Translate this page into:
Simultaneous HPLC quantitative analysis of mangostin derivatives in Tetragonula pagdeni propolis extracts
⁎Corresponding author. Tel.: +66 3810 2610x2610; fax: +66 3810 2610x109. boonyadist@buu.ac.th (Boonyadist Vongsak)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Propolis has been used as indigenous medicine for curing numerous maladies. The one that is of ethnopharmacological use is stingless bee propolis from Tetragonula pagdeni. A simultaneous high-performance liquid chromatography (HPLC) investigation was developed and validated to determine the contents of bioactive compounds: 3-isomangostin, gamma-mangostin, beta-mangostin, and alpha-mangostin. HPLC analysis was effectively performed using a Hypersil BDS C18 column, with the gradient elution of methanol–0.2% formic acid and a flow rate of 1 ml/min, at 25 °C and detected at 245 nm. Parameters for the validation included accuracy, precision, linearity, and limits of quantitation and detection. The developed HPLC technique was precise, with lower than 2% relative standard deviation. The recovery values of 3-isomangostin, gamma-mangostin, beta-mangostin, and alpha-mangostin in the extracts were 99.98%, 99.97%, 98.98% and 99.19%, respectively. The average contents of these mixtures in the propolis extracts collected from different seasons were 0.127%, 1.008%, 0.323% and 2.703% (w/w), respectively. The developed HPLC technique was suitable and practical for the simultaneous analysis of these mangostin derivatives in T. pagdeni propolis and would be a valuable guidance for the standardization of its pharmaceutical products.
Keywords
HPLC
Mangostin
Propolis
Quantitative analysis
Stingless bee
Tetragonula pagdeni
1 Introduction
Nowadays, propolis has been wildly utilized as medicine and dietary supplement because of its broad biological activities, including antimicrobial, anti-inflammatory (Paulino et al., 2006), immunomodulatory (Ma et al., 2011), and antioxidant (Potkonjak et al., 2012) activities. Previous phytochemical studies demonstrated that propolis contains predominantly complex phenolic compounds, such as caffeic acid phenethyl ester and prenylflavanone group which are responsible for its activities (Athikomkulchai et al., 2013).
To standardize propolis extracts of European honeybee (Apis mellifera), analytical methods, such as capillary electrophoresis, near infrared spectroscopy and high performance liquid chromatography coupled with different detectors, have been developed for the quality assessment (Adelmann et al., 2007; Cai et al., 2012; Sun et al., 2014). However, chemical constituents and its related bioactivities of each type of propolis depend on the bee species and their different preference for resin and food plants, geographical regions, variation in the plant resin compositions and accessible plant species (Ayaad et al., 2012; Silici and Kutluca, 2005).
The stingless bee (Tetragonula pagdeni Schwarz, Apoidea) is another bee species that is widely distributed and commercially cultivated in artificial hives in fruit gardens. The propolis of T. pagdeni has also been used as indigenous medicine and marketed in several preparations in Thailand (Thummajitsakul et al., 2010). Several mangostin derivatives have been reported to possess biological activities such as antioxidant, anti-inflammatory and anti-cancer activities (Aisha et al., 2012a,b; Jindarat, 2014) and could be used as active chemical markers for the quality assurance. Therefore, the HPLC method for the quantitative determination of four active components: 3-isomangostin, gamma-mangostin, beta-mangostin, and alpha-mangostin in the propolis extract of T. pagdeni from mangosteen orchard was developed and validated in this research.
2 Materials and methods
2.1 Chemical and reagents
HPLC grade methanol was obtained from Labscan (Thailand). Deionized water was purified by Ultra Clear™ system (Siemen Water Technologies Corp.). Formic acid was purchased from Labscan (Thailand) while all reagents were of analytical grade. 3-Isomangostin (1), gamma-mangostin (2), beta-mangostin (3), and alpha-mangostin (4), purity more than 98%, were purchased from Chengdu Biopurify Phytochemicals Ltd., Sichuan, China.
2.2 Propolis collection and extraction
Propolis of T. pagdeni was collected from an apiary in the mangosteen garden in the summer (April), rainy season (August) and winter (December) from Makham district, Chanthaburi province, eastern Thailand, and was kept in cold room at 4 °C until use.
Propolis (10 g) was cleaned and cut into small pieces and was then extracted with ethyl acetate (200 ml) at 40 °C by shaking at 100 rpm for 30 min. The suspension was centrifuged at 5000 rpm for 5 min at 20 °C. The supernatant was stored while the residual was re-extracted using the same procedure again. The supernatants were pooled together and evaporated in a rotary evaporator. The extracts were weighed and kept in the dark at 0 °C.
2.3 Preparation of sample solutions
Each sample was prepared by accurately weighing 30 mg of T. pagdeni extract and dissolving in methanol (5 ml). To enable complete dissolution, each sample was sonicated for 60 min. Each extraction was done in triplicate. Prior to the injection, each solution was filtered through a 0.22 μm nylon membrane filter and then analyzed in triplicate.
2.4 HPLC apparatus and chromatographic conditions
HPLC was achieved on an Agilent 1260 Series (Agilent Technologies) equipped with a 1260 Quat pump VL quaternary pump, 1260 ALS autosampler, 1260 TCC column thermostat, and 1260 DAD VL diode array detector. The separation was done on a Hypersil BDS C18 column (4.6 × 100 mm i.d., 3.5 μm) with a C18 guard column. The mobile phases were (A) 0.2% formic acid in water and (B) methanol using gradient elution: 75% B in A to 90% B in A for 10 min; 90% B in A to 100% B for 5 min; 100% B for 10 min. This column was re-equilibrated with 75% B in A for 10 min prior to each analysis and the flow rate was set at 1.0 ml/min with controlled temperature at 25 °C. DAD detector was set at the wavelength of 245 nm and injection volume was 5 μl for every sample and standard.
Stock solutions of standard compounds (1)–(4) were prepared by accurately weighing and dissolving the compounds in methanol to obtain the final concentration of 1000 μg/ml. Working solutions of standard compounds were obtained by diluting the stock standard solutions with methanol to achieve the desired concentrations.
2.5 Method validation
The analytical method was validated according to the International Conference on Harmonization guideline (ICH, 1996/2005). The method validation parameters were accuracy, precision, linearity, limit of quantitation (LOQ) and limit of detection (LOD).
2.5.1 Accuracy
Accuracy was evaluated across the specified range of the analytical procedure by recovery study. Pre-analyzed standard solutions were used for standard addition. Three different concentrations of standard mixtures were spiked to the sample extract. Each spiked samples were prepared in triplicate. The recovery was calculated as follows: recovery (%) = 100 × (amount found − original amount)/amount spiked.
2.5.2 Precision
Investigation of method precision was done by analyzing intra- and inter-day multiple injection of 50 μg/ml standard solutions. The intra-day precision was evaluated from seven consecutive injections within 1 day, while the inter-day precision was studied by analyzing for three different days by the proposed method. Percent relative standard deviation (%RSD) was used to expressed the method precision.
2.5.3 Linearity
Evaluation of linear relationship was studied across the range of 0.78–100 μg/ml for compounds (1)–(3) and 1.5–1000 μg/ml for compound (4). Seven known concentrations of each analyte were injected in triplicate. The calibration curves were constructed from the peak area versus the amount of the standards by least square regression.
2.5.4 Limit of quantitation (LOQ) and limit of detection (LOD)
Determination of signal-to-noise ratio was performed by comparing signals from samples with known low concentrations of analyte with those of blank samples and establishing the minimum concentration at which the analyte can be reliably detected under the proposed chromatographic condition. A concentration of analyte which establishes signal-to-noise ratio of 3:1 was considered for LOD and 10:1 for LOQ.
3 Results and discussion
HPLC technique was developed for the simultaneous analysis of the major bioactive components: 3-isomangostin (1), gamma-mangostin (2), beta-mangostin (3), and alpha-mangostin (4) in T. pagdeni propolis extract from mangosteen orchard. The analysis method has been optimized from previous reports (Walker, 2007; Yodhnu et al., 2009) with slight modifications. From several trials, the mobile phase gradient system of 0.2% formic acid in methanol was the optimal condition. It provided symmetrical peaks and has the most efficient separation and speed. The maximum absorbance of alpha-mangostin (4) 245 nm was used for wavelength detection. The chromatograms of propolis extract and authentic compounds are shown in Fig. 1.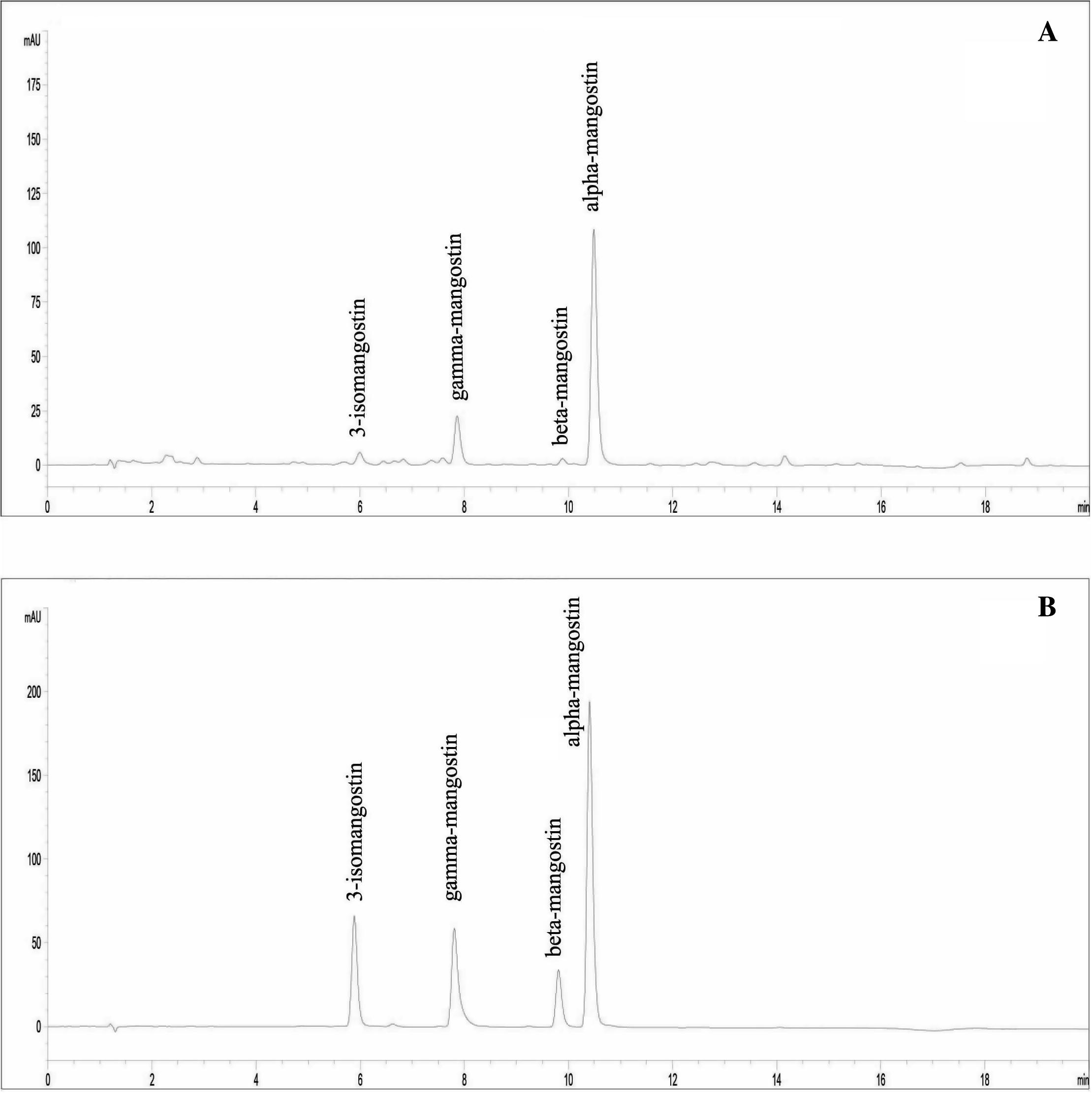
HPLC chromatogram of (A) propolis extracts of T. pagdeni from mangosteen plantation and (B) authentic compounds: 3-isomangostin, gamma-mangostin, beta-mangostin, and alpha-mangostin.
In order to ensure that the method is suitable for its intended use, method validation has been performed according to the ICH guideline (ICH, 1996/2005). The method validation parameters were linearity, precision, accuracy, LOD and LOQ. The calibration curves were constructed from the peak area versus the concentration of the standards and showed that the developed method was linear across the range of 0.82–105, 0.74–95, 0.80–102, and 1.53–986 μg/ml, for compounds (1), (2), (3), and (4), respectively, with good correlation coefficient (r2 ⩾ 0.9995) (Table 1). Method precision was studied using the 50 μg/ml standard solutions. Percent relative standard deviation (%RSD) values lower than 2% (Table 2) showed the acceptable precision of the method. Selectivity of the method was assessed by peak purity using UV spectrum obtained from diode array detector. The accuracy of the method, represented by recovery studies ranged between 99.40% and 100.32% (average 99.98%), 99.04% and 100.56% (average 99.73%), 97.93% and 100.04% (average 98.98%), and 98.66% and 99.84% (average 99.19%) for compounds (1)–(4), respectively (Table 3). The LOQ and LOD for compounds (1)–(4), were found to be 0.3 and 0.09, 0.4 and 0.12, 0.3 and 0.09, and 0.2 and 0.067 μg/ml, respectively (Table 1), indicating the high sensitivity of the method. Therefore, the developed method could be used to ensure and assess the quality of T. pagdeni propolis extract.
Parameters
Results
(1)
(2)
(3)
(4)
Regression equationa
Y = 18.523X + 6.7416
Y = 23.552X + 4.4897
Y = 10.559 + 0.9815
Y = 32.981X − 13.737
Correlation coefficient (r2)
0.9999
0.9999
0.9999
0.9995
Linear range (μg/ml)
0.82–105
0.74–95
0.80–102
1.53–986
LOQ (μg/ml)
0.3
0.4
0.3
0.2
LOD (μg/ml)
0.09
0.12
0.09
0.067
Compounds
Intra-day
Inter-day
Day 1
Day 2
Day 3
(1)
0.90
1.97
0.75
0.67
(2)
0.91
1.82
0.81
0.56
(3)
0.95
1.71
0.79
0.49
(4)
0.82
1.59
0.74
0.46
Serial No.
Compound
Theoretical (μg/ml)
Found (μg/ml)
Recovery (%)
1
(1)
7.8058
7.8229 ± 0.0629
100.22 ± 0.81
(2)
26.5313
26.6809 ± 0.4911
100.56 ± 1.85
(3)
11.6767
11.6811 ± 0.2267
100.04 ± 1.94
(4)
88.8509
88.0272 ± 1.0331
99.07 ± 1.16
2
(1)
10.6609
10.5966 ± 0.0170
99.40 ± 0.16
(2)
35.5928
35.2510 + 0.1712
99.04 ± 0.48
(3)
16.1011
15.9345 ± 0.0247
98.97 ± 0.15
(4)
121.1392
119.5184 ± 0.1209
98.66 ± 0.10
3
(1)
13.4853
13.5287 ± 0.0649
100.32 ± 0.48
(2)
44.7131
44.5237 ± 0.4016
99.58 ± 0.90
(3)
20.2571
19.8374 ± 0.1238
97.93 ± 0.61
(4)
182.8559
152.7446 ± 0.7051
99.84 ± 0.46
Average
(1)
99.98
(2)
99.73
(3)
98.98
(4)
99.19
The proposed HPLC technique was applied for quantitative analysis of the contents of the compounds (1)–(4) in T. pagdeni propolis extract collected from different seasons. The contents of these compounds are shown in Table 4. The amount of the compounds (1)–(4) in the propolis extracts ranged from 0.0870 to 0.1709, 0.6010–1.4604, 0.2208–0.4631 and 1.9103–3.1496, respectively. Additionally, in the propolis, the concentrations of these compounds (1)–(4) were found to be in range of 0.0206–0.0796%, 0.2798–0.3455%, 0.0852–0.1095% and 0.7451–0.9155% w/w, respectively.
Season (month)
Contents in extracts, and in propolis (% w/w)
(1)
(2)
(3)
(4)
Summer (April)
0.1709 ± 0.0073,
0.6010 ± 0.0137,
0.2208 ± 0.0065,
1.9103 ± 0.0161,
0.0796 ± 0.0034
0.2798 ± 0.0064
0.1028 ± 0.0030
0.8893 ± 0.0075
Rain (August)
0.0870 ± 0.0024,
1.4604 ± 0.0351,
0.4631 ± 0.0123,
3.1496 ± 0.0841,
0.0206 ± 0.0006
0.3455 ± 0.0083
0.1095 ± 0.0029
0.7451 ± 0.0199
Winter (December)
0.1236 ± 0.0019,
0.9640 ± 0.0149,
0.2836 ± 0.0067,
3.0477 ± 0.0444,
0.0371 ± 0.0006
0.2896 ± 0.0045
0.0852 ± 0.0020
0.9155 ± 0.0133
Average
0.1272 ± 0.0421,
1.0085 ± 0.4314,
0.3225 ± 0.1257,
2.7025 ± 0.6880,
0.0458 ± 0.0015
0.3049 ± 0.0064
0.0992 ± 0.0026
0.8500 ± 0.0136
4 Conclusions
According to the validated parameters of accuracy, precision, linearity, LOQ and LOD, the proposed technique was successful in simultaneously and quantitatively analyzing four major mangostin derivatives (3-isomangostin, gamma-mangostin, beta-mangostin, and alpha-mangostin) in propolis extracts of T. pagdeni from mangosteen plantation. This method would be beneficial in the quality control and the standardization of propolis extracts and preparations from mangosteen orchard.
Acknowledgements
The authors would like to thank Faculty of Pharmaceutical Sciences, Burapha University for financial support and Mr. Wisit Tanooard, Ms. Jirattikorn Laiwattanaphaisal, Ms. Nipatha Issaro as well as Thai local bee keeper of Kon Chan Channarong (stingless bee) in Chantaburi province, Thailand for facilities and propolis collection. We also thank Drug Discovery and Development Center, Thammasat University for laboratory facilities and Mr. Panupon Khumsupan for his kind proofreading of the manuscript.
References
- Exotic flora dependence of an unusual Brazilian propolis: the pinocembrin biomarker by capillary techniques. J. Pharm. Biomed. Anal.. 2007;43:174-178.
- [Google Scholar]
- In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthone extract. BMC Complement Altern. Med.. 2012;12:104.
- [Google Scholar]
- Alpha-mangostin enhances betulinic acid cytotoxicity and inhibits cisplatin cytotoxicity on HCT 116 colorectal carcinoma cells. Molecules. 2012;17:2939-2954.
- [Google Scholar]
- Isolation of antimicrobial peptides from Apis florea and Apis carnica in Saudi Arabia and investigation of the antimicrobial properties of natural honey samples. J. King Saud. Univ. Sci.. 2012;24:193-200.
- [Google Scholar]
- Rapid quantification of flavonoids in propolis and previous study for classification of propolis from different origins by using near infrared spectroscopy. Anal. Methods. 2012;4:2388-2395.
- [Google Scholar]
- ICH, 1996/2005. International Conference on Harmonization, Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology. ICH, Geneva, Switzerland.
- Xanthones from mangosteen (Garcinia mangostana): multi-targeting pharmacological properties. J. Med. Assoc. Thai.. 2014;97:S196-S201.
- [Google Scholar]
- The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell. Immunol.. 2011;270:13-18.
- [Google Scholar]
- Evaluation of the analgesic and anti-inflammatory effects of a Brazilian green propolis. Planta Med.. 2006;72:899-906.
- [Google Scholar]
- Antioxidant activity of propolis extracts from Serbia: a polarographic approach. Food Chem. Toxicol.. 2012;50:3614-3618.
- [Google Scholar]
- Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol.. 2005;99:69-73.
- [Google Scholar]
- Simultaneous determination of eight flavonoids in propolis using chemometrics-assisted high performance liquid chromatography–diode array detection. J. Chromatogr. B. 2014;962:59-67.
- [Google Scholar]
- Development of a species-diagnostic marker for identification of the stingless bee Trigona pagdeni in Thailand. Biochem. Genet.. 2010;48:181-192.
- [Google Scholar]
- HPLC analysis of selected xanthones in mangosteen fruit. J. Sep. Sci.. 2007;30:1229-1234.
- [Google Scholar]
- Validation of LC for the determination of α-mangostin in mangosteen peel extract: a tool for quality assessment of Garcinia mangostana L. J. Chromatogr. Sci.. 2009;47:185-189.
- [Google Scholar]







