Translate this page into:
Simulation and modeling of physiological processes of vital organs in organ-on-a-chip biosystem
⁎Corresponding authors. ftekhari@ymail.com (Aziz Eftekhari), armankhan0301@gmail.com (Ameer Khusro), umar.sahibzada@gmail.com (Muhammad Umar Khayam Sahibzada), mariopharma92@gmail.com (Márió Gajdács) gajdacs.mario@stoma.szote.u-szeged.hu (Márió Gajdács)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The limited adequacy of animal cell cultures and models to mimic the complexity of human bodies in laboratory conditions has emphasized researchers to find its quintessential bioelectronic alternative with improved competence. In this regard, tissue engineering has emerged as one of the most precise biomaterial technologies in terms of creating new tissues to model vital organs. An organ-on-a-chip biosystem has shown a plethora of applications in tissue engineering and drug delivery. Organ-on-a-chip is a microfluidic device that provides a completely controlled microenvironment, similar to the natural tissues for the cultured cells of an organ, by amalgamating cell biology and biomaterial science. The device contains several microchambers and microchannels embedded in a layer of a biocompatible polymer, such as polydimethylsiloxane. Microchambers house the cells, while microchannels provide nutrients and growth factors. Over the past few years, organ-on-a-chip technology has displayed ample applications in the field of biomedicine, not only by simulating the normal functions of disparate organs, but also by understanding the inter-relation between diversified systems. In this review, we have spotlighted recent advancements and applications of organ-on-a-chip biosystems to construct physiological models for the heart, lung, kidney, liver, and brain. Part of this review is also concentrated on abridging the desperate essentiality as well as future perspectives of organ-on-a-chip technology in biomedicine, disease modeling, and drug development process.
Keywords
Biomaterials
Microfluidics
Modeling
Organ-on-a-chip
Tissue engineering
1 Introduction
At present, the conventional methods of drug development by pharmaceutical industries are facing unprecedented challenges not only due to the escalating costs but also irrefutable implementation of extensive pre-clinical trials (Eftekhari et al., 2019). The use of animal cell culture techniques has often become unsuccessful to mimic the complexity of human bodies and is unable to model situations where organ-organ and tissue-tissue communications are significant (Mittal et al., 2019). Despite certain efficacy in animal models, the elucidation of the precise mechanism of action of drugs is often difficult, which leads to misleading observations and data. In the past, traditional two-dimensional (2D) cell cultures system had established a pivotal platform for extensive life science studies and showed colossal contributions in the medical system by reducing the utilization of animal models. Unfortunately, these techniques failed to simulate the physiological expressions of living cells and did not facilitate studying the microenvironment of tissues or the inter-relationships between disparate tissues and organs of humans (Rajan et al., 2020). Considering the certain inefficiency of animal model’s researches, it has emphasized the critical necessity for novel testing strategies and intermediate human in vitro models in the early phase of the drug development process, for predicting a molecule’s or a test drug’s effectiveness and safety in humans that could diminish the adverse impacts observed in different pre-clinical trial phases.
Over the past few years, organ-on-a-chip, lab-on-a-chip, or tissue-on-a-chip systems have emerged as one of the most promising tissue engineering technologies, which include findings from material science and biological systems (Jodat et al., 2019). This biomimetic technology is considered as an auspicious alternative to the conventional approaches of the drug development process, because it aims to mimic the functional attributes as well as physiological conditions of tissues and organs by regulating disparate parameters (Wu et al., 2020). The system is prepared on a microfluidics chip, which constitutes perfused chambers filled with living cells, simulating the physiology of tissues and organs (Bhatia and Ingber, 2014). The device consists of several microchambers and microchannels embedded in a layer of polymer, most commonly polydimethylsiloxane (PDMS) polymer. This polymer is mainly used due to its unique transparency and biocompatibility properties. Microchambers house the cells, while microchannels provide nutrients and growth factors. To improve the cell culture medium and cell adhesion, the chip surface is coated with the materials derived from the extracellular matrix (Wu et al., 2020). For a detailed study, various types of biosensors, digital sensors, imaging devices, and microelectrodes are installed on the chip. Usually, nutrient transfer and waste disposal are done by creating a continuous flow in the microchannels. At the same time, the flow-pressure values and dynamic changes of the cells are measured and controlled using different sensors (Shay, 2017).
This emerging technology not only provides paramount information about the morphology and differentiation of cells but also induces gene expression and cellular functions (Mittal et al., 2019). Most importantly, the organ-on-a-chip system exhibits unique potentiality to reduce the utilization of animal models, and even human beings in the development process of novel drug candidates (Zhang et al., 2018). The system offers pronounced therapeutics for disparate types of infectious, cardiovascular, neurological, and metabolic diseases (Shay, 2017). Recently, during the coronavirus disease-19 (COVID-19) pandemic, this system was implied for obtaining fast and reliable outcomes towards the treatment process (Monteil et al., 2020).
Nanoparticle-based drug delivery systems have offered incredible potential in the treatment of different diseases including cancer (Maleki Dizaj et al., 2019). However, the clinical application of nanomedicine is limited due to the problems in controlled drug release in relation to high batch-to-batch differences, inadequate synthesis rate of the conventional preparation techniques and the lack of delivery monitoring technologies for nanomedicines in correlation with animal tests (Ejeta, 2021). The microfluidic technology might bring new hopes in the elimination of these challenges, as they can generate nanoparticles with high controlling capability, reproducibility and efficacy as well as creating 3-D environments to simulate cellular processes (Ejeta, 2021).
Considering the cutting-edge researches in microfabrication technology and the desperate necessity of microfluidics techniques for human health, this review spotlights the recent advancements of the organ-on-a-chip system and its role in precision medicine as a future rational drug development approach.
2 Design concept and components of the organ-on-a-chip system
Controlling the external and internal cellular environment is imperative for the dynamic activity of the cell culture system. Different parameters and physiological environments are often controlled by the co-action of micromachinery and cellular biology (Bhatia and Ingber, 2014). The fluid shear stress, concentration gradient, mechanical stress, and cells patterning are primarily considered for the physiological functioning of the chip.
The dynamic nature of culture cells is enabled by microfluidics through micropump perfusion. It is known to administer essential nutrients and excrete waste materials. Fluid shear forces induce the polarity of organs. The organ-on-a-chip system applies essential pressure on endothelial cell functionality by stimulating signaling molecules. In a like manner, the fluid added into the organ-on-a-chip system allows biological activities at the organ level (Haddrick and Simpson, 2019). The fluid causes the formation of stable gradients of molecules at the microscale level. The concentration gradients induce biochemical signals, causing the processing of different biological phenomena. Complex physiological process is stimulated by microfluidics in the human body. It alters the flow velocity and achieves biochemical concentration gradients (Yang et al., 2014). Pressure in the blood vessels of the human body is the major indication of stress levels in the vital organs and tissue. This system uses elastic porous membranes for creating dynamic mechanical stress, which is considered an important factor of differentiations during the physiological process (Yang et al., 2017). Ordered arrangements of cells are essential for forming a functional body. Cell patterning on the chip is carried out by surface modification, template, and three-dimensional (3D) printing. Among them, the 3D printing technique shows multi-scale cells patterning by forming hydrogel scaffolds with the complex channel. Most importantly, 3D printing provides versatility in the cellular pattern, which is critical for in vitro reconstruction of the cellular microenvironment (Wu et al., 2020).
Microfluidics, living cell tissues, simulation, and senses are the most important components of an organ-on-a-chip system. Microfluidics helps deliver targeted cells towards a pre-designed location and constitutes fluid input and waste liquid release. The living cells tissues component aligns a specific cell type in a 2D or 3D system. Hydrogels are generally used to create 3D systems. Despite more accuracy of 3D tissue structures, living cells tissues are extensively cultivated in the 2D system due to the cost limitations and vasculature formation. The physical or chemical signal is essential for simulating the microenvironment for some tissues. Various signal stimuli can be observed from drug-screening processes. The detection and compilation of data are done by the sensing component (Wu et al., 2020).
Mitochondria as distinct organelles in mammalians may form organized tubular network that are continually changing under specific conditions including mitophagy or biogenesis, separation (fission) and fusion (Ahmadian et al., 2017; Fard et al., 2016). Previous studies showed that organ-on-a-chip devices can monitor the mitochondrial dysfunction dynamics and therefore simulates real-time organ damage (Clapp et al., 2021). For example, the levels of lactose and glucose in the liver chip could be measured via controlling microfluidic alteration (Cong et al., 2016).
The cell viability and metabolic activity are markers of mitochondrial function in relation to the oxidative phosphorylation process (Eftekhari et al., 2016). Moreover, screening of the toxicity and efficacy of drugs/xenobitoics is possible using liver chips (Cong et al., 2016). Microfluidic bead electrochemical immunosensors have been used to detect cell-released biomarkers along with liver chips. The applied system exhibited high accuracy and efficiency (Cong et al., 2016).
3 Organ-on-a-chip devices
Studying different biological barriers is a pivotal step to understanding the pharmacology of drugs. Researchers have developed 3D compartmentalization with membrane-based multi-layer compartments to mimic varied biological barriers viz. blood–brain barrier, kidney transport barrier, and the lung's alveolar-capillary interface. Recent discoveries in membrane and muscular thin films for recapitulating the physicochemical interface of different organs are discussed below:
3.1 Kidney-on-a-chip (KOC)
The kidney is an active metabolic organ and is involved in gluconeogenesis, erythropoietin, and renin synthesis. It is also the site of clearance for the drugs. The kidneys control body fluid balance and excrete waste products through three mechanisms: filtration, reabsorption, and secretion. The functional unit of the kidney is the ‘nephron’ which consists of the renal corpuscle (filters blood plasma), and the renal tubules that are involved in the reabsorption and secretion of substances. The kidney performs its hemostatic mechanism through the activity of different tubular parts of the nephrons (Feher, 2017). However, renal tubules are complementary in reabsorption and secretion of substances, but the role of proximal tubules is more important than other tubules. Proximal tubules reabsorb approximately three-fifth of the filtered water, salt, glucose, amino acids, and low molecular weight proteins. These tubules play a fundamental role in the acid-base balance process by reabsorbing four-fifth of the filtered bicarbonate. This reabsorption occurs due to the presence of pumps and channels in the basolateral (facing the interstitial space) and apical (facing the tubular lumen) membrane (Zhuo and Li, 2013). The distal tubule helps homeostasis of sodium, potassium, and divalent cations with 5–10% sodium and chloride reabsorption. It also helps in the secretion of potassium cations and maintains the systemic balance of magnesium and calcium (McCormick and Ellison, 2015).
Nephrons remove waste products from the body by filtering 180 L of blood plasma. This ultrafiltration and high fluid flow in the tubules exposes the apical surface of the nephron cells to the shear stress of the fluid (Raghavan et al., 2014). Fluid shear stress causes the polarity of nephron cells through the expression of different genes and thus the reorganization of the cytoskeleton (Jang et al., 2011). In humans, fluid shear stress is estimated from 0.7 to 1.2 dyne/cm2. The apical surface of the proximal tubule cells is continuously exposed to fluid shear stress and osmotic pressure. These cells detect the high flow of tubular fluid by apical surface sensors, send messages into the cell to reorganize the connections and cytoskeleton, and increase apical endocytosis (Raghavan and Weisz, 2015).
The KOC model represents the use of cell culture on a chip that is similar to the microenvironment of the kidney. The KOC model allows the culturing of renal cell types and provides cell–cell functional communication (such as the interaction between endothelial cells and podocytes in the renal corpuscle), expression of different membrane carriers, and normal endocrine and metabolic functions (Wilmer et al., 2016). KOC models may be used to screen and test drug toxicity, study glomerular filtration processes, analyze different parts of the nephron, and determine pharmacokinetics as well as the effective dose of the drug. Because the model is very similar to the human condition, it predicts more accurately the physiological processes and drug-induced renal toxicities and reduces the number of animals used in the clinical trials. Various models of different parts of the nephron (glomerulus, proximal, and distal tubule) have been designed, but a complete model of nephron-on-a-chip that integrates these components has not yet been developed. Currently, most KOC models based on microfluidic systems rely on three main structures: (i) single-layer systems, (ii) multilayer systems consisting of two chambers separated by a permeable membrane, (iii) systems made up of a single straight pipe structure, and (iv) systems consisting of a tubular structure with a vascular network. Among these systems, cell culture is the main focus of the design. Cell culture is 2D in monolayer systems and 3D in others. The design of these systems enables the researchers to study and create several functions from various parts of the nephron.
The development of KOC systems that mimic different physiological compositions and the environmental situation has the possibility of its colossal applications in the medical field. To simulate and reconstruct different environmental conditions, KOC systems must simultaneously consider five attributes of renal physiology: (i) tubular fluid flow mode, (ii) cell-matrix interaction with tubular microenvironments (such as extracellular matrix and growth factors), (iii) biophysical properties associated with tubular microenvironments, such as mechanical stiffness and porosity, (iv) multidimensional geometric properties of individual renal tubules (such as size and local and general curvatures), and (v) cell–cell and tissue-tissue relationships due to the structural organization of adjacent separate renal tubules (Sochol et al., 2016).
3.1.1 Glomerulus-on-a-chip (GOC)
The glomerulus is the filtering part of the nephron, which consists of a capillary network, basement membrane, and podocytes. By selectively filtering the blood, the glomerulus produces a filtrated liquid and eventually urine (Greka and Mundel, 2012). The design of an in vitro model that simulates the function of the glomerular filtration barrier is of immense interest to investigators. The filtration barrier has selective permeability and is always exposed to shear stress due to the liquid flow.
A GOC model was designed to study hypertensive nephropathy. In this model, endothelial cells and podocytes form two cell layers which are separated by a thin membrane coated with an extracellular matrix, thereby simulating the structure of a glomerulus. This model successfully simulated cytoskeleton rearrangement, cell damage, and glomerular leakage with the fluid flow (Zhou et al., 2016). Another GOC model was designed to study diabetic nephropathy. This model contained a glomerular filtration barrier that was affected by the fluid flow and produced pathological responses to high glucose (Wang et al., 2017a; Wang et al., 2017b). Recently, a GOC device used podocytes differentiated from the pluripotent human-derived stem cells. These podocytes, when cultured with human glomerular endothelial cells in a microfluidic apparatus, produce glomerular basement membrane collagen and mimic the interaction of normal glomerular tissues and different albumin and inulin clearance (Musah et al., 2017).
In a different study, the glomerular filtration barrier was modeled on GOC using co-cultures of human glomerular endothelial cells and podocytes. This model consisted of two vascular and urinary channels. There is a type-1 collagen layer between the two channels. Podocytes, basement membrane, and glomerular endothelial cells are located on the collagen layer. In long-term culture, the cells retained their morphology, formed capillary-like structures, and expressed cleft diaphragm proteins. This system simulated the structure and functions of the glomerulus (such as selective infiltration). When the filtration barrier of the glomerulus was exposed to the serum of patients with anti-podocyte antibodies, the chips showed that the amount of albuminuria was proportional to the proteinuria of the patients. Using this chip, it is possible to study the pathophysiology of the glomerulus, identify other therapeutic targets, and screen high-throughput therapeutic compounds (Petrosyan et al., 2019).
3.1.2 Proximal tubule-on-a-chip (PtOC)
The first site of drug clearance and the front line against drug toxicity in nephrons is the proximal tubule. Therefore, it is very important in the clinical evaluation of drug compounds. An essential aspect for accurate simulation of proximal tubule function in vitro is the ability to control the reabsorption and secretion of molecules (Ng et al., 2013). Specific carriers and pumps on both sides of the basal and apical sides of the proximal tubules transfer organic cations and anions, as well as supply a mechanism for the excretion of biological and endogenous substances (Jacobsson et al., 2007).
In another PtOC model, human epithelial cells of proximal tubule were implanted in the upper surface of the polyester membrane coated with the extracellular matrix. The polyester membrane separated the main chamber of the chip into apical and basal chambers. In this study, cells exposed to the fluid shear stress (dynamic culture conditions) acquired specific physiological properties such, as polarity, primary cilia, columnar shape, and functional carriers. While cells were cultured under static conditions, they did not acquire these characteristics. Therefore, creating static and dynamic culture conditions in a platform can simulate tissue physiological processes effectively (Jang et al., 2013) (Fig. 1A-C).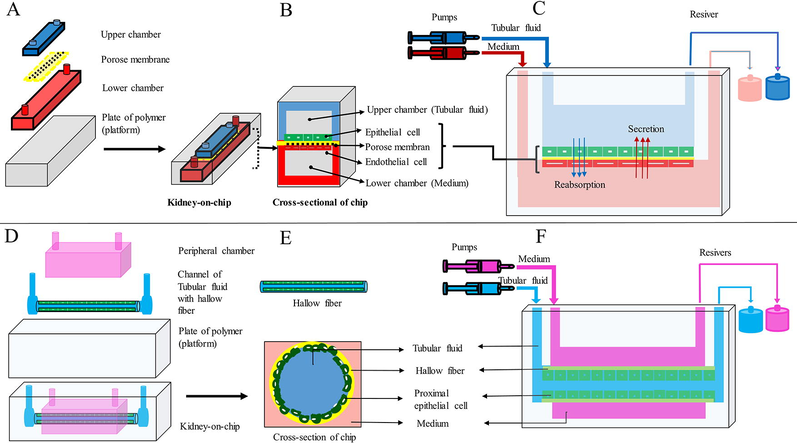
(A, B, and C) KOC design using a porous membrane located between two chambers. Renal tubular epithelial cells and vascular endothelial cells were cultured in the upper and lower chambers, respectively, and (D, E, and F) KOC design using hollow fiber. Renal tubular epithelial cells were cultured inside the hollow fiber. The hollow fibers were perfused by a tubular fluid stream and the chamber around the hollow fiber by a medium.
Hollow fibers may be used as scaffolds for cell culture and imitation of renal tubules due to their tubular shape. Proximal tubular epithelial cells have a key role in the reabsorption of ions and proteins that have passed through the filtration barrier of the glomerulus. The proximal tubule cells contain lots of megalin and colin multi-ligand receptors, which effectively absorb low molecular weight proteins and other ligands from the filtered fluid. Megaline and cubilin bind to vitamin-carrying proteins to reabsorb them. Impaired absorption of these proteins leads to vitamin deficiency (Eshbach and Weisz, 2017). In one model of PtOC, human epithelial cells of proximal tubule were cultured on the inner surface of hydrogel-coated hollow fibers. The cells cultured on the inner surface of the hollow fibers formed a functional transfer layer, which was demonstrated by albumin reabsorption and the secretion of albumin-bound uremic toxins (Jansen et al., 2015; Jansen et al., 2016). In a microfluidics device, human epithelial cells of proximal tubule were cultured in a tubular structure surrounded by a chamber. In the study of the physiological activity of this device, the transport of substances at the basal and apical levels, cellular polarity and morphology, and function of intracellular enzymes were well demonstrated (Weber et al., 2016) (Fig. 1D-F).
Bioprinting is an alternative method of making PtOC (Sochol et al., 2016). In one model of PtOC, the structural components of the proximal tubule were designed using bioprinting, in which the tubules were first printed on an extracellular matrix of gelatin-fibrinogen using volatile ink. Further, an additional extracellular matrix was poured around the printed design. After draining the volatile ink, a hollow tube was formed. Proximal tubule cells were implanted in a hollow tube and perfused for a long time. This system was stable for 2 months which indicates the durability and survival of cells. Thus, this system has the potential to improve and build other organ-on-a-chip systems (Homan et al., 2016). In another model of PtOC, a proximal tubule containing epithelial cells, endothelial cells, and renal fibroblasts were designed using bioprinting. This model which mimicked the features of renal fibrosis was stable for at least 30 days. This model used proximal tubule cells along with several types of renal interstitial cells to investigate cell–cell interaction and paracrine signaling between constituent cells. This model also can confirm key physiological aspects of the proximal tubule, diagnose renal toxicity, and evaluate renal fibrosis (King et al., 2017). In another study, using this type of design, two channels were created side by side on an extracellular matrix substrate. The epithelial cells of the proximal tubule were cultured in the first canal, and vascular endothelial cells were cultured in the second canal and perfused independently using a closed-loop system to evaluate the reabsorption of the renal tubule. This model showed active reabsorption through the tubular-vascular exchange of salts. The epithelium-endothelium relationship was also investigated by placing the epithelial cells of the proximal tubule in the hypoglycemic conditions (Lin et al., 2019) (Fig. 2).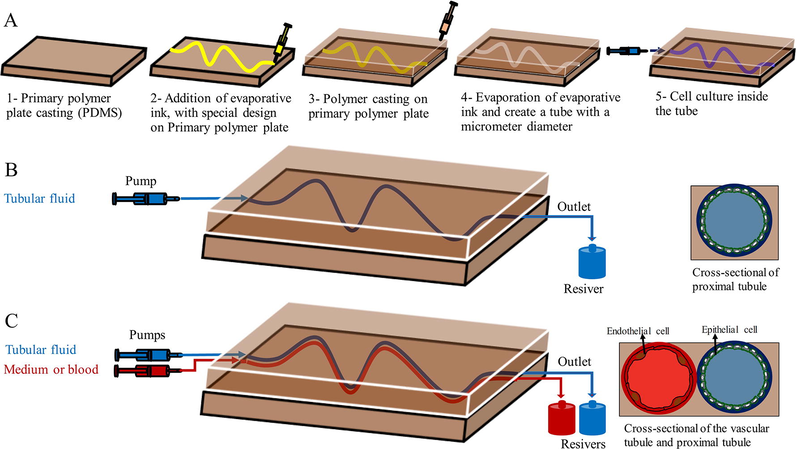
Making a KOC using bioprint technology. (A) steps of making a KOC using a bioprint, (B) PtOC model using only proximal epithelial tubule cells, and (C) PtOC model in which proximal epithelial tubule cells were used along with vascular endothelial cells.
3.1.3 Distal tubule-on-a-chip (DtOC)
So far, the use of DtOC model is very limited. Dog kidney cells were first used to make DtOC. In this model, the cells were cultured inside polydimethylsiloxane microchambers coated with fibronectin. Tiny ducts connect the culture chambers to a fluid circulation system but disrupt cell proliferation and survival at flow rates similar to in vivo conditions (50 μL/min) (Baudoin et al., 2007). A DtOC model was designed for the analysis of renal tubular cells using a microfluidic canal, a porous membrane, and central collecting duct cells of rat kidneys. After culturing the cells in the canal to simulate the tubular environment in vivo, the cells were exposed to a shear stress of 1 dyne/cm2 for 5 h to provide optimal fluid conditions for the cultured cells. These conditions increase cellular polarity and reorganize the cytoskeleton. This device simulates renal tubules in vivo and can be used specifically in drug screening and tissue engineering (Jang and Suh, 2010).
Microfluidic systems evaluate the physiological functions of biological components under in vivo and in vitro conditions. These systems are created on the micrometer scale and contain microliter scale materials. A KOC system is a microfluidic system designed to examine at least one physiological variable of a kidney on a platform. The material of these platforms is mainly polymer and biocompatible, and cells of one organ or tissue are easily cultured on it. For optimal cell adhesion, the polymer surface is mainly covered by an extracellular matrix component. A platform is a plate made primarily of polymer on which chambers are designed for cell culture, tissue or organ maintenance, and reservoirs for medium flow. On this plate, various biosensors are installed to measure and evaluate physiological variables. The plate is generally clear and transparent, and changes can be easily captured with a confocal microscope. Although 2D models and some 3D models may be useful in screening drug compounds, they cannot completely simulate the physiological condition of an organ. These models are mostly designed from the beginning to study the effect and determine the toxicity of drug compounds. If the purpose of designing these models was to study the function and knowledge of physiological mechanisms to build new organs for transplantation into the human body, success and results would be far more practical and effective.
3.2 Lung-on-a-chip (LuOC)
For the first time, a LuOC model was designed using soft lithography technique on polydimethylsiloxane polymer. A thin membrane (10 μm), coated with an extracellular a matrix was placed between the upper and lower microchannels. Human alveolar epithelial cells and lung endothelial cells were cultured in the upper and lower parts of this membrane, respectively. When the alveolar cells were connected, an air-fluid interface was created on the surface of the alveolar cells by circulating air in the upper channel. In the lower vascular canal, a constant medium current was maintained. The use of a periodic vacuum in two side hollow chambers resulted in regular mechanical tension of the lateral walls (flexible polydimethylsiloxane polymer) and the central porous membrane. The tension of the central porous membrane together with the cell layers attached to it mimics physiological respiratory movements (Huh et al., 2010). Using the same method, another microfluidic lung model was designed that contained airway epithelial cells (air-fluid interface), vascular endothelial cells, and lung fibroblasts cultured in three vertical sections. Each vertical part was separated by a nanoporous membrane (Sellgren et al., 2014). In another model, a reversible alveolus-on-a-chip model was designed to simulate the pulmonary parenchyma microenvironment. A micro diaphragm was used to simulate in vivo diaphragmatic movements and dilation of the alveolar wall (respiratory barrier) (Stucki et al., 2015) (Fig. 3 A-C).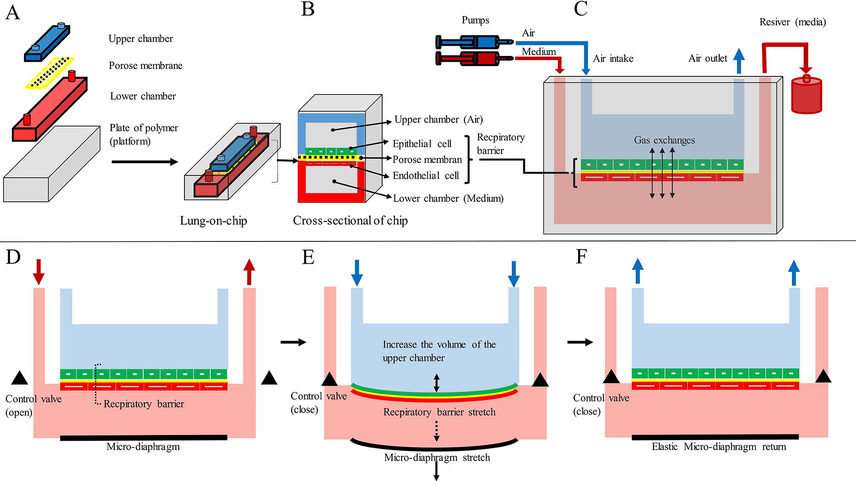
LuOC model. (A) the chip is made by engraving chambers and channels on a polymer platform, (B) cross-section of the chip, (C) connecting the chip to the pump and receiver, and (D, E, and F) alveolar-on-chip which mimics respiratory movements (cyclic strain) using micro diaphragm on the chip. Medium flow is regulated by valves. As the micro diaphragm is stretched, air enters the upper chamber. Note: Figures match the original models in terms of functionality but do not match in appearance.
A lung airway-on-a-chip model was designed to simulate the airway microenvironment and analyze the interaction of epithelial cells and airway smooth muscle with the extracellular matrix. The microdevice consisted of three vertical microfluidic chambers (layers), an upper chamber for culturing epithelial cells (air-fluid interface), a thin middle hydrogel layer, and a lower chamber for culturing airway smooth muscle cells. The device is completely assembled after the solvent is added to three different layers of polymethyl methacrylate (Humayun et al., 2018). An airway-on-a-chip model containing a vascular network was designed using 3D cell printing. In this model, an avascular platform using endothelial cells, lung fibroblasts, polycaprolactone, and polydimethylsiloxane polymer was printed directly in a three-dimensional aspect on the extracellular matrix. The platform consisted of a central microchamber and two lateral microchambers for culturing epithelial cells and lung fibroblasts. These microchambers were separated by microchannels to create a media stream and an area for connecting the polydimethylsiloxane polymer to the top polydimethylsiloxane chip. In this model, the airway successfully established a functional connection with the vascular network (Park et al., 2018a; Park et al., 2018b).
Researchers have developed various models to mimic the physiological functions within the chips. The presence of air in the epithelial chamber and the exposure of cultured cells to air as compared to cultured cells exposed to fluid, increases cell survival, pulmonary surfactant production, the electrical resistance of different tissue layers, and structural integrity and permeability of the natural barrier (Benam et al., 2017; Hiemstra et al., 2019). Structural and functional simulation of a model and its approach to the natural state improves the results. For example, in one model, exposing the alveolar epithelial cells (respiratory barrier) and airway epithelial cells to airflow and cyclic strain better mimic pulmonary physiological conditions and also improves the interaction and function of different parts of the pulmonary microenvironment (epithelial surface, basement membrane, and vascular surface).
Simulation of lung alveoli in vitro is difficult due to the complex architecture of the dynamic lung environment. The alveolar wall with only a few micrometers thickness is an extremely thin barrier between air and blood in vivo. It constitutes tight and semi-selective layers of epithelial cells (forming the alveolar wall), endothelial cells (forming the endothelial wall), and base membrane. The alveolar wall expands and contracts rhythmically to allow air to enter and exit the alveoli. Many in vitro models are designed to simulate the alveolar barrier of the lung in vitro, but only a few models use mechanical forces for respiratory movements (Konar et al., 2016; Bhowmick and Gappa-Fahlenkamp, 2016).
In 2010, a microfluidic system was developed that simulated diaphragmatic action. The device contained a thin porous elastic membrane (resembling an alveolar barrier) in which alveolar epithelial cells were cultured on the apical side of the membrane and endothelial cells were cultured on the basal side of the membrane. This alveolar barrier was periodically stretched in one direction to simulate diaphragmatic breathing movements (Huh et al., 2010) (Fig. 3D-F). In another model, respiratory movements were simulated by periodic deformation of the micro diaphragm, similar to the diaphragmatic movements in vivo. In this model, alveolar epithelial cells of a patient's lung were cultured on a device under physiological mechanical strain (Stucki et al., 2015). The alveolar model was used to simulate pulmonary thrombosis, primary alveolar epithelial cells, and lung endothelial cells. But in this model, the cells were not subjected to cyclic strain (Jain et al., 2018). In another alveolar model, the system automatically provided a passive medium flow at selected time points. Unlike previous models, the new alveolar model is equipped with a passive medium exchange that not only reproduces periodic mechanical stress but also allows long-term cell culture between air and fluid. Therefore, specific features of the lung microenvironment are simulated more accurately. These features have important effects on many biological processes, such as differentiation and polarity of epithelial cells, which play a key role in identifying physiological processes (Ravasio et al., 2011; Hobi et al., 2012).
3.3 Heart-on-a-chip (HOC)
Statistical studies have shown that drug toxicity is the prime reason for halting the development and production of drugs. Enhancing the efficacy of cardiovascular drugs and ensuring drug safety are important factors in the drug screening process (Ferri et al., 2013). Animal models and 2D cell cultures are common methods in the drug development process (Pirzad Jahromi et al., 2015). But they are inadequate and inefficient to simulate human physiological mechanisms. One of the most advanced methods for evaluating heart function and drug screening is the HOC model (Sidorov et al., 2017).
Biofabricating 3D heart tissues from cardiomyocytes [derived from human induced pluripotent stem cells (iPSCs)] are a suitable method to screen personalized medications. Vascularization of the heart tissue is necessary for the survival of cardiomyocytes because the arteries provide nutrients and eliminate metabolic waste products. Combining HOC with microfluidics simulates true biology and physiology in vivo by creating a circulatory or distribution system (Raasch et al., 2015).
Contractile behavior is the most important feature of cardiac function and it can be an important criterion for evaluating drug safety and efficacy during screening. Also, it is an indicator of cardiac functions that comprise variables such as contraction frequency, force, and synchronization over time. Until now, several reports have been published to evaluate contractile behavior with HOC (Zhang et al., 2017a; Zhang et al., 2017b). The main method for evaluating contractile behavior is the detection of deformation of flexible materials during cardiac contraction. In the cardiomyocyte culture medium, the contractile behavior is calculated by stretching a traction-sensitive sensor (Lind et al., 2017). The contractile behavior of cardiac muscle is measured on HOC by microsensors such as microcantilever of an atomic force microscope and pillar arrays. These microsensors are stimulated by the deformity caused by the contraction of cardiomyocytes. Pillar arrays are microsensors that are sensitive to pressure and tension and bend by force. They are implanted regularly and attached to the cardiac myocytes in a culture medium or HOC model. Contraction of myocytes causes displacement of pillar arrays. The contractile force can be calculated by measuring the amount of displacement or curvature of pillar arrays (Ribeiro et al., 2016). The microcantilever of an atomic force microscope is a tiny sensor that creates tiny indentations inside the tissue. This device acts as a probe to detect the deformation of the heart tissue and also causes synchronous contraction by sending stimulus messages in different sizes and frequencies. Increasing the frequency and amount of indentation significantly increases the contraction rate (Galie et al., 2015) (Fig. 4).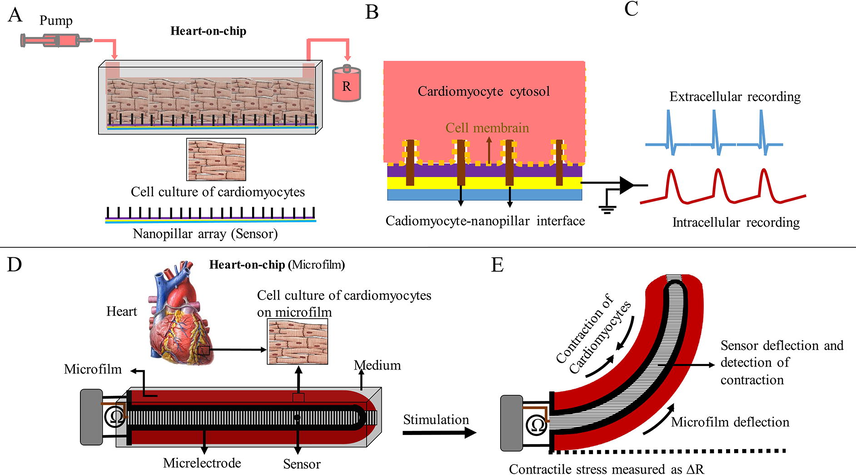
Two types of HOC models to measure the contractile behavior of cardiomyocytes. (A, B, and C) fabrication of a HOC model using the nanopillar array and (D and E) fabrication of a HOC model using sensory elastic microfilm.
The image processing system can analyze the contractile behavior of myocytes in the HOC model using a video recording system. Sensors or other devices (endoscopes) may be used to improve the efficiency of this system (Kim et al., 2011). In a recent report, a HOC model was developed containing a microchannel and cell culture chamber equipped with intracellular and extracellular bioelectronic devices. This model can mimic coronary occlusion diseases by changing the temporal oxygenation of the cell culture medium under normoxic, hypoxic, and recovery/perfusion conditions. This model may also accurately record electrical activity inside and outside the cell using various bioelectronic devices. For example, these devices can detect the initial period of tachycardia by detecting a decrease in the level of oxygen in the fluid inside the device. The design of this model or platform represents progress in understanding electrophysiological responses in different states of heart function and can be used in disease modeling and drug development (Liu et al., 2020). A model designed to study live and simultaneous cardiac contractile behavior uses image processing and piezoelectric sensing systems to identify the contractile behavior of a unit and the whole heart tissue, respectively. Measurements with two different systems provided complete information about the evaluation of the cardiac tissue’s contractile behavior (Sakamiya et al., 2020). The use of these two HOCs from bioelectronic and biomechanical devices has resulted in the optimal recording of the electro-mechanical activity of cardiac myocytes. The major concern is how to use electrical and mechanical sensors on these two devices without affecting the quality of data recording. Engineering the installation of sensors without any defects in data recording and cell damage is a significant step in the progression of other HOCs.
Recent HOC systems have efficiently simulated the structure and function of the normal heart muscle. These are also potent devices for modeling heart diseases. But the process of connecting and merging different measuring instruments and different sensors on one organ chip is difficult, in addition, to damaging the cells. Therefore, current devices are not suitable for examining the electrophysiological processes of the heart.
3.4 Brain-on-a-chip (BrOC)
The most complex organ in the human body is the brain and together with the spinal cord, it forms the central nervous system (CNS). The brain receives, interprets, mixes, and harmonizes information and then controls the activity of each organ of the body by sending appropriate signals. The brain consists of a large number of neurons that communicate unilaterally through synapses, where the axon end of one neuron communicates with the dendrites and the cell body of another neuron in a special direction (Swanson and Lichtman, 2016). In addition, the neurons communicate with other neural cells such as oligodendrocytes, astrocytes and microglia (Jäkel and Dimou, 2017). The most important feature of neurodegenerative disorders (Alzheimer's and Parkinson's disease) is the progressive cell death of parts of the brain. Parkinson's disease causes synaptic changes and neurons death in the corticosteroid network. On the other hand, Alzheimer's disease causes neuritis, neurons death, and deposition of neurotoxic protein plaques. These diseases mainly affect humans and the only way to study them is through animal models and to examine the brains of the people after death (post-mortem). Animal models differ from human models in studying the mechanism of action and drug screening (Pirzad Jahromi et al., 2012). Because of the ethical problems associated with the use of animal models, very limited information has been obtained about the mechanism and progression of these diseases (Franco and Cedazo-Minguez, 2014; Lage et al., 2018).
Recently, many advances in BrOC technologies have been made using a simulated blood–brain barrier (BBB) and neural circuits in engineered models. So far, five types of BrOC models have been designed to study the components of brain tissue and drug screening: (i) models that study BBB using cultures of nerve and endothelial cells in two chambers separated by a porous membrane, (ii) models that study different parts of neurons (cell body and axons) by dividing the culture medium into separate sections, (iii) models that study the components of brain tissue on a hydrogel, by studying the cell-matrix and cell–cell communication with three-dimensional printing, (iv) models that study the interaction of different cells by merging multiple chips and creating a medium flow between them using a pump, and (v) models that are used in high-throughput screening studies by integrating advanced BrOC models with traditional models (Bang et al., 2019).
The brain and spinal cord are made up of different neurons and glial cells. So far, no model has been proposed to simulate the complete structure and function of the brain. But it is possible to simulate part of the brain or part of brain processes using existing techniques. Therefore, the plasticity and connection of neurons in the neural network, the function of the BBB, and the myelination process of neurons on an engineered and isolated system can be examined independently. With the advent of cell culture technology, neurons could be cultured in vitro, and with the invention of soft lithography, cell culture was performed in a microphysical environment, such as microchannels (Whitesides et al., 2001). Later, using multistage lithography, microchannels of different heights and widths were fabricated on a BrOC. It was also possible to separate the soma and axon physically using this technology. By constructing microchannels at high and low levels on a BrOC and by placing neurons on it, soma and axons are placed in the upper and lower microchannels, respectively. Therefore, it is possible to study a soma and an axon independently (Park et al., 2009; Kim et al., 2016). By dividing a platform into separate parts and placing a neuron in these separate parts, different portions of the neuron can be examined independently. In addition, engineering and modifying the geometry of channels (through which only axons pass) allows for one-way axonal growth. In this way, axonal growth, direction, and axonal communication can be examined more carefully (Jadhav et al., 2016; Paranjape et al., 2019).
The most important function of the nervous system is to coordinate different organs by receiving sensory messages, processing, and sending motor commands. The speed of signal transmission plays an important role in the functioning of the nervous system and the coordination of different organs. The myelination of neurons increases the speed of signal transmission. Impaired myelin production leads to neurodegenerative diseases such as multiple sclerosis. Simulation of the myelination process in BrOC is one of the effective techniques in the basic studies related to myelination. The BrOC chips allow the direct study of axon myelination under a microscope due to their transparency (Kerman et al., 2015). Therefore, increased myelin production and differentiation of oligodendrocytes can be observed under a microscope by applying electrical and optical stimulation to a compartmentalized BrOC system (Lee et al., 2017).
Selective permeability of the blood–brain barrier (BBB) leads to normal brain function. This structural barrier facilitates the entry of necessary nutrients and the removal of waste products. It also prevents the entry of neurotoxins and large hydrophilic molecules and the invasion of pathogens (Pardridge, 2009; Daneman, 2012). Many drugs developed to treat neurological diseases often fail and are avoided during the drug screening process due to lack of transmission from the BBB and entry into brain tissue (Liu et al., 2012).
A microfluidic model with a porous membrane was designed to show how materials are transported through the BBB. The presence of pores in this membrane causes the cells in two microchannels to communicate with each other through the membrane pores in a cytokine-dependent manner. This BrOC simulates the structure of the BBB (Brown et al., 2015). The BBB trans-endothelial electrical resistance can be measured by placing one electrode in each channel (Wang et al., 2017a; Wang et al., 2017b). When cells cultured in two microchannels attach to the lateral surfaces of a porous membrane, 2D cell culture media become 3D. The interaction between different cells on both sides of the pores (endothelial cells and astrocytes) is established in the form of physical contact (direct) and chemical contact (indirect). In physical contact, two cells on either side of the membrane connect through pores, but in chemical contact, two cells communicate with each other through chemicals in the form of paracrine (Vatine et al., 2019) (Fig. 5).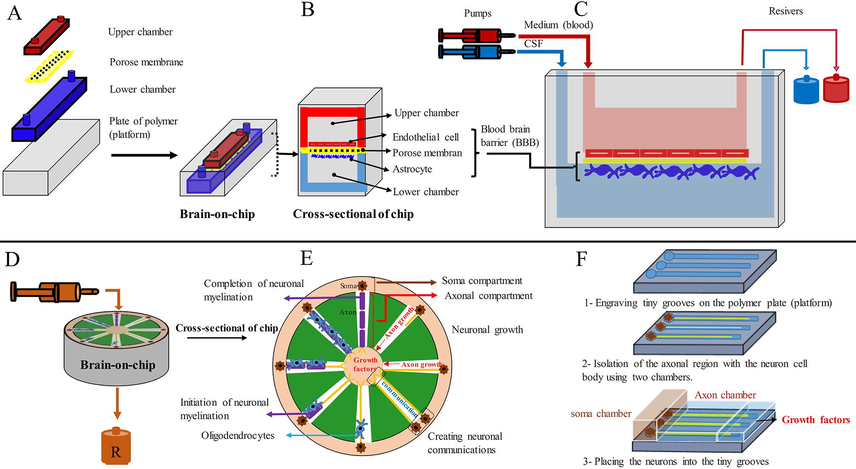
(A, B, and C) BrOC model using two chambers and a porous membrane between them and culturing endothelial cells in the upper chamber and astrocytes in the lower chamber, the BBB is simulated on a platform, (D and E) a segmented BrOC model with a cylindrical structure that can study neuronal growth, neural connections, and myelination in detail, and (F) isolation steps of different parts of neurons for detailed study in a BrOC model.
An important point in modeling 3D neural circuits is how axons are positioned in the 3D environment. In early models, neurons were placed between walls to study neuronal growth accurately. In this case, the neural network created was very limited. But in newer models, neurons are planted in the hydrogel substrate. The neural network and the vascular network created in the hydrogel substrate simulate the microstructure of neural tissue effectively. Models, in which components of the nervous system are implanted in the hydrogels, simulate the function and the structure of nervous tissue well. The vessels made in this bed are similar to the vessels of the body. Also, the relationship of the vascular system with the neural network created in this hydrogel substrate is functionally and structurally very similar to the neural tissue. These models are called high content models (Uwamori et al., 2017). In the previous models, porous membranes were used to simulate the BBB, but in the recent models, BrOC-compatible hydrogel channels have been placed as barriers for vascular transport. The vascular networks formed in the hydrogel ducts are functionally and structurally similar to the body vascular system and increase the communication between the brain vascular cells and the nerve cells. When the neural circuit and the vascular network are placed together in a model, it increases axonal growth and coordination of neural electrical activity (Osaki et al., 2018; Sances et al., 2018).
Connecting several different chips is another way to study different parts of an organ or the physiological and pharmacological interaction of several organs. Therefore, by connecting multiple chips on a single platform, it is possible to simulate several functions of an organ or connect several organs. The process of designing and connecting multiple organs on a chip and building a multi-chip system to simulate the interaction between different cells or organs is in progress. These systems connect several organs on the chip and flow the culture medium to facilitate cellular and tissue interactions (Maoz et al., 2018). Newer models use the connection of multiple brain chips (membrane type) to study the connection between different brain cells. Also, to study the intercellular communication of the brain with different organs, the connection of several organ chips is used together (Oleaga et al., 2016; Vernetti et al., 2017). Various platforms have been developed and used to study the cerebral vascular system, neural network, and myelination.
To simulate the structure and physiology of brain tissues accurately on a BrOC, three important factors must be considered: (i) cell source, (ii) cell–cell interaction, and (iii) cell-matrix interaction. The first step in simulating brain tissue and production of personalized drugs is to extract cells from human resources. As the cellular functions and genetic characteristics of animal cells are different from human cells and extracting early human cells is difficult, therefore, the iPSCs are a good source of human cells (Takahashi and Yamanaka, 2006). The iPSCs may be obtained by stimulating stromal cells with key growth factors and then differentiating them into the cell of our interest. Because of the preservation of genetic information of cells during induction and differentiation, the production of personalized drugs will be possible (Singh et al., 2015). Patient-specific BBB models are currently designed that use the patient's iPSCs. These models make it possible to prepare a patient-specific medication (Vatine et al., 2019). In Alzheimer's disease, microglial cells are activated by beta-amyloid as an active marker. Microglia cells accelerate neuronal death by interacting with nerve cells via neurotoxic inflammatory agents and microglia activators (Park et al., 2018a; Park et al., 2018b). Due to the direct observation of the cell population in the organ-on-a-chip technology, it is possible to study the response of each cell type to develop drugs. On the other hand, it is possible to commercialize the drugs by choosing effective drugs and minimizing undesirable side effects. The extracellular matrix is a key factor in the physical adhesion and transmission of messages between cells. Therefore, it is necessary to determine which component of the extracellular matrix should be used for in vitro culture (Boudreau and Bissell, 1998). The cellular matrix of the brain can be used for neurons produced by direct reprogramming. The neurons cultured in a cellular matrix of the brain are differentiated better than traditional 2D culture and collagen-based 3D culture. This method also ensures the structural integrity and functional maturity of the neurons (Jin et al., 2018). Accurate simulation of the tissue environment is necessary to improve cell interaction with the extracellular matrix.
Various models have been designed using microfluidics and microfabricated technology that are used to study various diseases of the nervous system. It is not currently possible to study the entire nervous system on one model, the only way to study this complex system is to design models that simulate parts of the system's activity. So far, various platforms have been developed to study the cerebral vascular system, neural network, and myelination. The connection of several platforms was used to study various brain activities. With the advancement of technology, hydrogel-based platforms were used for implanting different components of the nervous system.
Due to the lack of physiologically-relevant brain tumors models and unclear mechanism of progression and resistance, increasing computational and in vitro modeling are developing for preclinical prediction. Using minitumors with physiologically relevant vascularized environment, decrypting mechanisms of brain tumors and speed up the drug development and prediction of response to anticancer agents (Clarke et al., 2021). Numerous bioengineered 3D platforms including collagen, gelatin and hyaluronan have been produced to assess the responses of patient-derived brain tumor (Neves et al., 2021). Immaturity, need for validation and high reproducibility are some of the challenges for biofabrication technologies (Clarke et al., 2021).
3.5 Liver-on-a-chip (LiOC)
Parenchymal cells (hepatocytes) and non-parenchymal cells (endothelial, Kupffer, satellite cells, and bile epithelial cells) make up 60 and 40% of the liver cells, respectively. The most important physiological function of the liver is related to hepatocytes. Hepatocytes are involved in many physiological processes such as synthesis (bile, plasma proteins, urea, and glucose), storage (glucose, iron, and vitamins), metabolism (amino acids), breakdown (xenobiotics and drugs), and detoxification of various substances (LeCluyse et al., 2012; Materne et al., 2013). Non-parenchymal cells maintain tissue structure, regulate physiological processes, and modulate the phenotype of liver cells. The main non-parenchymal cells are sinusoidal endothelial cells, and by creating a unique tubular structure in the walls of the sinuses, they cause the optimal exchange of substances between the blood flow and hepatocytes (Poisson et al., 2017). The Kupffer cells are the specific macrophages of the liver with a frequency of 12%. These cells kill the microorganisms in the liver sinusoids by phagocytosis and neutralize the toxic effects (xenobiotics, drugs, and toxins) by endocytosis. They also activate the immune system and other cells by producing various cytokines (Dixon et al., 2013). The satellite cells, with a frequency of 1.4% of non-parenchymal liver cells, are responsible for storing Vitamin A and fat droplets and regulating microcirculation. They are also the most important cause of liver fibrosis (Maschmeyer et al., 2011; Huang et al., 2017). The bile epithelial cells line the walls of the bile ducts. These cells modify the bile made by the liver cells through complex chemical and physiological processes. Recent studies have identified their role in hepatotoxicity and cholestatic liver disease (Masyuk et al., 2018).
In vitro liver models are critical for hepatology studies and drug development for liver diseases. An important aspect of these models is the cell source. There are three major types of cells to rebuild liver tissue in vitro: primary human hepatocytes, hepatic-derived cell lines, and stem cell-derived hepatocytes (Prot et al., 2011; Garnier et al., 2018). Hepatic cell lines, such as HepG2 (derived from human hepatocellular carcinoma of a 15-year-old male) and HepaRG (terminally differentiated hepatic cells derived from a hepatic progenitor cell line of a female hepatocarcinoma), have been widely used in toxicological investigations because they have a stable phenotype and are easily manipulated with the unlimited proliferation (Desai et al., 2017; Weltin et al., 2017). By differentiating induced pluripotent stem cells into the liver cells, hepatic organoids are made that have physiological functions of hepatic tissue such as urea production, albumin secretion, liver-specific metabolic activities, and gene expression (Foster et al., 2019).
The LiOC systems are used for recognizing physiological processes, screening and testing for drug toxicity, predicting drug metabolism, establishing models for liver disease, and constructing multi-organs-on-a-chip (Deng et al., 2019). The standard factors (standard liver cells and standard metabolic responses) are used to screen and determine the responses of the liver towards the drug compounds. For example, cytochrome P450 (CYP) enzyme levels are a standard factor in analyzing the metabolism of hepatocytes. Although cultured hepatocytes and native hepatocytes have phenotypic and metabolic differences, the levels of CYP enzymes are not different in vitro and in vivo. In addition, they are often unaffected by the chemical stimuli in vitro (Wrighton and Stevens, 1992; Shoemaker et al., 2020).
Au et al. (2014) designed a digital microfluidic device for culturing and producing three-dimensional liver tissues. Inhibitors and inducers of CYP activity were used to perform enzymatic tests and determine acetaminophen-induced hepatotoxicity. In this study, significant changes in the enzymatic activities were observed in cultured 3D tissues. But these changes were not observed in the standard hepatocytes monolayer models. These results showed that environmental conditions and the 3D geometry of tissues affected the enzymatic activities and physiological functions of the tissues. Probably, different environmental and geometric conditions in in vitro systems are responsible for these differences, and cell–cell, as well as cell-extracellular matrix interaction, is better established in 3D than in 2D culture medium.
Another valuable strategy for engineering an in vivo liver model for drug screening and other studies is to mimic the architecture of the hepatic lobule in terms of the contribution of the different types of cells that make up the lobule. Co-culture of hepatocytes with other liver cells increases the efficiency of in vitro liver models (Soldatow et al., 2013). The most important cells that interact with the hepatocytes are endothelial cells, non-parenchymal cells such as fibroblasts, Kupffer cells, and hepatic stellate cells (Kang et al., 2013; Zhou et al., 2015). Designing the exact architecture of liver tissues (size and geometry of building blocks) and using key factors in liver cells (presence of all tissue-forming cells and continuous flow through the vascular-like network) is an important step in providing advanced models for studying the physiological functions of the liver and understanding biochemical mechanisms in the clearance and screening of drug compounds.
Ma et al. (2016) designed a microdevice to simulate the placement of hepatocytes and endothelial cells in a liver lobule. Hepatocytes and endothelial cells were cultured radially on 3D hydrogels. Endothelial cells that formed a hepatic sinusoid-like structure were located in radial structures with hepatocytes. Hepatocytes cultured in this device exhibited higher baseline CYP activity, compared to standard hepatocytes controlled under standard conditions. Standard conditions include 2D culture of hepatocytes or culture of hepatocytes alone in 3D hydrogels (Fig. 6).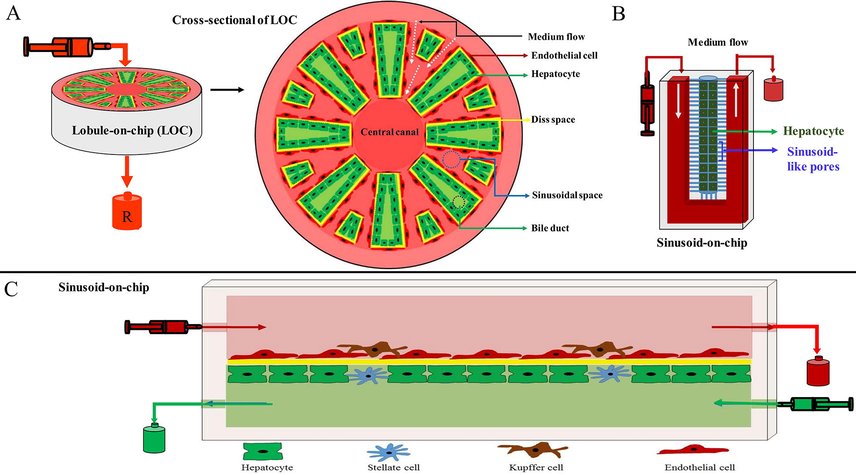
LiOC model. (A) a lobule-on-chip model was designed on a radial platform. Hepatocytes were cultured with endothelial cells, (B) a LiOC (sinusoid-on-chip) in which hepatocytes were cultured in a cell chamber and sinusoidal-like pores were used to simulate vascular endothelium, and (C) this LiOC model used all the cells that make up the liver lobule, a porous membrane between two chambers (upper and lower), and two opposing medium currents (in two chambers).
Another key physiological aspect is the continuous perfusion of cultured cells. Blood flow in vivo continuously cools liver cells, provides nutrients, and eliminates waste products. However, standard cell culture media are not able to produce these characteristics in vitro. Lack of constant flow in a standard cell culture medium causes unstable conditions such as reduction of nutrients, increase productions of waste materials, and elevation of tissue temperature. Creating a constant medium flow in the cell culture media and organ-on-a-chip devices by creating stable environmental conditions have improved tissue function and increased cell survival (Lee et al., 2007; Baudoin et al., 2014).
In a project to determine the mechanisms associated with liver cancer metastasis, a liver-on-a-chip model was designed to investigate how tumor-derived extracellular vesicles affect liver metastasis. Extracellular vesicles are cell-derived membrane vesicles that are secreted in almost all cell types, including cancer cells. The use of this liver-on-a-chip technique showed that high levels of TGFβ1 in the extracellular vesicles derived from breast cancer-induced liver metastasis by increasing greater adhesion of breast cancer cells to the sinus endothelial cells of the liver (Kim et al., 2020).
The functional units of the liver are the lobules. The hepatic lobule consists of a central canal, the radial columns, and sinusoids between the columns. Blood from the portal vein and hepatic artery (from the periphery to the center of the lobule) enters the central canal through the sinusoids. The blood in the central canal then enters the vena cava through the hepatic vein. The lobular radial columns are made up of hepatocytes and bile ducts exist inside these columns. There is an opposite current (from the center to the periphery of the lobule) inside the radial columns of the lobule that enters the bile made by hepatocytes through the canals into the bile ducts. The bile ducts are connected and bile enters the gallbladder.
In most liver-on-a-chip models, several important physiological points, such as the bile secretion process, bile duct architecture, and bile flow direction in the hepatic lobule are not considered. Bile is the most important secretory substance in the liver, and most chemicals and drugs are converted to water-soluble substances by complex liver mechanisms and then secreted into the bile. Therefore, mixing hepatocyte secretions with other substances in the medium prevents the correct extraction of information and the results of these models are challenged in terms of validity. However, the model proposed by Ma et al. (2016) seems to be the best and closest model for simulating the structure of hepatic lobule provided that the bile flow is separated from the medium flow. Hence, to separate the bile flow from the medium, in this model, channels can be created in the area between the center and periphery of the lobule.
The perisinusoidal space is the area in the liver between the hepatocytes and the sinusoidal endothelium that contains blood plasma. Hepatocyte microvilli expand into this space, allowing proteins and other plasma components to be absorbed by the hepatocytes from the sinusoids. The presence of pores and discontinuities in the endothelium, as well as its basement membrane, facilitates this transmission. This space may be lost in liver disease, leading to the reduced absorption of nutrients and excretion of waste products such as bilirubin by hepatocytes. The perisinusoidal space plays a key role in the function of hepatocytes and prevents the rapid flow of interstitial fluid and rapid changes in the areas around hepatocytes (Sanz-García et al., 2021).
In a LiOC model proposed by Boeri et al. (2019), two chambers were separated using a permeable membrane. Endothelial cells were cultured with Kupffer cells in the upper chamber and hepatocytes with the stellate cells in the lower chamber. Inside these two chambers, two medium currents were circulated in opposite directions. This design was able to prevent the mixing of the sinusoidal space medium and the bile duct (Fig. 6). But in this model, the permeable membrane between the sinusoidal endothelium and the hepatocytes cannot simulate the perisinusoidal space. It seems that another membrane or scaffold can be used to solve this problem and create space. Also, to improve the performance of the model, a collagen scaffold can be used instead of a polymer membrane.
Hepatocellular carcinoma-on-chip models have been utilized to assess the effect of tumor microenvironment conditions on the viability of tumor-specific T cell receptor (TCR) expressing T cells (Clapp et al., 2021). This model has been successfully used to evaluate the effect of inflammation and hypoxia (tumor-related niche condition) on the cytotoxicity of the aforementioned cells. According to the result hypoxia diminished the functionality of cells and the inflammatory condition resulted in optimal T cell-mediated cytotoxicity (Clapp et al., 2021)
3.6 Bone marrow-on-a-chip
Hematotoxicity is a remarkable issue, in which various circulating mature blood cells or immature sensitive ones in the bone marrow are targeted by direct or indirect cytotoxicity from chemical compounds, such as drugs and other xenobiotics (Mahalingaiah et al., 2018). The evaluation of hematotoxicity as a routine part of drug safety assessment for early pre-clinical development and investigative hematopathology process is very critical because toxic effects to the hematopoietic system result in human severe damage due to myelosuppression, pancytopenia, infection, or clotting deficiencies (Gödel et al., 2021). Despite the straightforward monitoring of hematotoxicity, it is sometimes associated with severe complications, therefore this process is mainly performed in a therapeutic area or in vitro situation, which has a high profit-loss ratio. Due to the high proliferation rate and constant differentiation of immature blood cells in the bone marrow, as well as the giant diversity of the cells, which are in different stages of differentiation, it is not surprising that the effect of the toxicant on the cells would be cell-type selective related to their sensitivity to the drugs (Skaggs et al., 2019). Given the various mechanism of hematotoxicity on the hematopoietic stem cells, many protocols have been extended.
Given that the hematopoietic stem cells (HSCs) of the bone marrow have a rapid rate of proliferation and renewal, they could strongly be affected by the adverse effects of chemical compounds, which targeted their proliferation, differentiation, and function from the progenitors to the mature cells (Ruiz-Argüelles et al., 2018). Previously, for preclinical toxicity studies, different laboratory animals were used, which were associated with many limitations. In another developed model as a humanized bone marrow xenograft system, immunodeficient mice were transplanted with human CD34 cells, and the toxicity of the drugs was assessed on the human hematopoietic stem cells in vivo by examining the human mature chimeric cells in the peripheral blood of the mice (Martinov et al., 2021). The major differences between the drug sensitivity of the mouse- and human-derived cells were one of the main limitations of this model (Lõhmussaar et al., 2020). Subsequently, to bridge the gap between animal and clinical investigations, various in vitro methods were examined with desirable results (Kolle and Landsiedel, 2020). To gain more accurate and closer results to the human-species clinical context, as well as to reduce the rate of species-specific cross-reactive reagents and the number of animals used, it is worthy to apply the human base hematotoxicity assays, which employs human cells such as bone marrow or umbilical cord blood cells in vitro (Starling et al., 2020).
Various systems have been studied to achieve truthful and commercially viable in vitro assays for the evaluation of hematotoxicity (Mahalingaiah et al., 2018). In another achievement for toxicity assessment in human hematopoietic stem cells, two transcription factors (GATA2 and ETV2) were induced in human pluripotent stem cells (hiPSCs) to differentiate the cells to myeloid-hematopoietic ones in a suspension medium through a lentivirus (Elcheva et al., 2014; Slukvin and Uenishi, 2019). The test items were applied to the cells during the differentiation and the various adverse effects of the chemical compounds were studied by evaluating the cell surface markers fluctuation, viability, and analysis of target gene expression (Elcheva et al., 2014). These transcription factor-based differentiation systems provided a useful platform, which allowed monitoring the effects and mechanisms of myelosuppressive drugs on hiPSC-derived myeloid progenitor cells (Elcheva et al., 2014).
Unreliability of the animal models as a preclinical pattern and the failure of the 2D cell culture models to simulate dynamic modes of the native cellular microenvironment has led to the establishment of various 3D models in the evaluation of hematotoxicity (Dehne et al., 2019). Among the various developed methods, the bone marrow-on-chip model is considered a clinically-reliable model (Aleman et al., 2019). The human bone marrow-on-a-chip system as a micro physiological system (MPS) is an advanced in vitro model of the hematotoxicity assessment procedure, which uses 3D culturing methods and specific extracellular matrix ingredients with constant media perfusion that provide them to keep homeostasis (Dehne et al., 2019; Maharjan et al., 2020; Kefallinou et al., 2020). This artificial niche scaffold co-culture with stromal and mesenchymal cells (MSCs) mimics the in vivo biological properties under in vitro conditions (Bui et al., 2021). Given that the hematopoietic stem/progenitor cells (HSPCs) can sustain for prolonged culture periods in the in vivo-like microenvironment system, the effects of a chemical compound could be comprehensively evaluated, even in very late-stage maturation of the cells (Zhang et al., 2017a; Zhang et al., 2017b; Nies and Gottwald, 2017). To provide a more physiological relevance and increase the promoting factors in the system, vascular bed and further cell type could be added (Cochrane et al., 2019). To investigate the effects of the various metabolites on the HSPCs in bone marrow-on- a chip devices, the bone marrow model is co-cultured with the other organ models (Geraili et al., 2018). So far, different devices of the bone marrow-on-chip system have been developed. In such a model, the upper cell culture contained a cylindrical-shaped microchamber, detached from the lower medium perfusion one with a 20 μm thickness poly dimethyl siloxane porous membrane (Kefallinou et al., 2020). The median membrane with 6–8 μm pores not only acts as a dwelling for the MSCs but also allows the cells to be continuously nourished from the lower medium perfusion microchamber. The whole structure is sealed with the glass slide at the upper microchamber layer to monitor the expansion of the cells using a microscope. In this system, the MSCs are initially cultured in the upper microchamber to expand the stromal cell-matrix and subsequently to host the HSCs (Fig. 7) (Kefallinou et al., 2020).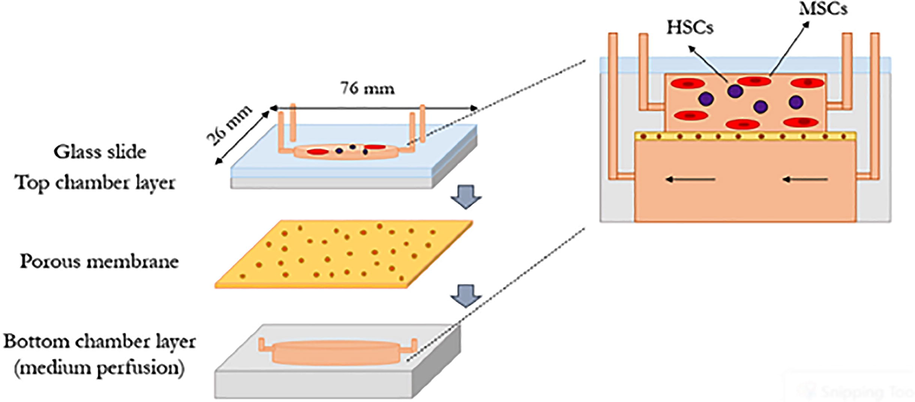
3D model of the bone marrow-on-a-chip device consisting of the upper cell culture microchamber layer and the lower medium perfusion microchamber layer, which are separated by a porous membrane.
Although MPS-based hematotoxicity screening is a comprehensive method to assay the toxicity of the drugs on the bone marrow hematopoietic stem cells, major challenges are involved in it (Lõhmussaar et al., 2020). Due to the extensive intercellular signaling and high complexity of the bone marrow niche, the reproducibility of a microenvironment similar to in vitro conditions is very troublous (Dehne et al., 2019). At present, in the field of MPS research, high efforts are being made to execute the body-on-a chip model as well as to set up the patient's bone marrow-on-a chip to evaluate the therapeutic effects of various drugs on the cells in the system.
4 Organ-on-a-chip technologies: Why are they necessary?
Microfluidics technologies are considered a gigantic step towards biomedicine. No doubt, the extraction of extensive information regarding biological systems requires novel innovations in biomedicine. In this regard, the organ-on-a-chip system has been proved as an emerging technology and helped understand the biological processes at the molecular level. This device has shown great potentiality to alter the healthcare system worldwide. The Organ-on-a-chip system is playing a paramount role in healthcare due to its rapid analyses characteristics as compared to the other traditional biomedical techniques. High data processing and storage approaches can be achieved by microfluidics techniques. In addition, this technology not only reduces the necessity of more reagents and bulky equipment but also saves time for performing extensive procedures while maintaining the sensitivity and specificity of conventional protocols. In a like manner, these miniaturized devices may contribute an auspicious role in the healthcare systems of developing nations by providing cost-effective and sophisticated analytical tools, especially in the context of the Sustainable Development Goals (SDGs). Despite the lower ability to recreate the complex nervous, immune, and endocrine systems as compared to the in vivo models, the organ-on-a-chip system may transform the biomedical field in the next few years due to its pronounced applications viz. high throughput drugs screening, therapeutics, biosensing and diagnostics.
5 Future perspectives
The extensive applications of organ-on-a-chip systems may provide a significant opportunity to model and explore different organs that have yet to be studied in their desired fields. Organ-on-a-chip biosystem has shown enormous advantages over in vivo model organisms. Currently, traditional cell culture methods are unable to provide controlled stimuli for stem cells. Organ-on-a-chip technology may not only establish new approaches for studying the differentiation of stem cells but also improve the existing stem cell therapy. In addition, these devices may provide an efficacious platform for investigating the genetic state of stem cells in the future by maintaining an ideal microenvironment. It is proposed that organ-on-a-chip technologies may be used in developing several human-disease models including pediatric and other rare diseases. Of note, designing and applications of human-body-on-a-chip would be the pivotal applications of organ-on-a-chip biosystems in the near future.
6 Conclusions
Organ-on-a-chip technologies have shown significant ability to translate in vitro studies into clinical research. Given the potentiality to maintain the organs’ microenvironment, this microfluidic device has been proved as a primary model to investigate the pharmacological attributes of specific drugs of interest. Several organ-on-a-chip systems have been prepared and the technology is creating a strong partnership between academia and industries. These microfluidics technologies may play decisive roles and pose an absolute platform in the discovery of effective drugs for vital organs associated with deadly diseases in a limited time. They can also be linked with an actuator system for on-demand or non-stop drug release. Microfluidics have opened new avenues in the fabrication of drug carriers and the development of direct drug administration chips. Manufacturing drug carriers that can produce a repeatable release pattern as well as the controlled release of several agents with different release profiles requires the application of microfluidic technology. In a nutshell, organ-on-a-chip technology is creating an exquisite picture of tissue engineering for establishing the foundation of a new era in the field of biomedicine.
Acknowledgement
M. G. was supported by the János Bolyai Research Scholarship (BO/00144/20/5) of the Hungarian Academy of Sciences. The research was supported by the ÚNKP-21-5-540-SZTE New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund. M.G. would also like to acknowledge the support of ESCMID’s “30 under 30” Award. The APC of this paper has been supported by the University of Szeged Open Access Fund.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mechanistic approach for toxic effects of bupropion in primary rat hepatocytes. Drug Res.. 2017;67(04):217-222.
- [Google Scholar]
- Deconstructed microfluidic bone marrow on-a-chip to study normal and malignant hemopoietic cell–niche interactions. Small Methods. 2019;15(43):1902971.
- [CrossRef] [Google Scholar]
- Hepatic organoids for microfluidic drug screening. Lab Chip. 2014;14(17):3290-3299.
- [Google Scholar]
- Brain-on-a-chip: A history of development and future perspective. Biomicrofluidics. 2019;13(5):051301.
- [CrossRef] [Google Scholar]
- Development of a renal microchip for in vitro distal tubule models. Biotechnol. Prog.. 2007;23(5):1245-1253.
- [Google Scholar]
- Evaluation of a liver microfluidic biochip to predict In vivo clearances of seven drugs in rats. J. Pharm. Sci.. 2014;103(2):706-718.
- [Google Scholar]
- Human lung small airway-on-a-chip protocol. Methods Mol. Biol. (Clifton, NJ).. 2017;1612:345-365.
- [Google Scholar]
- Cells and culture systems used to model the small airway epithelium. Lung. 2016;194(3):419-428.
- [Google Scholar]
- Advanced organ-on-a-chip devices to investigate liver multi-organ communication: focus on gut, microbiota and brain. Bioengineering. 2019;6(4):91.
- [CrossRef] [Google Scholar]
- Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol.. 1998;10(5):640-646.
- [Google Scholar]
- Recreating blood-brain barrier physiology and structure on chip: A novel neurovascular microfluidic bioreactor. Biomicrofluidics. 2015;9(5):054124.
- [CrossRef] [Google Scholar]
- Challenges and limitations of strategies to promote therapeutic potential of human mesenchymal stem cells for cell-based cardiac repair. Korean Circ. J.. 2021;51(2):97-113.
- [Google Scholar]
- Organ-on-chip applications in drug discovery: an end user perspective. Biochem. Soc. Trans.. 2021;49(4):1881-1890.
- [Google Scholar]
- Advancement of sensor integrated organ-on-chip devices. Sensors. 2021;21(4):1367.
- [CrossRef] [Google Scholar]
- Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev.. 2019;140:68-77.
- [Google Scholar]
- Microphysiological systems in the evaluation of hematotoxicities during drug development. Curr. Opin. Toxicol.. 2019;17:18-22.
- [Google Scholar]
- Engineered liver-on-a-chip platform to mimic liver functions and its biomedical applications: A review. Micromachines (Basel).. 2019;10(10)
- [Google Scholar]
- Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. Int. J. Mol. Sci.. 2017;18(5):1085.
- [CrossRef] [Google Scholar]
- Bioassay of saliva proteins: The best alternative for conventional methods in non-invasive diagnosis of cancer. Int. J. Biol. 2019;124:1246-1255.
- [Google Scholar]
- In vitro/vivo studies towards mechanisms of risperidone-induced oxidative stress and the protective role of coenzyme Q10 and N-acetylcysteine. Toxicol. Mech. 2016;26(7):520-528.
- [Google Scholar]
- Recent advances of microfluidic platforms for controlled drug delivery in nanomedicine. Des. Devel. Ther.. 2021;15:3881-3891.
- [Google Scholar]
- Direct induction of haematoendothelial programs in human pluripotent stem cells by transcriptional regulators. Nat. Commun.. 2014;5(1)
- [CrossRef] [Google Scholar]
- Receptor-mediated endocytosis in the proximal tubule. Annu. Rev. Physiol.. 2017;79(1):425-448.
- [Google Scholar]
- Triazole rizatriptan induces liver toxicity through lysosomal/mitochondrial dysfunction. Drug Res.. 2016;66(09):470-478.
- [Google Scholar]
- Mechanism of concentration and dilution of urine. In: Feher J., ed. Quantitative Human Physiology (second ed.). Boston: Academic Press; 2017. p. :730-739.
- [Google Scholar]
- Drug attrition during pre-clinical and clinical development: understanding and managing drug-induced cardiotoxicity. Pharmacol. Ther.. 2013;138(3):470-484.
- [Google Scholar]
- Integrated in vitro models for hepatic safety and metabolism: evaluation of a human Liver-Chip and liver spheroid. Arch. Toxicol.. 2019;93(4):1021-1037.
- [Google Scholar]
- Successful therapies for Alzheimer's disease: why so many in animal models and none in humans? Front. Pharmacol.. 2014;5:146.
- [Google Scholar]
- Mechanically stimulated contraction of engineered cardiac constructs using a microcantilever. IEEE Trans. Biomed Eng.. 2015;62(2):438-442.
- [Google Scholar]
- Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Sci. Rep.. 2018;8(1)
- [CrossRef] [Google Scholar]
- Controlling differentiation of stem cells for developing personalized organ-on-chip platforms. Adv. Healthc Mater.. 2018;7(2):1700426.
- [CrossRef] [Google Scholar]
- Hematologic rescue of CAR T-cell-mediated prolonged pancytopenia using autologous peripheral blood hematopoietic stem cells in a lymphoma patient. Hemasphere. 2021;5(3):e545.
- [Google Scholar]
- Organ-on-a-chip technology: turning its potential for clinical benefit into reality. Drug Discov. Today. 2019;24(5):1217-1223.
- [Google Scholar]
- Interfacial stress affects rat alveolar type II cell signaling and gene expression. Am. J. Physiol. Lung Cell. Mol. Physiol.. 2012;303(2):L117-L129.
- [Google Scholar]
- Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res.. 2017;352(2):420-426.
- [Google Scholar]
- Reconstituting organ-level lung functions on a chip. Science (New York, NY).. 2010;328(5986):1662-1668.
- [Google Scholar]
- Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab Chip. 2018;18(9):1298-1309.
- [Google Scholar]
- Identification of six putative human transporters with structural similarity to the drug transporter SLC22 family. Genomics. 2007;90(5):595-609.
- [Google Scholar]
- Compartmentalized platforms for neuro-pharmacological research. Curr. Neuropharmacol.. 2016;14(1):72-86.
- [Google Scholar]
- Primary human lung alveolus-on-a-chip model of intravascular thrombosis for assessment of therapeutics. Clin. Pharmacol. Ther.. 2018;103(2):332-340.
- [Google Scholar]
- Glial cells and their function in the adult brain: a journey through the history of their ablation. Front. Cell. Neurosci.. 2017;11:24.
- [Google Scholar]
- Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integr. Biol.. 2011;3(2):134-141.
- [Google Scholar]
- Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr. Biol.. 2013;5(9):1119-1129.
- [Google Scholar]
- A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10(1):36-42.
- [Google Scholar]
- Human proximal tubule epithelial cells cultured on hollow fibers: living membranes that actively transport organic cations. Sci. Rep.. 2015;5(1)
- [CrossRef] [Google Scholar]
- Bioengineered kidney tubules efficiently excrete uremic toxins. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Three-dimensional brain-like microenvironments facilitate the direct reprogramming of fibroblasts into therapeutic neurons. Nat. Biomed. Eng.. 2018;2(7):522-539.
- [Google Scholar]
- Human-derived organ-on-a-chip for personalized drug development. Curr. Pharm. Des.. 2019;24(45):5471-5486.
- [Google Scholar]
- Layered long-term co-culture of hepatocytes and endothelial cells on a transwell membrane: toward engineering the liver sinusoid. Biofabrication. 2013;5(4):045008.
- [CrossRef] [Google Scholar]
- Fabrication of a 3D microfluidic cell culture device for bone marrow-on-a-chip. Micro Nanoeng.. 2020;9:100075.
- [CrossRef] [Google Scholar]
- Kerman, B.E., Kim, H.J., Padmanabhan, K., Mei, A., Georges, S., Joens, M.S., et al., 2015. In vitro myelin formation using embryonic stem cells. Development (Cambridge, England). 142(12), 2213-2225.
- A microchip for high-throughput axon growth drug screening. Micromachines. 2016;7(7):114.
- [Google Scholar]
- Three-dimensional human liver-chip emulating premetastatic niche formation by breast cancer-derived extracellular vesicles. ACS Nano. 2020;14(11):14971-14988.
- [Google Scholar]
- A cell-based biosensor for real-time detection of cardiotoxicity using lensfree imaging. Lab Chip. 2011;11(10):1801.
- [CrossRef] [Google Scholar]
- 3D proximal tubule tissues recapitulate key aspects of renal physiology to enable nephrotoxicity testing. Front. Physiol.. 2017;8
- [CrossRef] [Google Scholar]
- Human-derived in vitro models used for skin toxicity testing under REACh. Handb. Exp. Pharmacol.. 2020;265:3-27.
- [Google Scholar]
- Lung-on-a-chip technologies for disease modeling and drug development. Biomed. Eng. Comput. Biol.. 2016;7(Suppl 1):17-27.
- [Google Scholar]
- current screening methodologies in drug discovery for selected human diseases. Mar. Drugs. 2018;16(8):279.
- [CrossRef] [Google Scholar]
- Organotypic liver culture models: meeting current challenges in toxicity testing. Crit. Rev. Toxicol.. 2012;42(6):501-548.
- [Google Scholar]
- Subcellular electrical stimulation of neurons enhances the myelination of axons by oligodendrocytes. PloS One. 2017;12(7):e0179642.
- [Google Scholar]
- An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng.. 2007;97(5):1340-1346.
- [Google Scholar]
- Renal reabsorption in 3D vascularized proximal tubule models. roc. Natl. Acad. Sci. U.S.A.. 2019;116(12):5399-5404.
- [Google Scholar]
- Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater.. 2017;16(3):303-308.
- [Google Scholar]
- Heart-on-a-chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett.. 2020;20(4):2585-2593.
- [Google Scholar]
- Human-derived model systems in gynecological cancer research. Trends in Cancer. 2020;6(12):1031-1043.
- [Google Scholar]
- On-chip construction of liver lobule-like microtissue and its application for adverse drug reaction assay. Anal. Chem.. 2016;88(3):1719-1727.
- [Google Scholar]
- An in vitro model of hematotoxicity: differentiation of bone marrow–derived stem/progenitor cells into hematopoietic lineages and evaluation of lineage-specific hematotoxicity. Curr. Protoc. Toxicol.. 2018;76(1):e45
- [Google Scholar]
- 3D immunocompetent organ-on-a-chip models. Small Methods. 2020;4(9):2000235.
- [CrossRef] [Google Scholar]
- An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin Drug Deliv. 2019;16(4):331-345.
- [Google Scholar]
- A linked organ-on-chip model of the human neurovascular unit reveals the metabolic coupling of endothelial and neuronal cells. Nat. Biotechnol.. 2018;36(9):865-874.
- [Google Scholar]
- Building the next generation of humanized hemato-lymphoid system mice. Front. Immunol.. 2021;12
- [CrossRef] [Google Scholar]
- Physiology of Cholngiocytes. In: Said H.M., ed. Physiology of the Gastrointestinal Tract (Sixth Edition). Academic Press; 2018. p. :1003-1023.
- [Google Scholar]
- Chip-based liver equivalents for toxicity testing – organotypicalness versus cost-efficient high throughput. Lab Chip. 2013;13(18):3481-3495.
- [Google Scholar]
- Distal convoluted tubule. Compr Physiol.. 2015;5(1):45-98.
- Organ-on-chip models: Implications in drug discovery and clinical applications. J. Cell. Physiol.. 2019;234(6):8352-8380.
- [Google Scholar]
- Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181(4):905-913.e7.
- [Google Scholar]
- Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng.. 2017;1(5)
- [CrossRef] [Google Scholar]
- A fibrin-based tissue-engineered renal proximal tubule for bioartificial kidney devices: development, characterization and in vitro transport study. Int. J. Tissue Eng.. 2013;2013:1-10.
- [Google Scholar]
- Microphysiological systems to study tumor-stroma interactions in brain cancer. Brain Res. Bull.. 2021;174:220-229.
- [Google Scholar]
- Artificial hematopoietic stem cell niches-dimensionality matters. Adv. Tissue Eng. Regen. Med. Open Access. 2017;2(5):236-247.
- [Google Scholar]
- Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Engineered 3D vascular and neuronal networks in a microfluidic platform. Sci. Rep.. 2018;8(1):5168.
- [Google Scholar]
- Compartmentalization of human stem cell-derived neurons within pre-assembled plastic microfluidic chips. J. Vis. Exp.. 2019;147:e59250
- [Google Scholar]
- Alzheimer's disease drug development and the problem of the blood-brain barrier. Alzheimers. Dement.. 2009;5(5):427-432.
- [Google Scholar]
- Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomed. Microdevices. 2009;11(6):1145-1153.
- [Google Scholar]
- A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer's disease. Nat. Neurosci.. 2018;21(7):941-951.
- [Google Scholar]
- Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication. 2018;11(1):015002.
- [CrossRef] [Google Scholar]
- A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun.. 2019;10(1)
- [CrossRef] [Google Scholar]
- Pirzad Jahromi, G., Seidi, S., Sadr, S.S., Shabanzadeh, A.P., Keshavarz, M., Kaka. G.R., et al., 2012. Therapeutic effects of a combinatorial treatment of simvastatin and bone marrow stromal cells on experimental embolic stroke. Basic Clin. Pharmacol. Toxicol.. 110(6), 487-493.
- Multipotent bone marrow stromal cell therapy promotes endogenous cell proliferation following ischemic stroke. Clin. Exp. Pharmacol. Physiol.. 2015;42(11):1158-1167.
- [Google Scholar]
- Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol.. 2017;66(1):212-227.
- [Google Scholar]
- A cocktail of metabolic probes demonstrates the relevance of primary human hepatocyte cultures in a microfluidic biochip for pharmaceutical drug screening. Int. J. Pharm.. 2011;408(1-2):67-75.
- [Google Scholar]
- Microfluidically supported biochip design for culture of endothelial cell layers with improved perfusion conditions. Biofabrication. 2015;7(1):015013.
- [CrossRef] [Google Scholar]
- Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc. Natl. Acad. Sci. U.S.A.. 2014;111(23):8506-8511.
- [Google Scholar]
- Flow stimulated endocytosis in the proximal tubule. Curr. Opin. Nephrol. Hypertens.. 2015;24(4):359-365.
- [Google Scholar]
- Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform. Acta Biomater.. 2020;106:124-135.
- [Google Scholar]
- Interfacial sensing by alveolar type II cells: a new concept in lung physiology? Am. J. Physiol. Cell Physiol.. 2011;300(6):C1456-C1465.
- [Google Scholar]
- For whom the cells pull: Hydrogel and micropost devices for measuring traction forces. Methods (San Diego, Calif).. 2016;94:51-64.
- [Google Scholar]
- Modifications to the “Classical” autologous hematopoietic stem cell transplantation in multiple sclerosis: a less toxic approach is feasible and improves the neurological condition. A Mexican perspective. Biol. Blood Marrow Transplant.. 2018;24(3):S125-S126.
- [Google Scholar]
- A heart-on-a-chip platform for online monitoring of contractile behavior via digital image processing and piezoelectric sensing technique. Med. Eng. Phys.. 2020;75:36-44.
- [Google Scholar]
- Human iPSC-derived endothelial cells and microengineered organ-chip enhance neuronal development. Stem Cell Rep.. 2018;10(4):1222-1236.
- [Google Scholar]
- A biomimetic multicellular model of the airways using primary human cells. Lab Chip. 2014;14(17):3349-3358.
- [Google Scholar]
- Organs-on-a-Chip: A future of rational drug-design. J. Biosci. Med.. 2017;05(09):22-28.
- [Google Scholar]
- A 3D cell culture organ-on-a-chip platform with a breathable hemoglobin analogue augments and extends primary human hepatocyte functions in vitro. Front. Mol. Biosci.. 2020;7:568777
- [Google Scholar]
- I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology. Acta Biomater.. 2017;48:68-78.
- [Google Scholar]
- Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol.. 2015;3:2.
- [Google Scholar]
- Comparison of immune system development in nonclinical species and humans: Closing information gaps for immunotoxicity testing and human translatability. Reprod. Toxicol.. 2019;89:178-188.
- [Google Scholar]
- Arterial identity of hemogenic endothelium: a key to unlock definitive hematopoietic commitment in human pluripotent stem cell cultures. Exp. Hematol.. 2019;71:3-12.
- [Google Scholar]
- A role for 3D printing in kidney-on-a-chip platforms. Curr. Transplant. Rep.. 2016;3(1):82-92.
- [Google Scholar]
- Prenatal exposure to per-and polyfluoroalkyl substances, umbilical cord blood dna methylation, and cardio-metabolic indicators in newborns: the healthy start study. Environ. Health Persp.. 2020;128(12):127014.
- [CrossRef] [Google Scholar]
- A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip. 2015;15(5):1302-1310.
- [Google Scholar]
- Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663-676.
- [Google Scholar]
- Integration of neurogenesis and angiogenesis models for constructing a neurovascular tissue. Sci. Rep.. 2017;7(1):17349.
- [Google Scholar]
- Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 2019;24(6):995-1005.e6.
- [Google Scholar]
- Functional coupling of human microphysiology systems: intestine, liver, kidney proximal tubule, blood-brain barrier and skeletal muscle. Sci. Rep.. 2017;7:42296.
- [Google Scholar]
- A disease model of diabetic nephropathy in a glomerulus-on-a-chip microdevice. Lab Chip.. 2017;17(10):1749-1760.
- [Google Scholar]
- Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng.. 2017;114(1):184-194.
- [Google Scholar]
- Development of a microphysiological model of human kidney proximal tubule function. Kidney Int.. 2016;90(3):627-637.
- [Google Scholar]
- Accessing 3D microtissue metabolism: Lactate and oxygen monitoring in hepatocyte spheroids. Biosens. Bioelectron.. 2017;87:941-948.
- [Google Scholar]
- Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng.. 2001;3(1):335-373.
- [Google Scholar]
- Kidney-on-a-chip technology for drug-induced nephrotoxicity screening. Trends Biotechnol.. 2016;34(2):156-170.
- [Google Scholar]
- The human hepatic cytochromes P450 involved in drug metabolism. Crit. Rev. Toxicol.. 1992;22(1):1-21.
- [Google Scholar]
- Organ-on-a-chip: recent breakthroughs and future prospects. BioMed Eng. OnLine. 2020;19(1)
- [CrossRef] [Google Scholar]
- An experimental investigation of micro pulsating heat pipes. Micromachines (Basel).. 2014;5(2):385-395.
- [Google Scholar]
- Roles of fluid shear stress and retinoic acid in the differentiation of primary cultured human podocytes. Exp. Cell Res.. 2017;354(1):48-56.
- [Google Scholar]
- Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip. Future Sci. OA. 2017;3(2):FSO187.
- [CrossRef] [Google Scholar]
- Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceed. Natl. Acad. Sci. U.S.A.. 2017;114(12):E2293-E2302.
- [Google Scholar]
- Development of a functional glomerulus at the organ level on a chip to mimic hypertensive nephropathy. Sci. Rep.. 2016;6(1)
- [CrossRef] [Google Scholar]
- Liver injury-on-a-chip: microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip. 2015;15(23):4467-4478.
- [Google Scholar]
- Proximal nephron. Compr Physiol.. 2013;3(3):1079-1123.







