Selenium nanoparticles mitigate diabetic nephropathy and pancreatopathy in rat offspring via inhibition of oxidative stress
⁎Corresponding author at: Department of Zoology, College of Science, Building 05, King Saud University, Riyadh 11451, Saudi Arabia. ihazza@ksu.edu.sa (Ibrahim M. Alhazza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Gestational diabetes (G.D.) and its effects on the offspring are some of the major concerns in the mothers of the 21st century. The present study investigates if selenium nanoparticles (SeNPs) can prevent G.D.'s ill effect in the pups to the diabetic mother rats. For this study, the rats were distributed in three groups as per treatment given to their diabetic mothers: DM (p) [diabetic pups from diabetic mothers]; D.M. + Se-NP (pups from diabetic mothers treated with Se N.P. at the dose of 5 mg/kg body weight twice a week for a month) beside the control group (C.N.). The pups were sacrificed, and their blood, pancreas, and kidney were preserved for their biochemical analysis and histological evaluation. The biochemical study, including renal markers (urea, creatinine, and albumin in serum samples), showed a prominent positive effect of the N.P.s in the pups compared to the D.M. group. The redox parameters (reduced glutathione and malondialdehyde levels in tissue samples) were correctly modulated in the pups from N.P.s treated diabetic mothers. These findings were in agreement with the histological examination of the kidney and pancreas tissue samples. The N.P.s showed restoration of various histological details in the pups from the NPs-treated mothers. The current findings entail that the N.P.s counter the possible diabetic-related complications in the offspring by reorganizing the cellular redox status. Hence, the present study shows that SeNPs contain G.D. in diabetic mothers and don't allow the passage to their offspring.
Keywords
Selenium
Nanoparticles
Diabetes
Nephropathy
Pancreatopathy
Offspring
Oxidative stress
1 Introduction
The International Diabetes Federation (IDF) has estimated that Gestational diabetes (G.D.), one of the most common pregnancy-related complications, accounts for almost 14% of pregnancies globally in 2017. It represents approximately eighteen million births each year. It may either go off after delivery with diet control and regular work-out (class A1). Otherwise, it can make one person with diabetes for their whole life dependent on insulin and other supportive medications if not appropriately addressed (Battarbee et al., 2020). Females with obesity, overweight, advanced maternal age, family history of diabetes, lack of a balanced diet, and unhealthy lifestyle are susceptible to G.D. Generally, it resolves after delivery of the baby. It can have lifelong health concerns like increased risk for type 2 diabetes, obesity, and cardiovascular disease in the mother and the child later. Hence, complications can affect a larger population's health shortly worldwide (Cai et al., 2016; Assche et al., 2001). Despite addressing G.D. with lifestyle intervention (diet and exercise) and medications, a treatment strategy with higher efficacy and fewer side effects is still a challenge in the contemporary medical world (Ebaid et al., 2020a; Plows et al., 2018).
Selenium (Se) is one of the essential trace elements for a healthy life. The element occurs in various forms, different degrees of toxicities, and bioactivities. However, organic Se is biologically more accommodatable for its balanced biological activities with lower toxicity but higher bioavailability than inorganic Se. Hence, organic Se has excellent potential to prevent and treat many diseases and disorders (Behne et al., 2010). This element's physiological importance is evident from its presence in selenoproteins in the human body that consists of 25 eukaryotic genes in humans and mice (Bubenik et al., 2014). This broad family of selenoproteins attributes in function and regulation of thyroid hormones, growth factors, cellular development and differentiation, inhibition of abnormal immune response, regulation of inflammatory, chemotactic and phagocytic responses, reproduction, and redox status (Hariharan and Dharmaraj, 2020). Se plays a pivotal role in physiology and causative factors leading to T2DM, including oxidative stress. Hence, Se has a very bright perspective on the prevention and treatment of T2DM (Wang et al., 2015). Also, Se has been reported to relieve many diabetes-related complications, including elevated redox status, systemic inflammation, improved insulin resistance (Zho et al., 2016).
However, there is a very narrow margin between bioavailability and selenium's biological activities restrained by the thresholds of functionality and toxicity [8]. Hence, its dose must be below the toxicity range during the administration to treat diseases like T2DM. The selenium nanoparticles (SeNPs) promise a supposed balance between therapeutic potential and toxicity profiling with excellent biological activity, bioavailability, and low toxicity with nanotechnology. Many studies have shown that these SeNPs have efficient antioxidants with 3–7 fold less toxicity as compared to selenium compounds or organic Se (Al-Quraishy et al., 2015; Zhang et al., 2001). Diabetes mellitus affects two significant organs in the subjects- the kidney and pancreas. If these organ-related complications are not addressed on time, they can lead to diabetes-associated nephropathy and pancreatopathy (El-Borady et al., 2020; Mohapatra et al., 2016). The present investigation evaluates the efficacy of SeNPs in amelioration of diabetes-associated nephropathy and pancreatopathy in the offsprings of diabetic rats.
2 Materials and methods
2.1 Materials
All the used chemicals and reagents for this work were purchased either from Sigma Aldrich (St. Louis, MO, USA) or EMD Millipore Merck (Darmstadt, Germany). All the kits used in the biochemical analysis were bought from QCA and Linear diagnostic kits (Spain).
2.2 Methods
2.2.1 Preparation of citric acid modified selenium nanoparticles (SeNP)
SeNPs were prepared as per the reported procedures (Alagesan and Venugopal, 2019; Kong et al., 2014) with little modification. The selenium dioxide is dissolved in water and well mixed with citric acid by stirring for two h at room temperature. The condition was then changed to vigorous stirring, and ascorbic acid as a reducing agent is dropped slowly on the mixture. The mixture's temperature is raised to 80 °C, and the mixture was continuously stirred for 15 h. the nano-selenium is isolated from the mother solution by centrifugation, washed, and dried in an oven at 80 °C. The surface functional groups on the surface of the prepared nano-selenium were detected by FTIR spectroscopy.
2.2.2 Animal husbandry and treatment
A total of 12 pups (male Swiss albino, 80 ± 30 g, 5–6 weeks old) from the diabetic parent rats (induced by administration of a single dose of alloxan at 100 mg/kg as reported Abdullah et al., 2018) were reared from the Departmental Animal House, Zoology department, College of Sciences (KSU, Riyadh). All the housing, acclimatizing, and monitoring methods were conducted per our previous work (Ebaid et al., 2020b; Hassan et al., 2019). These pups were divided randomly into three groups as per treatment given to their diabetic mothers: DM (p) [diabetic pups from diabetic mothers]; D.M. + Se-NP (pups from diabetic mothers treated with Se N.P. at the dose of 5 mg/kg body weight twice a week for a month). Besides, C.N. (p) [control pups group without any treatment] was also formed from the healthy mother rats' pups. All visible and physical monitoring during and after treatment was keenly observed and recorded, including periodic body weight, food intake, physical activity, and color and texture of the hairs. All the animals, after completion of the treatment rounds, were sacrificed on the same day.
2.2.3 Collection of tissue samples
After sacrifice, the primary target organs of the treatment, kidneys and pancreas, were dissected-out and put immediately in chilled PBS. Then tissues were homogenized (Ika-Werke, Germany) in Tris–KCl buffer (pH 7.36) and stored in separately labeled vials at −80 °C until further analysis. Half of the tissues were saved for histological evaluation in an appropriate solution. Also, serum was prepared from the extracted from the animals as per previous methods (Alhazza et al., 2020).
2.2.4 Measurement of glucose level
It was carried out by the commercial kit (Catalogue number 998282; QCA kits, Spain) following the manufacturer's instructions.
2.2.5 Measurement of renal markers in serum
Urea (Catalogue number 993648; QCA kits, Spain), creatinine (Catalogue number 1123010; Linear kits, Spain) and albumin (Catalogue number 997258; QCA kits, Spain) were chosen as renal markers to assess the efficacy of Se-NPs in diabetic rats as per the provided instructions with the kits.
2.2.6 Measurement of redox parameters
The level of reduced glutathione (GSH) and malondialdehyde (MDA) was chosen as parameters to assess the involvement of oxidative stress in the study. Both were measured by established protocol (Jollow et al., 1974; Beuge and Aust, 1978).
2.2.7 Histological evaluation
The fresh tissue samples (kidney and pancreas) were processed, sectioned and stained as previously mentioned (Alhazza et al., 2020). The histopathological evaluation was conducted blindfolded using a light microscope (Leica DMRB/E Heerbrugg, Switzerland) attached with H.D. camera (Leica MC 170 HD, Singapore). Histomicrograph of the sections were snapped and then digitally improved by Adobe Photoshop (Adobe Systems, Mountain View, CA).
2.2.8 Statistical analysis
The data shown in work is as mean ± S.D. analyzed by GraphPad Prism 5 software, including one-way ANOVA analysis with Tukey's post hoc multiple comparison test. The asterisk marks “*, **, and ***” were used as asterisk marks to show a significant difference from negative control (C.N.) at P less than 0.05, 0.005, and 0.001. The marks “#, ##, and ###” were used as asterisk marks to show a significant difference from positive control (D.M.) at P less than 0.05, 0.005, and 0.001.
3 Results
3.1 Characterization of SeNP
The structural–functional groups on the surface of the prepared nano-selenium was analyzed by the FTIR (Fig. 1). The spectrum showed peaks at 1052, 1633, and 3435 Cm-1, which are related to C-O stretching, C = O stretching, and O–H stretching, respectively. The occurrence of these functional groups on the surface of nano-selenium confirms the successful covering of nano-selenium with an organic citric acid layer. This combination of the nano-selenium with the organic citric acid enables the long term stability of the nano-selenium as well as provide the energetic effect of the nano-selenium and citric acid (Yang et al., 2012; Tang et al., 2019, 2020; Shahverdi et al., 2018; Zhang et al., 2020).
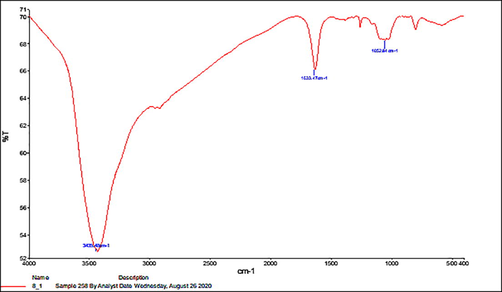
- Showing the FTIR of Selenium nanoparticles.
3.2 Effect on glucose level
D.M. exhibited an increase in its level by 32.46% with respect to the control, C.N., while DM + Se-NP showed a decrease in its status by 14.34% in comparison to the D.M. group (Fig. 2).
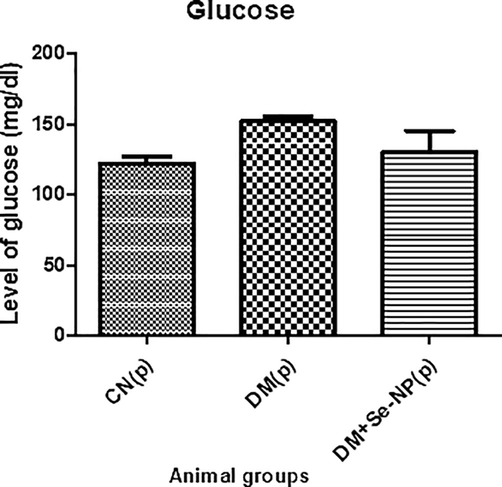
- Bar graphs of the glucose level of the different animal groups in mg per 100 ml. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.3 Effect on renal markers
3.3.1 Urea
The pup group, D.M., showed enhancement in its level by 51.25% as compared to C.N.; however, the combination group, DM + Se-NP, demonstrated a decline in its level by 23.27% concerning to D.M. group (Fig. 3).
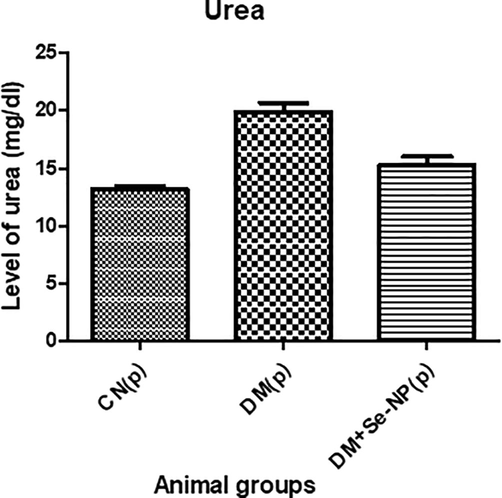
- Bar graphs of the level of urea of the different animal groups in mg per 100 ml. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.3.2 Creatinine
D.M. showed an elevation in its level by 4.59% compared to C.N. while the combination group showed a decrease in its level by 5.49% with respect to D.M. (Fig. 4).
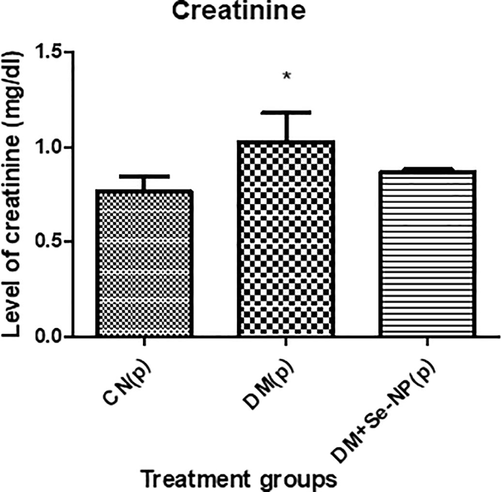
- Bar graphs of the creatinine level of the different animal groups in mg per 100 ml. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.3.3 Albumin
D.M. group showed a decrease in its level by 1.53%, while DM + Se-NP demonstrated 1.99% of enhancement in its level (Fig. 5).

- Bar graphs of the level of albumin of the different animal groups in mg per 100 ml. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.4 Effect on redox parameters
3.4.1 Effect of GSH level in pups
D.M. group demonstrated a decline in GSH level by 92.63% as compared to the C.N. whereas, the DM + Se-NP group exhibited a staggering increase in its level by 995.15% concerning to D.M. group (Fig. 6).
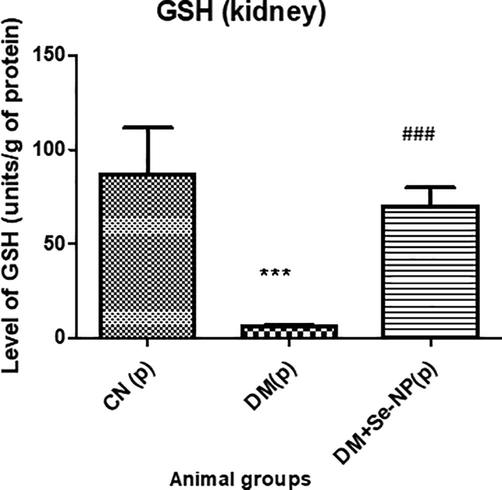
- Bar graphs of the level of glutathione reduced of the different animal groups. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.4.2 Effect on MDA levels
The group D.M. displayed an increase in its level by 54.11% concerning the control group, C.N. whereas, DM + Se-NP showed a decline in its level by 29% compared to the D.M. group (Fig. 7).
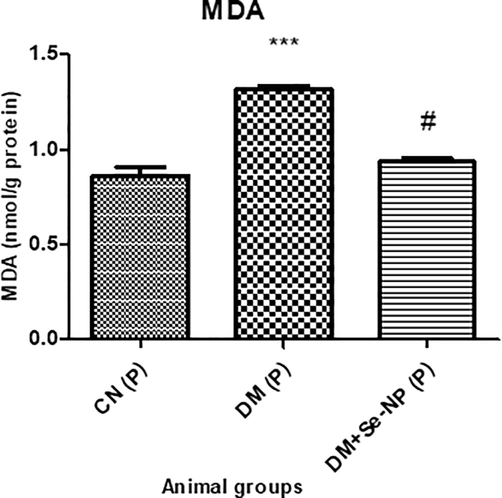
- Bar graphs of the level of malondialdehyde of the different animal groups. * indicates significantly different from the control, group I, while # indicates significantly different from group II. * and # p ≤ 0.05; ** and ## p ≤ 0.01; *** and ### p ≤ 0.001.
3.5 Effect on histological integrity
3.5.1 Pancreas
Fig. 8 shows that islets of Langerhans are typically distributed with regular sizes in the control sections (C.N.). Examinations showed that β-cells are normal in size and shape with normal rounded central nuclei (Fig. 8A-C). Severe damage in the islets of Langerhans was observed in the pancreas sections from the D.M. group. The islets are narrow and small in size in most of these sections (Fig. 8D, E), in low and middle power. In the diabetic-born pups, cells of Langerhans islets are seemed irregular in shape with dark stains. Nuclei have degenerated with darkly stained granulated chromatin fibers (Fig. 8F). Diabetes has disturbed the cellular and nuclei components of the pancreas islets in this group (Fig. 8D–F).
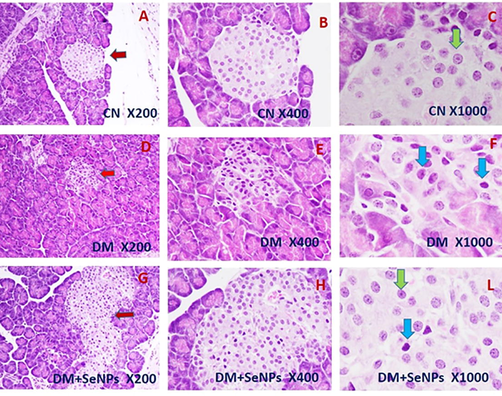
- Representative microscopic sections of the pancreas from pups born to control, diabetic and diabetic treated with selenium nanoparticle mothers. The red arrows show the islets of Langerhans in the three groups (H&E; X 200); High magnification shows the normal Langerhans cells marked with the green arrows and the abnormal Langerhans cells marked with blue arrows (H&E; X 1000).
On the other hand, a partial and clear improvement was observed in the pancreas sections taken from pups born to DM + SeNPs mothers. The histopathological examinations showed that most cells in islets of Langerhans are more regular in shape than those in D.M. pups. However, some sections are shown darkly stained apoptotic cells, entailing a partial improvement as a result of SeNPs treatment (X 1000) (Fig. 8G–L).
3.5.2 Kidney
Fig. 9(A–C) depicts the typical histological structure of the kidney in the present study. The kidney sections of the diabetic pups showed severe shrinkage of the glomeruli with some vacuolation and lobulation (Fig. 9D–F). The urinary space appeared narrower in this group than in the control rats. Apparent edema of the glomeruli leads to limited urinary spaces. Slight edema was observed in the tubular cells that were more pronounced in diabetic rats. Renal tissues from diabetic pups treated hemorrhage in the interstitial tissue. Renal tissues from diabetic pups treated with SeNPs showed clear improvement since the urinary spaces typically appeared like those of the control pups. No remarkable edema in the tubular cells (Fig. 9 G-L). Slight degeneration in some tubular epithelial cells was observed in the renal tissue of this group.
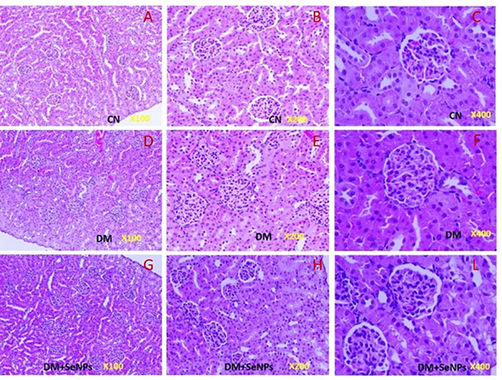
- A–C: Representative kidney sections showing the normal appearance of the glomerulus and renal tubules) in control rats. D–F: Representative kidney sections from pups born to diabetic mothers showing most of the edematous glomeruli with wide urinary space. G–L: Representative kidney sections of the pups born to diabetic mothers treated with SeNPs showing degeneration in some glomeruli, with normal urinary spaces.
4 Discussion
Nephrotoxicity is one of the significant complications in progressive diabetes that might lead to renal failure in its advanced stage (Lim, 2014; Ebaid et al., 2020b). Hence, the complication has to be dealt with before it gets severe in the patients for their long life span and quality of life. The organic source of selenium has been reported to be effective antidiabetic with significant function restoration of crucial organs, including the pancreas and kidney (Hariharan and Dharmaraj, 2020). Hitherto, the higher dose of selenium is toxic. At the same time, Se-NPs, in contrast, has been applauded for its biocompatibility and enhanced efficacy at low dose attributed to their tiny size with high surface area (El-Borady et al., 2020). Hence, we have tried to investigate if the pups from the diabetic mothers treated with Se-NPs show any amelioration from diabetic nephropathy and functional recovery of the target organs (kidney and pancreas).
A great deal of literature suggests that Se is an integral component in the biosynthesis of GSH and its Sulphur- containing amino acids (methionine and cysteine) in organs like the pancreas (Socha et al., 2014; Arteel and Sies, 2001). Besides, AlBasher et al. (2020) has shown that Se can bind to ROS generated due to oxidative stress and counters the same by replenishment of GSH by converting oxidized glutathione (GSSG) into reduced glutathione (GSH). Further, SeNP has been reported to demonstrate the superior performance attributed to their small particle size and the large surface area leading to enhanced mucosal permeability and intestinal absorption as well as tissue deposition (Mohapatra et al., 2014). Also, SeNPs enhanced the expression of GPx, significantly restoring to the normal level in diabetic rats compared to the diabetic untreated rats (El-Borady et al., 2020). Additionally, demonstrated that dietary selenium-rich diet given to the broilers in the poultry farm not only elevates glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) activity in their pancreas but also potentiates the activities of the enzymatic and non-enzymatic antioxidants to protect against deteriorative reactions like lipid peroxidation (Ahmed et al., 2016; Wang et al., 2011). In the present investigation, we have that there is a staggering enhancement in GSH level in the pups from the mothers treated with Se-NPs. In contrast, the MDA level was significantly reduced in the same group with the pups from untreated diabetic mothers. As the oxidative stress and accumulation of free radicals (ROS and RNS) are essential in the pathogenesis and etiology of diabetes mellitus, the regulation of the same after treatment with Se-NPs (in the D.M. + Se-NPs pups), restores the kidney and pancreas functions. That is why most renal markers (urea, creatinine and albumin) were found near the control in the pups to the treated mothers. Besides, Se has a unique ability to mimic insulin's functionality and removes the clinical features of pancreatopathy (Mohapatra et al., 2016). Further, it has been reported to regulate glucose metabolism effectively and enhance insulin secretion and sensitivity by the restoration of pancreatic β- cells significantly (Al-Quraishy et al., 2015). These are the reasons for the significant lowering of serum glucose in treated mothers' pups (DM + Se-NP) in the present study.
All these biochemical results are well supported by the histological analysis of the target organs. On the one hand, the N.P.s significantly restored the histological details in both the organs that are usually disturbed in diabetic conditions. On the other hand, the N.P.s enhance the biological functions of both the organs. Hence, the renal markers and the glucose level in the treated group's pups were improved upto the level that made them quite comparable to the normal values. Our findings are by previously reported works of various research teams (El-Borady et al., 2020; Vecchio et al., 2019; Al-Quraishy et al., 2015).
The present study establishes a protective effect of Se-NPs against nephropathy and pancreatopathy in the pups from the mothers with gestational diabetes treated with the N.P.s. All these effects of N.P.s have been ascribed to their proven antioxidant nature. The property restored the key histological details of both the organs that helped to attain the threshold functionality of pancreatic β cell and enhanced insulin secretion concomitant with the regulation of glucose metabolism and downregulation of pancreatic inflammation (Ahmed et al., 2016). Saifi et al., (2020) and Dhanya et al. (2014) separately have also demonstrated that Se has very effective hypoglycemic and anti-inflammatory effects in patients with G.D. These unique pharmacological properties further enhanced if Se is administered as SeNP (Ebokaiwe et al., 2020; El-Borady et al., 2020). This is quite evident from the present study. Nevertheless, the relationship between diabetes, oxidative stress, and selenium is very complicated. Hence, further studies with advanced techniques are required to investigate the in-depth mechanism involved (work in progress).
5 Conclusion
Se-NPs have strong anti-diabetic and anti-oxidant properties in expecting gestational diabetic mothers. The NPs regulate oxidative stress and inflammation-mediated diabetic condition of the mothers and protect their pups from diabetes and related complications.
6 Authors' contributions
Hossam Ebaid, Iftekhar Hassan and Ibrahim M. Alhazza conceived the research idea and designed the experiments. Mohamed Habila synthesized and characterized the nanoparticles. Iftekhar Hassan, Hossam Ebaid and Jameel Al-Tamimi performed the experiments as per their respective expertise. Iftekhar Hassan and Hossam Ebaid, analyzed all the data. Iftekhar Hassan, Hossam Ebaid and Mohamed Habila drafted the manuscript and prepared the figures as well as conducted statistical analysis of the generated experimental data according to their sections. Besides, Ibrahim M. Alhazza arranged the fund and required setup in the laboratory. All the authors have approved the final version of the manuscript.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1436-004).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed. Pharmacother.. 2018;105:1223-1231.
- [Google Scholar]
- Pre-clinical study for the antidiabetic potential of selenium nanoparticles. Biol. Trace Elem. Res.. 2016;177:267-280.
- [Google Scholar]
- Green synthesis of selenium nanoparticle using leaves extract of Withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9:105-116.
- [Google Scholar]
- Nephroprotective role of selenium nanoparticles against glycerol-induced acute kidney injury in rats. Biol. Trace Elem. Res.. 2020;194:444-454.
- [Google Scholar]
- Chemopreventive effect of riboflavin on the potassium bromate–induced renal toxicity in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020:1-10.
- [Google Scholar]
- Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine.. 2015;10:6741-6756.
- [Google Scholar]
- The biochemistry of selenium and the glutathione system. Environ. Toxicol. Pharmacol.. 2001;10:153-158.
- [Google Scholar]
- Long-term consequences for offspring of diabetes during pregnancy. Br. Med. Bull.. 2001;60:173-182.
- [Google Scholar]
- The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. J. Perinatol.. 2020;40:232-239.
- [Google Scholar]
- Long-term selenium supplementation of humans: selenium status and relationships between selenium concentrations in skeletal muscle and indicator ma- terials. J. Trace. Elem. Med. Bio.. 2010;24:99-105.
- [Google Scholar]
- Characterization of the UGA-recoding and SECIS-binding activities of SECIS-binding protein 2. RNA Biol.. 2014;11:1402-1413.
- [Google Scholar]
- The influence of gestational diabetes on neurodevelopment of children in the first two years of life: a prospective study. PLoS ONE. 2016;11:0162113.
- [Google Scholar]
- Selenium downregulates oxidative stress-induced activation of leukotriene pathway in experimental rats with diabetic cardiac hypertrophy. Biol. Trace Elem. Res.. 2014;161(1):107-115.
- [CrossRef] [Google Scholar]
- Ebaid, H., Abdel-Salam, B., Hassan, I., Al-Tamimi, J., Metwalli, A., Rady, A., Alhazza, I.M., 2020a. Diabetes-Mediated Toxicity Resulted in the Expression of CD80 and CD86 on Neutrophils after Delayed Wound Healing in Male Rats 3592425, 10.
- Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. Lond.. 2020;17:6.
- [Google Scholar]
- Selenium nanoparticles and metformin ameliorate streptozotocin-instigated brain oxidative-inflammatory stress and neurobehavioral alterations in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2020
- [CrossRef] [Google Scholar]
- Hypoglycemic potential of selenium nanoparticles capped with polyvinyl-pyrrolidone in streptozotocin-induced experimental diabetes in rats. Heliyon.. 2020;6(2020):e04045
- [Google Scholar]
- Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacol.. 2020;28:667-695.
- [Google Scholar]
- Ameliorative effect of zinc oxide nanoparticles against potassium bromate-mediated toxicity in Swiss albino rats. Environ. Sci. Pollut. Res.. 2019;26:9966-9980.
- [Google Scholar]
- Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4bromobenzene oxide as hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol.. 2014;65:155-162.
- [Google Scholar]
- Diabetic nephropathy – complications and treatment. Int. J. Nephrol. Renovasc. Dis.. 2014;7:361-381.
- [Google Scholar]
- Effects of dietary nano-selenium on tissue selenium deposition, antioxidant status and immune functions in layer chicks. Int. J. Pharmacol.. 2014;10:160-167.
- [Google Scholar]
- Diabetes mellitus is associated with an exocrine pancreatopathy: conclusions from a review of literature. Pancreas. 2016;45:1104-1110.
- [Google Scholar]
- The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci.. 2018;19:3342.
- [Google Scholar]
- Influence of selenium supplementation on carbohydrate metabolism and oxidative stress in pregnant women with gestational diabetes mellitus. J Med Biochem.. 2020;39(2):191-198.
- [Google Scholar]
- Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J.. 2014;13:62.
- [Google Scholar]
- Characterization of folic acid surface-coated selenium nanoparticles and corresponding in vitro and in vivo effects against breast cancer. Arch. Med. Res.. 2018;49:10-17.
- [Google Scholar]
- Construction of arabinogalactans/selenium nanoparticles composites for enhancement of the antitumor activity. Int. J. Biol. Macromol.. 2019;128:444-451.
- [Google Scholar]
- Development, structure characterization and stability of food grade selenium nanoparticles stabilized by tilapia polypeptides. J. Food Eng.. 2020;275:109878
- [Google Scholar]
- New evidence of exocrine pancreatopathy in pre-symptomatic and symptomatic type 1 diabetes. Curr. Diab. Rep.. 2019;19:1223-1225.
- [Google Scholar]
- Effects of selenomethionine and sodium selenite supplementation on meat quality, selenium distribution and antioxidant status in broilers. Czech. J. Anim. Sci.. 2011;56:305-313.
- [Google Scholar]
- Association between serum selenium level and type 2 diabetes mellitus: a non-linear dose–response meta-analysis of observational studies. Nutr. J.. 2015;15:48.
- [Google Scholar]
- Surface decoration by Spirulina polysaccharide enhances the cellular uptake and anticancer efficacy of selenium nanoparticles. Int. J. Nanomed.. 2012;7:835-844.
- [Google Scholar]
- Preparation and characterization of selenium nanoparticles decorated by Spirulina platensis polysaccharide. J. Food Biochem.. 2020;13363
- [Google Scholar]
- Organoselenium Small Molecules and Chromium(III) Complexes for Intervention in Chronic Low-grade Inflammation and Type 2 Diabetes. Curr. Top. Med Chem. 2016:16.
- [Google Scholar]







