Translate this page into:
Silver nanoparticles mediate differential responses in some of liver and kidney functions during skin wound healing
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 17 June 2010
Abstract
Silver has been used for the treatment of medical ailments for over 100 years due to its natural antibacterial and antifungal properties. In this study, silver nanoparticles were synthesized, evaluated for its wound healing activity and its effect in some functions of the liver and kidney. We investigated the wound-healing properties of silver nanoparticles in an animal model and found that rapid healing and improved cosmetic appearance occur within 15 days. Furthermore, we showed that silver nanoparticles exert positive effects through their antimicrobial properties, reduction in wound inflammation, and modulation in some of liver and kidney functions during skin wound healing. These results have given insight into the actions of silver and have provided a novel therapeutic direction for wound treatment in clinical practice.
Keywords
Silver nanoparticle
Wound healing
GOT
GPT
Creatinine
Urea
1 Introduction
Silver nanoparticles had been utilized in various aspects like spectrally selective coating for solar energy absorption, optical receptors, polarizing filters, catalysts in chemical reaction, biolabelling and antimicrobial agents (Liechty et al., 2000). Application of silver nanoparticles in these fields is dependent on the ability to synthesize particles with different chemical composition, shape, size and mono-dispersity. Development of simple and ecofriendly method would help in developing further interest in the synthesis and application of metallic nanoparticles. The use of silver in the past has been restrained by the need to produce silver as a compound, thereby increasing the potential side effects. Nanotechnology has provided a way of reducing pure silver nanoparticles. This system also markedly increases the rate of silver on release.
The ultimate goal for wound healing is a speedy recovery with minimal scarring and maximal function. Wound healing proceeds through an overlapping pattern of events including coagulation, inflammation, proliferation, matrix and tissue remodeling.
Fan and Bard (1999) published studies of silver nanoparticles on wound healing are sparse, and the mechanism of action remains unknown. Herein we report that silver nanoparticles can promote wound healing and reduce scar appearance in a dose-dependent manner. Furthermore, our studies show that silver nanoparticles act by decreasing inflammation and no side effect on the liver and kidney functions through rat model. The potential benefits of silver nanoparticles in all wounds can therefore be enormous.
2 Materials and methods
2.1 Animal experiment
Nine-week old Male Wistar rats (53.2–106.9 g) from the Laboratory Animal Unit of King Saud University, Research centre – Saudi Arabia – Riyadh. All animals were reared on a standard laboratory. They were kept in a room where the temperature (20 ± 10 °C), humidity (25–35%), and day:night cycle (12:12 light:dark) were controlled.
Injury template was fashioned from a plastic 60-mL syringe by cutting a window (10 × 5 mm) into the back with the opposite half removed. The dorsum of each rat was carefully shaved beside the tail and laid on the injury template following anaesthesia. This model would achieve approximately 10% deep partial thickness injury of total body surface area. The rate was injected with sterile saline intraperitoneally (1 mL) for fluid resuscitation.
2.2 Synthesis of silver nanoparticles
The formation of silver nanoparticles can be observed by a change in color since small nanoparticles of silver are yellow. Add 30 mL of sodium borohydride (NaBH4) to an Erlenmeyer flask. Add a magnetic stir bar and place the flask in an ice bath on a stir plate. Stir and cool the liquid for about 20 min. Drip 2 mL of silver nitrate (AgNO3) into the stirring NaBH4 solution at approximately drop per second. Stop stirring as soon as all of the AgNO3 is added. The addition of a few drops of 1.5 M sodium chloride (NaCl) solution causes the suspension to turn darker yellow, then gray as the nanoparticles aggregate. Transfer a small portion of the solution to a test tube. Add a drop of 0.3% polyvinyl pyrrolidone (PVP). PVP prevents aggregation. Addition of NaCl solution then has no effect on the color of the suspension. Add enough solid polyvinyl alcohol (PVA) to give a 4% solution (Solomon et al., 2007).
2.3 UV–visible spectral analysis
In metal nanoparticles such as in silver, the conduction band and valence band lie very close to each other in which electrons move freely. These free electrons give rise to a surface plasmon resonance (SPR) absorption band (Taleb et al., 1998; Noginov et al., 2006; Link and El-Sayed, 2003; Kreibig and Vollmer, 1995), occurring due to the collective oscillation of electrons of silver nano particles in resonance with the light wave (Nath et al., 2007). Classically, the electric field of an incoming wave induces a polarization of the electrons with respect to much heavier ionic core of silver nanoparticles. As a result a net charge difference occurs which in turn acts as a restoring force. This creates a dipolar oscillation of all the electrons with the same phase. When the frequency of the electromagnetic field becomes resonant with the coherent electron motion, a strong absorption takes place, which is the origin of the observed color. Here the color of the prepared silver nanoparticles is dark reddish brown. This absorption strongly depends on the particle size, dielectric medium and chemical surroundings (Noginov et al., 2006; Link and El-Sayed, 2003). Small spherical nano particles (<20 nm) exhibit a single surface plasmon band (He et al., 2002). The absorption peak (SPR) is obtained in the visible range at 410 nm in Fig. 1. With the above mentioned concentration. The stability of silver nanoparticles is observed for 4 months and it shows a SPR peak at the same wavelength.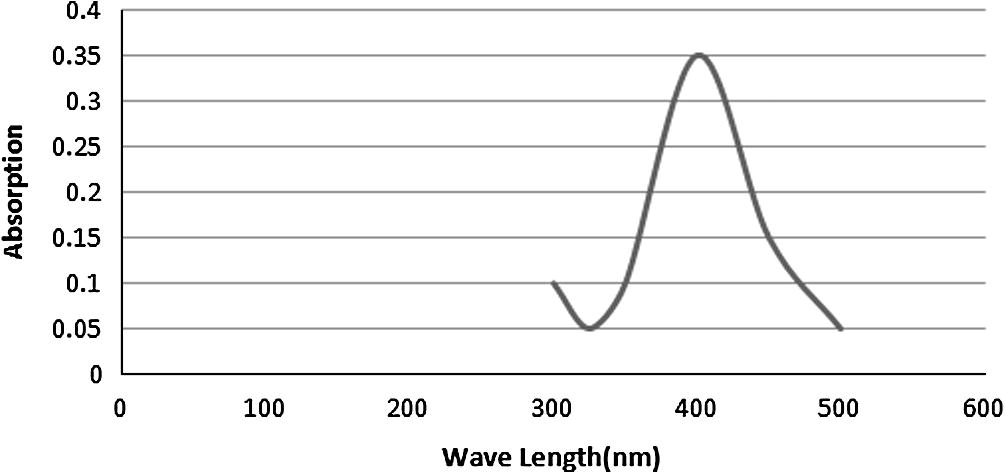
UV–vis absorption spectra.
2.4 Transmission electron microscopy (TEM) analysis
A TEM image of the prepared silver nano particles is shown in the Fig. 1. The Ag nano particles are spherical in shape with a smooth surface morphology. The diameter of the nano particles is found to be approximately 9 nm. TEM image Fig. 2 also shows that the produced nano particles are more or less uniform in size and shape.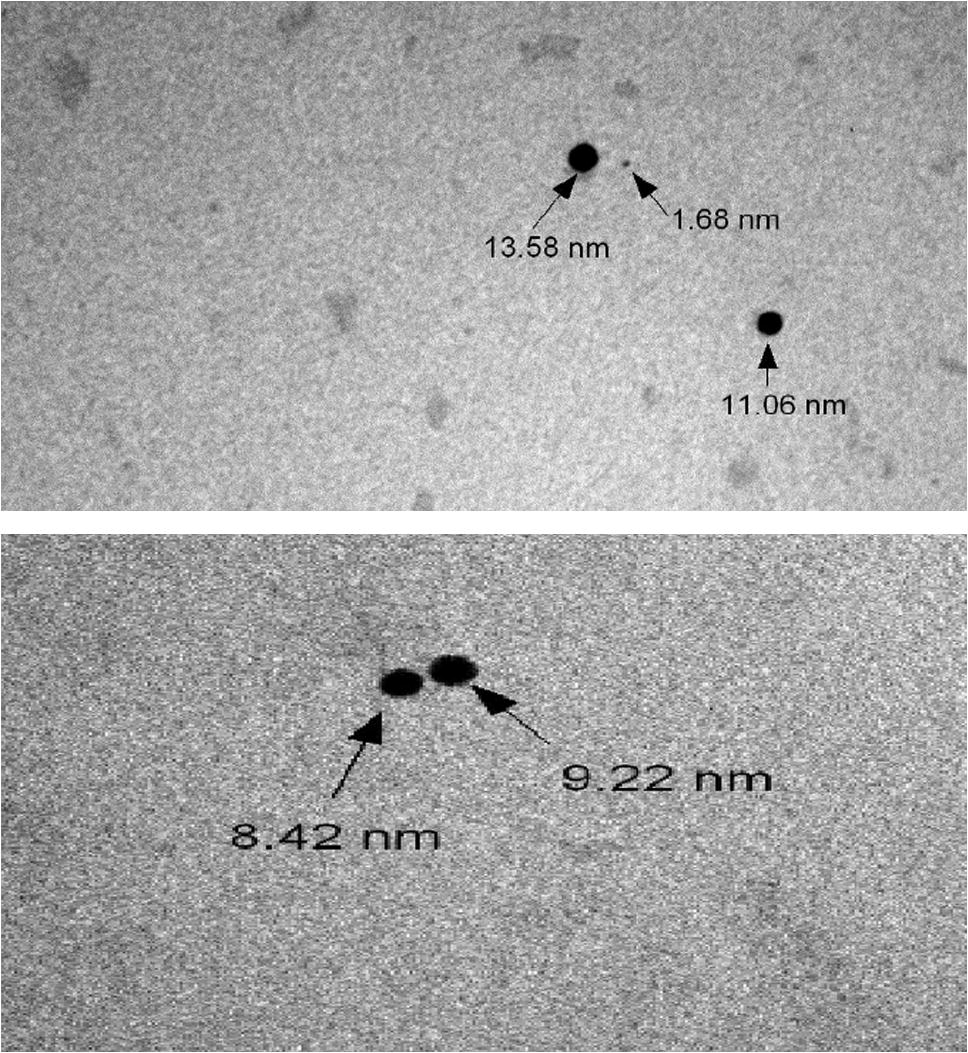
TEM image.
2.5 Statistical analysis
The results were expressed as mean (mean ± SD). Data were analyzed statistically using one-way analysis of variance followed by t test. A value of (P < 0.05) was considered statistically significant.
3 Results
Silver nanoparticles promote healing and achieve better cosmesis. In our control model (Noginov et al., 2006) rats, the deep partial-thickness wounds normally healed after (27 ± 1.99) days. In animals treated with silver nanoparticles (NP). A single dose of (5 g/kg) of body weight was orally gavaged into (Moore and O’Garra, 1993) rats and observed for any sign of toxicity for the next 14 days, these healed in (16 ± 68) days, whereas (Fig. 3A). The rate of healing in the two groups was also compared. As with healing time, rate of healing was increased in animals treated with NP (Fig. 3B). These observations indicate that wound healing is accelerated by silver nanoparticles.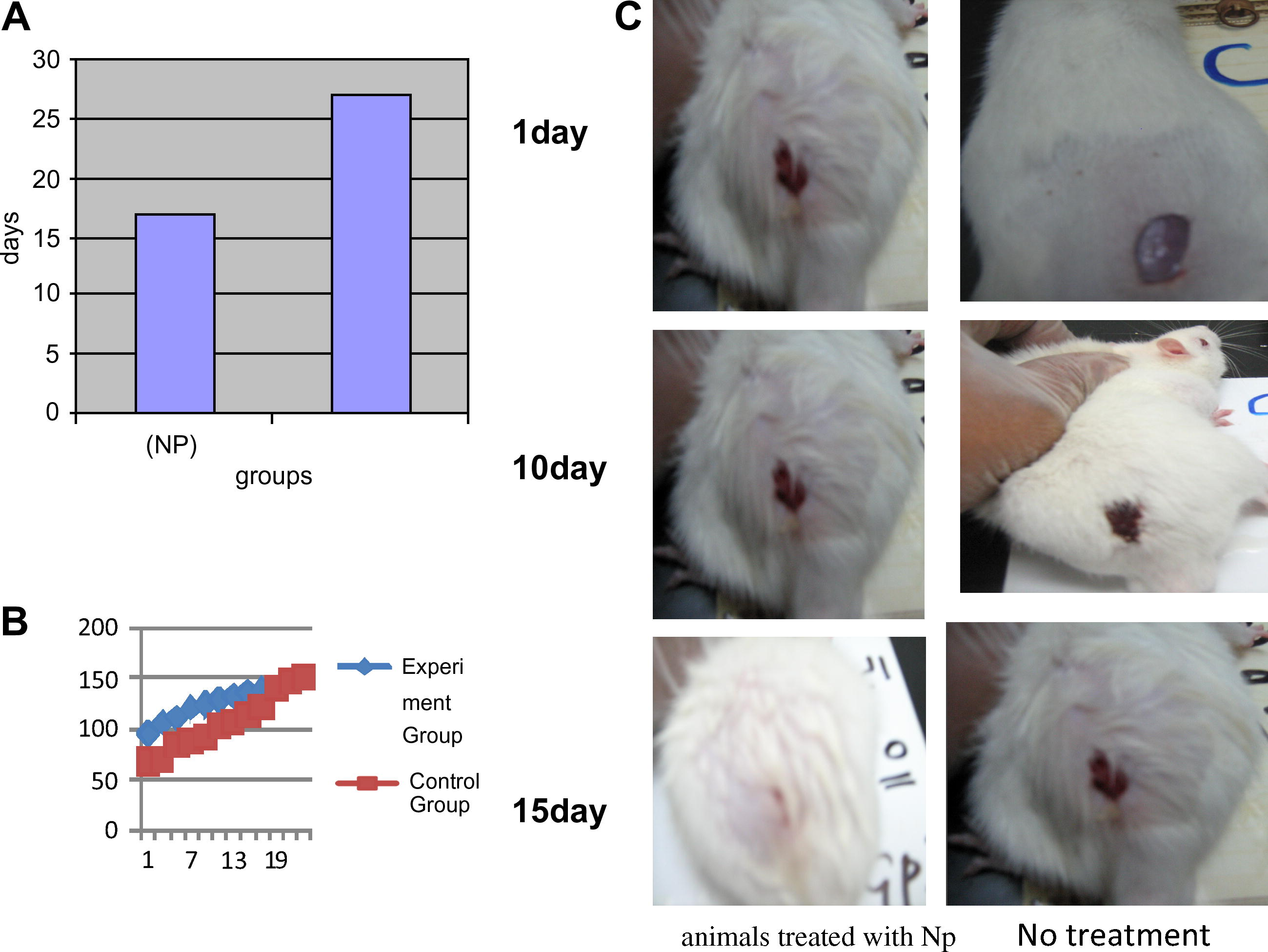
Silver nanoparticles accelerate wound healing and achieve superior cosmetic outcome: (A) time taken for wounds to heal in animals treated with silver nanoparticles (Np), and no treatment (NT). (B) The rate of wound healing in wound animals treated with (Np) or no treatment (NT). (C) Photographs of wounds from animals treated with Np, or no treatment on days 0, 10, and 15 after burn injury.
We next compared the appearance of healed wounds. We found that wounds in the Np group showed the most resemblance to normal skin, with less hypertrophic scarring and nearly normal hair growth on the wound surface, with a thin epidermis and nearly normal hair follicles (Fig. 3C).
The inflammatory response is an important component of wound healing. Within wounds, various inflammatory mediators are secreted to modulate the healing process.
In normal wound healing, the potential for pro- and anti-inflammatory cytokines is certainly present, and the inflammatory response is entirely appropriate.
To accomplish successful wound repair and tissue regeneration, the inflammatory response must be tightly regulated in vivo. Among the contributors to delayed wound healing, prolonged inflammatory response is undoubtedly one of the important factors.
A vital mediator in this anti-inflammatory cascade appears to be IL-10, which can be produced by keratinocytes as well as inflammatory cells involved in the healing process, including T lymphocytes, macrophages, and B lymphocytes (Moore and O’Garra, 1993). One of the unique actions of IL-10 is its ability to inhibit the synthesis of pro-inflammatory cytokines which also include IL-6 (Fiorentino et al., 1991; de Waal Malefyt et al., 1991). IL-10 also inhibits leukocyte migration toward the site of inflammation, in part, by inhibiting the synthesis of several chemokines, including monocyte chemo attractant protein-1 (MCP-1) and macrophage inflammatory protein-1a (MIP-1a).
Both of the C–C chemokines promote monocyte accumulation, and MIP-1a is also a potent neutrophil chemoattractant in rats (Ajuebor et al., 1999). Moreover, studies have shown that MCP-1 and MIP-1a play prominent roles in macrophage recruitment into wounds during wound repair (Alam et al., 1994). Within wounds, IL-6 is also secreted by polymer phonuclear cells (PMNs) and fibroblasts. IL-6 has been recognized as an initiator of events in the physiological alterations of inflammation after injury (DiPietro, 1995; Engelhardt et al., 1998). In fact, an increase in IL-6 concentration parallels the increase in PMN count locally within acute wounds. IL-6 promotes inflammation through monocyte and macrophage chemotaxis and activation (Paquet and Piérard, 1996). Decreased IL-6 may result in fewer neutrophils and macrophages recruited to the wound and less cytokines being released in the wound with subsequently lower paracrine stimulation of cellular proliferation, fibroblast and keratinocyte migration, and extracellular matrix production (Biswas et al., 1998). This lack of amplification of the inflammatory cytokine cascade may be important in providing a permissive environment for scar less wound repair to proceed. Silver induced neutrophil apoptosis and decreased MMP activity may also contribute to the overall decrease in the inflammatory response and as a consequence, an increased rate of wound healing.
In the study reported herein, better cosmetic appearance was observed in animals treated with silver nanoparticles. In terms of wound healing, enhanced expression of TGF-b1mRNA is found in both keloids and hypertrophic scars. Cumulative evidence has suggested that TGF-b1 plays an important role in tissue fibrosis and post-injury scarring. We think that lower levels of TGF-b coincided temporally with increased levels of IFN-g until wound closure in the ND group.
As IFN-g has been demonstrated as a potent antagonist of fibro genesis through its ability to inhibit fibroblast proliferation and matrix production, its control on TGF-b production may play a role (Duncan and Berman, 1985).
3.1 Body weight gain
The body weight gain of rats treated with silver nanoparticles (Np) was similar to that of controls no treatment (NT) (Tables 1 and 2) while combined administration of this treatment resulted in a significant progress ready during the first 2 weeks.
Test
1
2
3
4
5
Weight before treatment (g)
91.9
85.1
101.8
106.9
89.4
Weight after treatment (g)
141.7
145.9
158.2
162.8
140.4
Intermediate values used in calculations:
t = 27.5266, df = 4, standard error of difference = 1.990
Group
Group one
Group two
Mean
95.020
149.800
SD
9.039
10.109
SEM
4.042
4.521
N
5
5
Test
1
2
3
4
5
6
7
8
9
10
Weight before treatment (g)
82.8
70.1
53.2
83.1
74
63.7
65.5
72.2
58
53.7
Weight after treatment (g)
120.3
119.7
108.4
159.5
128.7
121.5
129.3
138.4
105.5
91.3
Intermediate values used in calculations:
t = 7.9659, df = 18, standard error of difference = 6.858
Group
Group one
Group two
Mean
67.630
122.260
SD
10.812
18.800
SEM
3.419
5.945
N
10
10
The final body weight of the rats was un treated increase by 34%, 35%, 36% and 42% (P < 0.0001). This difference is considered to be extremely statistically significant. The analysis of variance has shown that increase in body weight gain. While the experimental group treated with silver nanoparticles had increased in body weight gain 39%, 41%, 45% and 51% (P < 0.0001). This difference is considered to be extremely statistically significant. The increase in body weight gain the same result between two group (ND and NT) an effects the silver nanoparticles was also noted.
3.2 Liver function changes
GPT were significantly increased after 7th day and 15th days to experimental group (NP) when compared with the control group (NT) (Table 3, Figs. 4 and 5).
Time
1 day
7 day
15 day
GOT (Np)
167
182.58
162.2
GPT (Np)
25.38
27.78
32.16
GOT (NT)
152.11
161.1
155.1
GPT (NT)
22.26
33.71
24.27
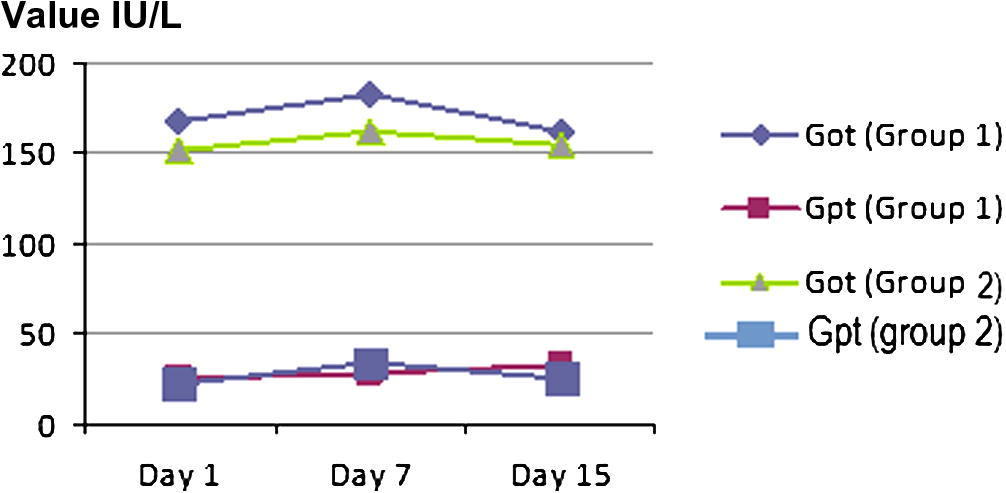
The value of (GOT and GPT) liver function in (1, 7, and 15) for the two groups.
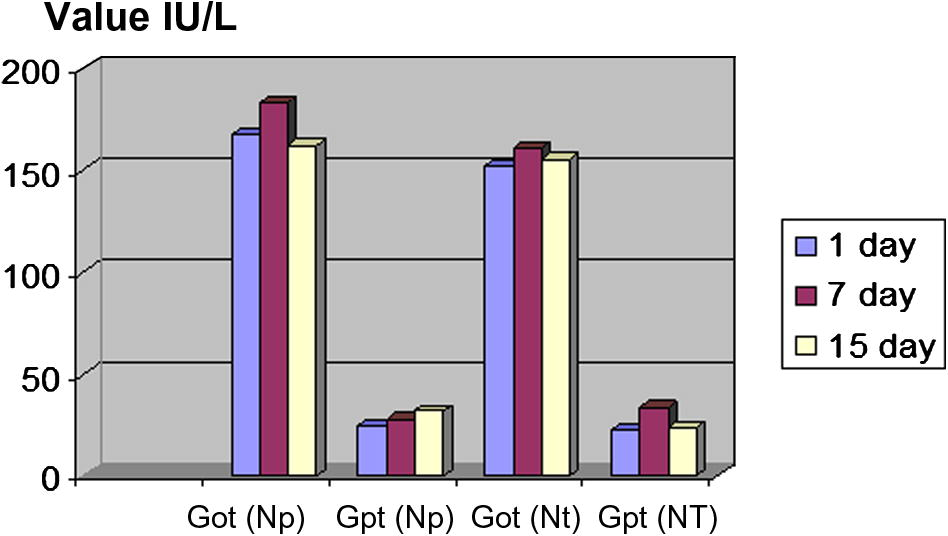
The value of (Got, GPT).
GOT were significantly increased after 7th day and then decreased 15th days to experimental group (NP) and the control group (NT) Table 3.
Glutamic–pyruvic transaminase (GPT) – also known as alanine aminotransferase or ALT – is a cytoplasmic hepatocellular enzyme, whose increase in blood is highly indicative for liver damage e.g. by hepatitis, cirrhosis or hepatic tumors.
Changes of liver function (GPT) no differed among rats treated with silver nanoparticles (Np), and rats no treatment with silver nanoparticles (NT). Unpaired t test results this difference is considered to be not statistically significant (95%) (t = 0.1125, df = 13, standard error of difference = 5.069).
In most types of liver disease (GPT) activity is higher than that of glutamicoxaloacetic transaminase (GOT); an exception is in alcoholic hepatitis. The ratio of GPT and GOT may provide further information about the severity of the disease and may serve as a prognostic indicator (Tables 4 and 5).
Group
Group one
Group two
Mean
28.420
27.850
SD
12.163
7.612
SEM
5.440
2.407
N
5
10
Group
Group one
Group two
Mean
170.580
163.300
SD
29.796
29.319
SEM
13.325
9.271
N
5
10
Changes of liver function (GOT) no differed among rats treated with silver nanoparticles (Np), and rats no treatment with silver nanoparticles (NT). Unpaired t test results this difference is considered to be not statistically significant (95%) t = 0.4511, df = 13, standard error of difference = 16.139.
3.3 Kidney function changes
Urea is a waste product formed from the breakdown of proteins. Urea is usually passed out in the urine. A high blood level of urea (‘uraemia’) indicates that the kidneys may not be working properly, or that you are dehydrated (have a low body water content). Creatinine is a waste product made by the muscles. Creatinine passes into the bloodstream, and is usually passed out in urine. A high blood level of creatinine indicates that the kidneys may not be working properly. Creatinine is usually a more accurate marker of kidney function than urea.
Changes of some kidney function (creatinine and urea) no differed among rats treated with silver nanoparticles (Np), and no treatment (NT) Tables 6 and 7.
Test
1
2
3
4
5
Creatinine
<44.2
<44.2
<44.2
<44.2
<44.2
Urea
50.3
54.1
52.2
47.5
55.6
Test
1
2
3
4
5
6
7
8
9
10
Creatinine
<44.2
<44.2
<44.2
<44.2
<44.2
<44.2
<44.2
<44.2
<44.2
<44.2
Urea
61
46.9
52.8
50.4
47.8
45.5
52.7
46.5
42.8
46.3
Unpaired t test results (95%) this difference is considered to be not statistically significant (t = 1.0430, df = 13, standard error of difference = 2.560) Table 8 and the same level of creatinine (Tables 6 and 7).
Group
Group one
Group two
Mean
51.940
49.270
SD
3.183
5.201
SEM
1.424
1.645
N
5
10
3.4 Size of the lesion
Size of the lesion for experimental group (P < 0.0001). By conventional criteria, this difference is considered to be extremely statistically significant (95%) (t = 8.4459, df = 12, standard error of difference = 0.399) (Table 9).
Group
Group one
Group two
Mean
6.2777
2.9092
SD
3.4212
2.1436
SEM
0.9489
0.5945
N
13
13
Size of the lesion for control group (P < 0.0001). By conventional criteria, this difference is considered to be extremely statistically significant (95%, t = 51.8715, df = 19, standard error of difference = 0.090) (Table 10) and that mean when we use silver nanoparticles we found. The rate of healing was increased in animals treated with NP. These observations indicate that wound healing is accelerated by silver nanoparticles (Table 11, Figs. 6 and 7).
Group
Group one
Group two
Mean
7.6700
2.9810
SD
2.2265
1.8288
SEM
0.4979
0.4089
N
20
20
Time
Experiment (NP)
Control (NT)
Length (mm)
Width (mm)
Length (mm)
Width (mm)
Day 1
10.16
5.5
10.7
5.54
Day 2
10.1
5.68
10.64
5.5
Day 3
9.59
5.24
10.46
5.24
Day 4
8.93
4.67
10.3
5.1
Day 5
8.42
4.16
10.02
4.92
Day 8
7.49
3.49
9.4
4.42
Day 9
7
3.06
9.02
4.18
Day 10
6.17
2.49
8.78
3.9
Day 11
5.53
1.86
8.52
3.64
Day 12
9.88
1.21
7.88
3.16
Day 15
2.63
0.16
7.36
2.62
Day 16
0.67
0
6.96
2.3
Day 17
0.04
0
6.7
2.16
Day 18
6.5
2
Day 19
6.12
1.74
Day 22
5.68
1.24
Day 23
5.24
0.96
Day 24
4.78
0.6
Day 25
4.38
0.34
Day 26
3.86
0.02
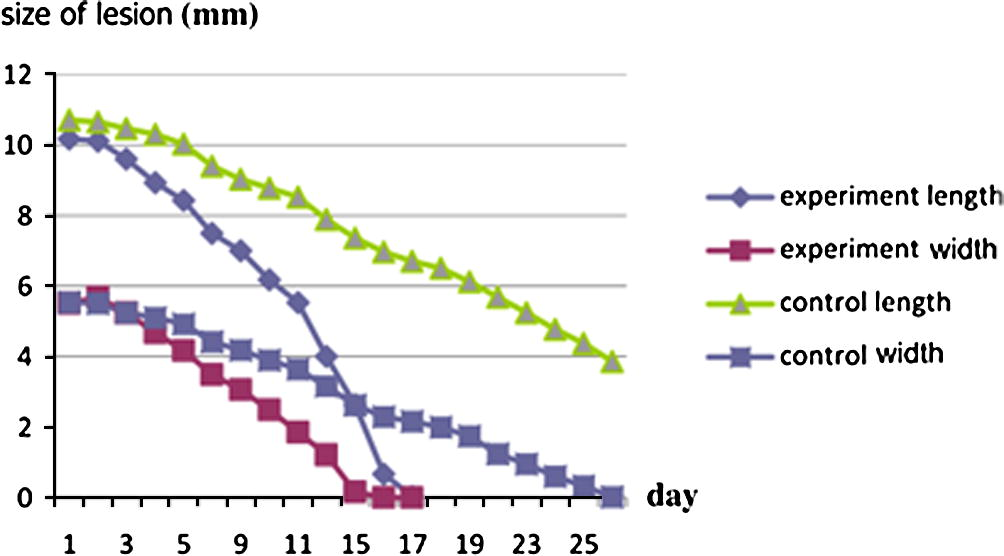
Size of lesion length and width between two groups.
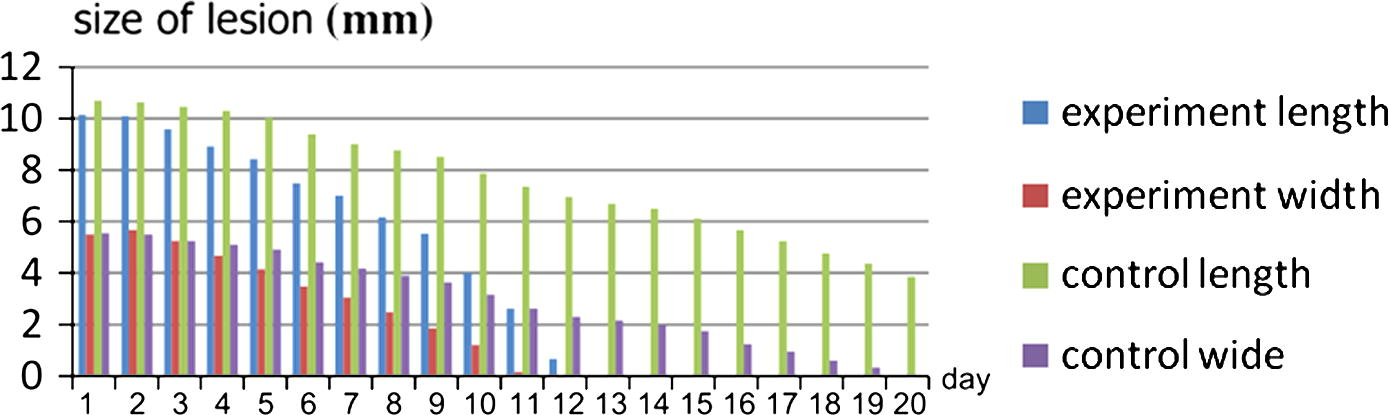
Length and width between two groups.
References
- Regulation of macrophage inflammatory protein-1a expression and function by endogenous interleukin-10 in a model of acute inflammation. Biochem. Biophys. Res. Commun.. 1999;255:279-282.
- [Google Scholar]
- Macrophage inflammatory protein-1 alpha and monocyte chemoattractant peptide-1 elicit immediate and late cutaneous reactions and activate murine mast cells in vivo. J. Immunol.. 1994;152(3):1298-1303.
- [Google Scholar]
- Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91(1):258-265.
- [Google Scholar]
- Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med.. 1991;174:1209-1220.
- [Google Scholar]
- Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4(4):233-240.
- [Google Scholar]
- Gamma-interferon is the lymphokin responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J. Exp. Med.. 1985;162:516-527.
- [Google Scholar]
- Chemokines IL-8, GROα, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am. J. Pathol.. 1998;153:1849-1860.
- [Google Scholar]
- Imaging of biological macromolecules on mica in humid air. Proc. Natl. Acad. Sci. USA. 1999;96:14222-14227.
- [Google Scholar]
- IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol.. 1991;146(10):3444-3451.
- [Google Scholar]
- Preparation of polychrome silver nanoparticles in different solvents. J. Mater. Chem.. 2002;12:3783-3786.
- [Google Scholar]
- Optical Properties of Metal Clusters. Berlin: Springer; 1995.
- Fetal wound repair results in scar formation in interleukin-10 (IL-10) deficient mice in a syngeneic murine model of scarless fetal wound repair. J. Pediatr. Surg.. 2000;35(6):866-873.
- [Google Scholar]
- Optical properties and ultrafast dynamics of metallic nanocrystals. Annu. Rev. Phys. Chem.. 2003;54:331-366.
- [Google Scholar]
- Synthesis of CdS and ZnS quantum dots and their applications in electronics. Nanotrends – J. Nanotechnol. Appl.. 2007;02(03)
- [Google Scholar]
- The effect of gain and absorption on surface plasmos in metal nanoparticles. Appl. Phys. B. 2006;86(3):455-460.
- [Google Scholar]
- Optical properties of self-assembled 2D and 3D superlattices of silver nanoparticles. J. Phys. Chem. B. 1998;102(12):2214-2220.
- [Google Scholar]







