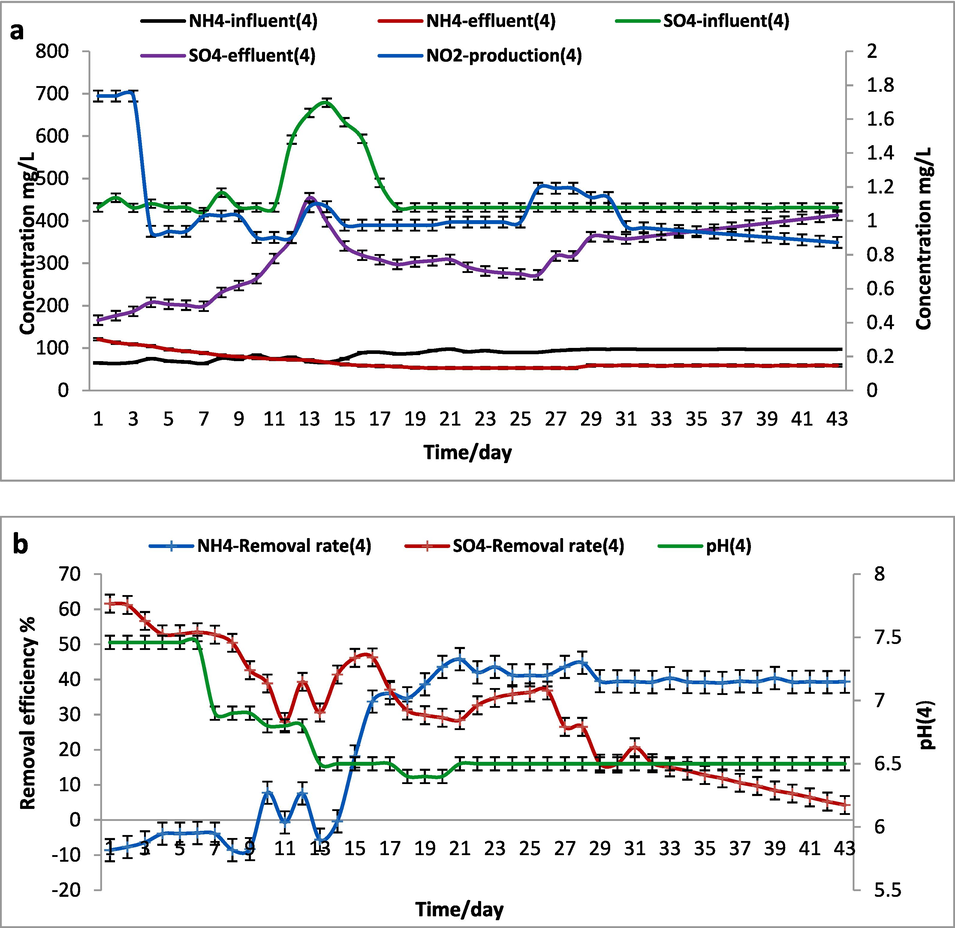
SCS-4 reactor (0.5 g.L-1 of (NH4)2SO4) a. Influent and effluent of NH4+, SO42– concentrations and NO2– production, b. The removal efficiency of NH4+ and SO42– and pH.
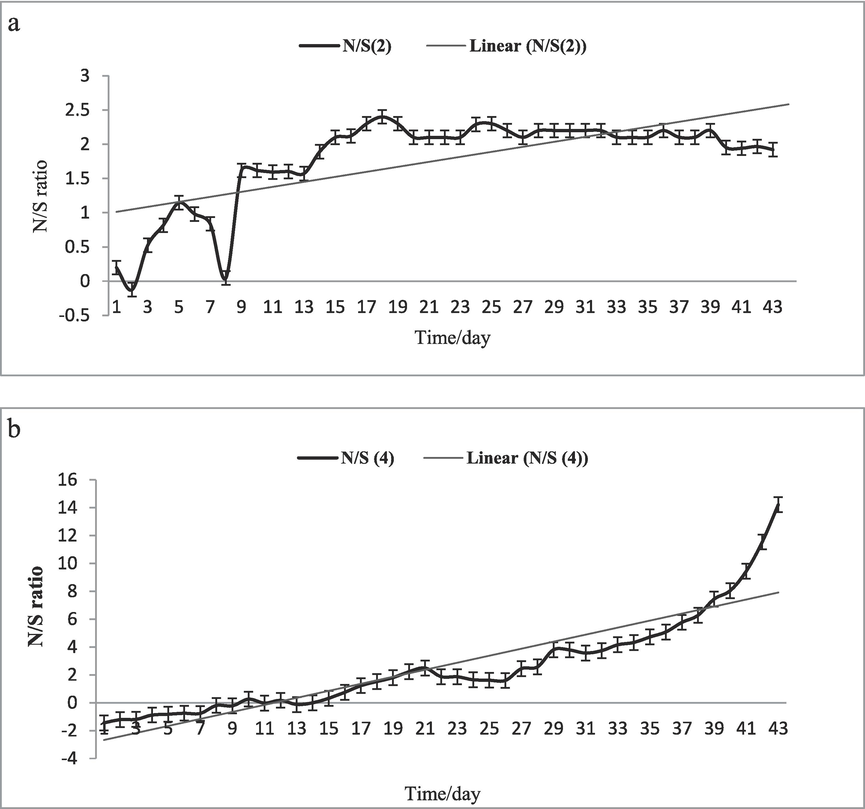
The N/S conversion ratio in a. SCS-2 reactor and b.SCS-4 reactor.
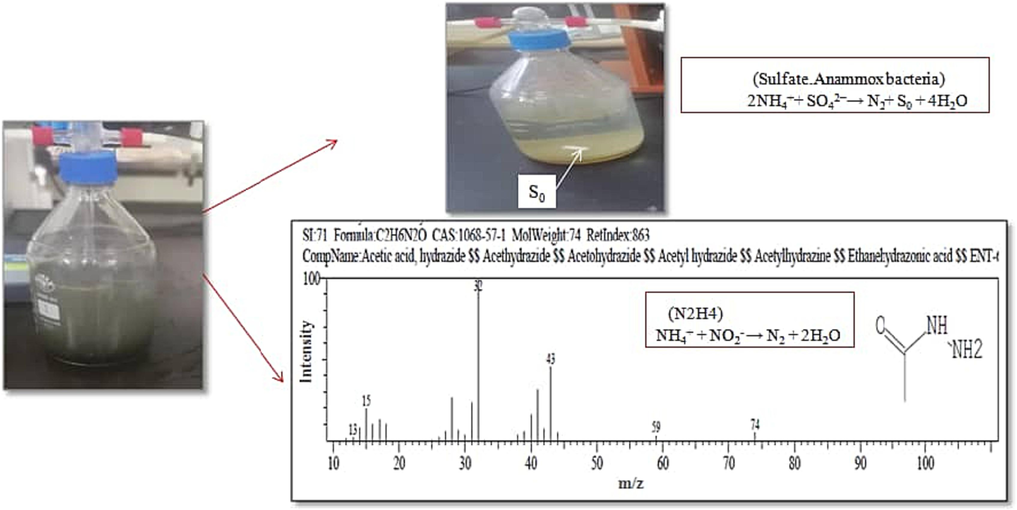
Hydrazine appearance by GC–MS and, color change of the sludge from black to yellow in SCS-2 and SCS-4 reactors.
All previous analyses and other studies refer to the complicated sulfammox process and undetectable combination pathways. The ratio of ammonium to sulfate consumption is one of the main evidence that supported the sulfammox process following equation which equals 1.8–2 dependent on the data assay that is in line with (Fdz-Polanco et al., 2001).
3.2 Aspect of sulfate –reducing anammox reactions
The hydrazine appearance in the effluent in both of SCS-2 and SCS-4 reactors indicating the anammox activity and may it diffuse through the ladderane lipids anammox bacterial cell. The gas chromatography has shown hydrazine as a Acetic acid, hydrazide shape due to reaction with (NH4)2SO4 modified supported co-media which involved NaHCO3(Guo et al., 2021). Also by the end of the reaction, the clear yellow sludge granules were changed from the black color which refers to S0 that not easily soluble as investigated which is supported by (Wang et al., 2022). Subsequently S0 granules could be dissolving in the organic solvent following (Wu et al., 2015) method illustrated in Fig. 8 as findings that fit with suggestions of (Rikmann et al., 2012) in sulfate –reducing anammox process. Taken together overall these results, it can be suggest that the below sulfammox interaction which pushed by Bacillus ceruse involving anammox reaction due to hydrazine appearance as shown Fig. 9.The majority of the research work done in the field was on development of strains, their mode of action and on explaining the science behind their potentiality.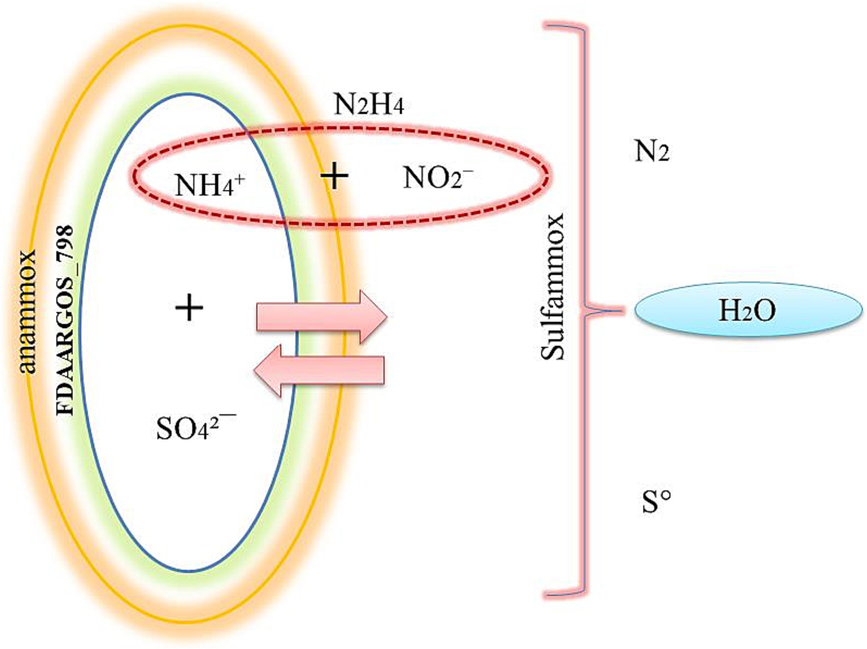
Suggested sulfammox reaction.
4 Conclusion
For the thorough treatment of wastewaters containing high levels of ammonium and sulfate, the sulfammox process was determined and confirmed using four different semi-continuous stirred reactors. The significant efficient simultaneously removal of NH4+ and SO42- were observed at SCS-2 reactor 77 % and 50 % ±1.0 and, 41.2 ± 0.1 % and 37 ± 0.5 % in 26 days at SCS-4 reactor which are treated by Bacillus ceruse. The appearance of hydrazine in SCS-2 and SCS-4 reactors is confirming the anammox reaction involve the sulfammox process. Our N/S ratio findings 1.8–2 were fitting with sulfammox obtained formula [n(NH4+-N)/n(SO4 2-)]. It seems that Bacillus ceruse enhances the sulfammox process. After these outcomes, further work needs on Bacillus ceruse immobilization and granulation to be applicable in the pilot-scale of sulfammox in wastewater treatment.
Acknowledgement
The authors are grateful to the LiaoNing Revitalization Talents Program Key R&D project of Liaoning Province of China (No. 2020JH2/10300079)/Liaoning BaiQianWan Talents Program.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Combined partial denitrification/anammox process for nitrogen removal in wastewater treatment. J. Environ. Chem. Eng.. 2023;11
- [CrossRef] [Google Scholar]
- Biological conversion pathways of sulfate reduction ammonium oxidation in anammox consortia. Front. Environ. Sci. Eng.. 2020;14:1-11.
- [CrossRef] [Google Scholar]
- Bureau., N.E., 2002. Analyzing Methods for Water and Wastewater, foutth ed. Beijing: China Environmental Science Press, 2002. 258–284 (in Chinese).
- Isolation and identification of bacteria responsible for simultaneous anaerobic ammonium and sulfate removal. Sci. China Chem.. 2010;53:645-650.
- [CrossRef] [Google Scholar]
- Chai, W.S., Bao, Y., Jin, P., Tang, G., Zhou, L., 2021. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels.
- Integration of the sulfate reduction and anammox processes for enhancing sustainable nitrogen removal in granular sludge reactors. Bioresour. Technol.. 2023;383
- [CrossRef] [Google Scholar]
- Sulfate reducing ammonium oxidation (SULFAMMOX) process under anaerobic conditions. Environ. Technol. Innov. 2021
- [CrossRef] [Google Scholar]
- New process for simultaneous removal of nitrogen and sulphur under anaerobic conditions. Water Res.. 2001;35:1111-1114.
- [CrossRef] [Google Scholar]
- Incorporation of the sulfur cycle in sustainable nitrogen removal systems - A review. J. Clean. Prod.. 2022;372
- [CrossRef] [Google Scholar]
- Hydrazine-containing heterocycle cytochalasan derivatives from hydrazinolysis of extracts of a desert soil-derived fungus Chaetomium madrasense 375. Front. Chem.. 2021;9:1-9.
- [CrossRef] [Google Scholar]
- Treatment of real industrial wastewater with high sulfate concentrations using modified Jordanian kaolin sorbent: batch and modelling studies. Heliyon. 2021;7:e08351.
- [Google Scholar]
- Modeling of sulfur-driven autotrophic denitrification coupled with Anammox process. Bioresour. Technol. 2022
- [CrossRef] [Google Scholar]
- Sulfidation forwarding high-strength Anammox process using nitrate as electron acceptor via thiosulfate-driven nitrate denitratation. Bioresour. Technol. 2022
- [CrossRef] [Google Scholar]
- Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal. Bioresour. Technol.. 2008;99:6817-6825.
- [CrossRef] [Google Scholar]
- Madani, R.M., 2021. Novel simultaneous anaerobic ammonium and sulfate removal process. https://doi.org/∗ Corresponding author. E-mail addresses: rayanmadani@yahoo.com (R.M. Madani), liangjiyan2017@126.com (J. Liang), sutcuili@163.com (L. Cui), 1601050156@qq.com (D. Zhang), timopd1@gmail.com (T.A. Otitoju), randa_9123@hotmail.com (R.H. Elsalahi), 2410658485@qq.com (X. Song). https://doi.org/10.1016/j.eti.2021.101661.
- Novel simultaneous removal of ammonium and sulfate by isolated Bacillus cereus strain from sewage treatment plant. Water Air Soil Pollut.. 2022;233:185.
- [CrossRef] [Google Scholar]
- Sulfate- reducing and nitrite-dependent anammox for ammonium removal. Commun. Agric. Appl. Biol. Sci.. 2012;77:227-230.
- [Google Scholar]
- Anaerobic ammonium oxidation linked to sulfate and ferric iron reduction fuels nitrogen loss in marine sediments. Biodegradation. 2018;29:429-442.
- [CrossRef] [Google Scholar]
- Sulfate-reducing ammonium oxidation: a thermodynamically feasible metabolic pathway in subseafloor sediment. Geology. 2009;37:939-942.
- [CrossRef] [Google Scholar]
- Challenges, solutions and prospects of mainstream anammox-based process for municipal wastewater treatment. Sci. Total Environ.. 2022;820:153351
- [CrossRef] [Google Scholar]
- Start-up of single-stage partial nitritation-anammox micro-granules system: Performance and microbial community dynamics. Environ. Res.. 2020;186:109581
- [CrossRef] [Google Scholar]
- Nitrate removal by anammox biomass with intracellular carbon source as electron donors via DNRA pathway. Environ. Res. 2021
- [CrossRef] [Google Scholar]
- Organics alleviate the inhibition of sulfate on ANAMMOX sludge. J. Environ. Sci. Heal. - Part A Toxic/hazardous Subst. Environ. Eng. 2022
- [CrossRef] [Google Scholar]
- A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour. Technol.. 2015;175:594-601.
- [CrossRef] [Google Scholar]
- Effect of nitrite and nitrate on sulfate reducing ammonium oxidation. Water Sci. Technol.. 2019;80
- [CrossRef] [Google Scholar]
- Study of sulfate-reducing ammonium oxidation process and its microbial community composition. Water Sci. Technol.. 2019;79:137-144.
- [CrossRef] [Google Scholar]
- Effect of nitrite and nitrate on sulfate reducing ammonium oxidation. Water Sci. Technol.. 2020;80:634-643.
- [CrossRef] [Google Scholar]
- Zhang, Z., 2022. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. https://doi.org/10.1016/j.jclepro.2022.134109.
- Sulfammox forwarding thiosulfate-driven denitrification and anammox process for nitrogen removal. Environ. Res.. 2022;214:113904
- [CrossRef] [Google Scholar]
- Zhu, Z., 2022. Sulfammox forwarding thiosulfate-driven denitrification and anammox process for nitrogen removal Zijian. https://doi.org/10.1016/j.envres.2022.113904.
Further reading
- Li, J., 2022. Synergism of hydrolytic acidification and sulfate reducing bacteria for acid production and desulfurization in the anaerobic baffled reactor: High sulfate sewage wastewater treatment. https://doi.org/10.1016/j.cej.2022.136611.
- Biological remediation of acid mine drainage: review of past trends and current outlook. Environ. Sci. Ecotechnology. 2020;2:100024
- [CrossRef] [Google Scholar]
- Comparison of sulfate-reducing and conventional Anammox upflow anaerobic sludge blanket reactors. J. Biosci. Bioeng.. 2014;118:426-433.
- [CrossRef] [Google Scholar]
- Sulfate-reducing anammox for sulfate and nitrogen containing wastewaters. Desalin. Water Treat.. 2016;57:3132-3141.
- [CrossRef] [Google Scholar]
- Anaerobic ammonia removal in presence of organic matter: a novel route. J. Hazard. Mater. 2007
- [CrossRef] [Google Scholar]
- Development of a novel process for anoxic ammonia removal with sulphidogenesis. Process Biochem.. 2008;43:984-991.
- [CrossRef] [Google Scholar]
- Application of the anammox-based process for nitrogen removal from anaerobic digestion effluent: a review of treatment performance, biochemical reactions, and impact factors. J. Water Process Eng.. 2020;38:101595
- [CrossRef] [Google Scholar]
- The development of a reverse anammox sequencing partial nitrification process for simultaneous nitrogen and COD removal from wastewater. Bioresour. Technol.. 2014;155:427-431.
- [CrossRef] [Google Scholar]
- Performance of sulfate-dependent anaerobic ammonium oxidation. Sci. China Ser. B Chem. 2009
- [CrossRef] [Google Scholar]
- Simultaneous removal of ammonium-nitrogen and sulphate from wastewaters with an anaerobic attached-growth bioreactor. Water Sci. Technol.. 2006;54:27-35.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102947.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







