Translate this page into:
Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress
⁎Corresponding author at: Botany and Microbiology Department, College of Science, King Saudi University, P. O. Box. 2460, Riyadh 11451, Saudi Arabia. parvaizbot@yahoo.com (Parvaiz Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Current study illustrates the impact assessment of TiO2 nano-particle seed priming on the overall germination, physiology in maize thriving under salinity stress.
Methodology
Seeds of maize cultivar were soaked in 40, 60 and 80 ppm aerated solution of TiO2nano-particle for one day and nano-primed seeds were then exposed to salinity stress, i.e., 200 mM NaCl on paper culture system.
Results
Results indicated that 60 ppm TiO2nano-priming treatment effect positively on the rate of germinate and growth of maize seedling under salinity stress. The experiment was carried out in sand as a growth medium with 60 ppm TiO2 priming. Results exhibited that germination percentage, germination energy, seedling vigor index, lengths of root and shoot, fresh and dry weights of seedling, potassium ion (K+) concentration, relative water content (RWC), total phenolic and proline and contents, superoxide dismutase (SOD), catalase (CAT) and phenylalanine ammonia lyase (PAL) activities were significantly enhanced and mean emergence time (MET), sodium ion (Na+) concentration, membrane electrolyte leakage (MEL) and malondialdehyde (MDA) content were decreased by TiO2 priming as compared to control under salinity stress.
Conclusion
So, nano-priming with TiO2 mitigates the salinity injury in maize and could be a significant alternate strategy to mitigate the deleterious impact of salinity stress in maize.
Keywords
Nano-priming
TiO2 nanoparticle
Maize
Salinity
Lipid peroxidation
Antioxidant enzymes
Proline
Total phenolic content
1 Introduction
Salinity, as major abiotic stress, not only restricts growth but also retarded developmental processes in important crops (Rameeh, 2012). On approximation 800 million hectare land at a global level is severely affected by salt stress; thus key threat to agricultural production ultimately result in reduced yield (Munns, 2005). Generally, through osmotic and ionic stress, salinity affects elementary metabolic processes, including protein synthesis as well as carbohydrates and lipid metabolism (Parida and Das, 2005). Due to a greater concentration of sodium ion (Na+) in plant tissues, inhibition of cellular metabolism and membrane dysfunction occur under salt stress. Also, higher Na+ concentration causes osmotic stress leading to water deficit in cells, thus reduce water potential (Munns and Tester, 2008). To mitigate the antagonistic effects of saline stress, various methods have been adopted to adjust the ion equilibrium and osmotic homeostasis to overcome the impact of salt injury (Liu et al., 2015; Ibrahim, 2016; Shah et al., 2020). Among different measures, an efficient and simple approach to recover the plant performance undLIU er stressful conditions is seed priming with nano-particles (Iqbal and Ashraf, 2007). Seed priming is generally defined as the controlled hydration of the seeds to the level that allows pre-germinative metabolic activity to continue while averting the surfacing of the radical.
TiO2 is a well-known nanoparticle which play a vital role not only in plant growth but also in development and it is ninth most abundant element on earth. So that is why it is important to track it toxic or beneficial effect on plant. TiO2 nanoparticles have several insightful impacts on the crop morphological, biochemical and physiological features (Mishra et al., 2014). Mahmoodzadeh et al., 2013 reported the elevated levels of germination and improved radicle and plumule development in canola seedlings when treated with TiO2 NPs application. TiO2 nanoparticles increased growth and production attributes like yield in wheat seedling thriving under water stress (Jaberzadeh et al., 2013). TiO2 NPs controls enzymes activity during nitrogen metabolism in plants such as glutamate dehydrogenase (NDH), nitrate reductase (NiR), glutamine synthase (GS), and glutamic-pyruvic transaminase (GPT) that supports the plants to uptake nitrate and facilitate and regulate the conversion of inorganic N to organic N in the form of chlorophyll and various amino acids ultimately proteins, that might increase plant biomass (Mishra et al., 2014). Hong-Bo et al., 2005 illustrated that TiO2 nano-particles shields the chloroplast from high light intensity by elevating the antioxidant enzyme activity such as peroxidases (POD), superoxidases (SOD) and catalases (CAT).
Maize is one of the significant crops used as food and forage worldwide and also known as salt-sensitive crop plants (Farooq et al., 2015). Maize productivity is hindered by different stresses like drought, floods, and heavy metals etc. but with all these stress salinity stress is directly or indirectly linked. Increased salt concentration leads to the significant decline in the rate of seed germination and delays the development of maize seedling (Zhang et al., 2007). Several previous research reports demonstrate the pre-treatment of seed with silicon, chloride salt, and gibberellic acid facilitates the maize development in the saline fields (Ashraf and Rauf, 2001; Zhang et al., 2007; Ghodrat and Rousta, 2012; Abdel Latef and Tran, 2016). However, no work regarding mitigation of salinity stress by seed priming through TiO2 has been reported until now. In the present research, we hypothesized that seed priming with TiO2 could help maize crops to overcome salt stress by increasing the production of various structural and protective compounds. Consequently, the prime aim of the current research is to investigate the effects of TiO2 seed priming overall seed development including both seed germination, seedling development and determining the optimized concentration of TiO2 for seed priming.
2 Materials and methods
2.1 Categorization and scanning electron microscopy (SEM) image of nano-particles (NPs)
Nano-particles of TiO2 of analytical grade employed for seed priming was purchased from Nanomaterials Pioneers Company NANOSANY (Mashhad, IRAN). Nano TiO2 exhibited the specificity in terms of specific surface size ranging from 200 to 240m2g−1, 0.1 ml g-pore size and purity higher than 99%. A scanning electron microscope (SEM) was employed for the quantification of the size of TiO2 nanoparticles and approximated about 10–25 nm (Fig. 1). XRD (X-ray Diffraction Method) (Xpert, PRO MPD, PAN analytical) was employed to illustrate the crystal characteristics of the nano-particles ranging from 30°-120° conceded at 40 kV and 40 mA current with Cu Kα radiation. The display of all the varied TiO2 nano-particles in the anatase form was represented by X- Ray Diffraction method (Fig. 2).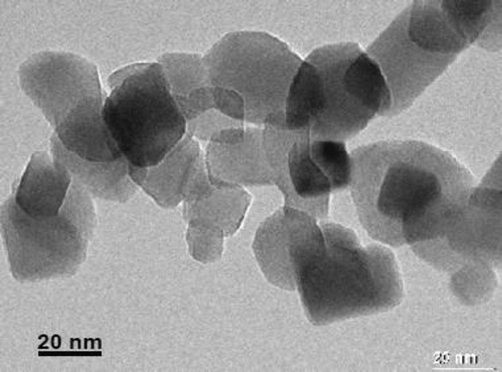
TEM micrograph of TiO2 NPs. Average size of the nanoparticles was 10–25 nm.
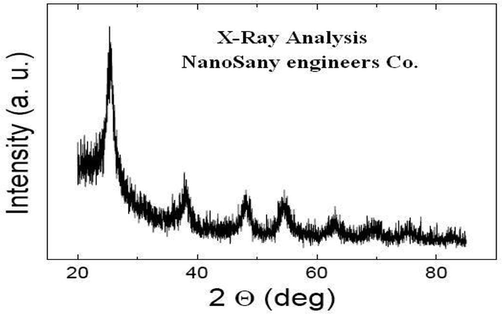
XRD pattern of TiO2 NPs. XRD measurement showed that used TiO2 NPs were all in the anatase phase.
2.2 Plant materials
Maize variety CS-200 seeds used in this study were purchased from Petal Seed Company (Mardan, Pakistan) and were harvested in 2015. The starting moisture content of seed was 11.09% and the starting germination percentage was 93.38%. The experimental location and soil analysis was same as published in our previous paper (Shah et al., 2018; Fig. 3). The climatic condition of the experimental area was sub-tropical.
Location of the study site in the Peshawar city of Pakistan (Taken from our previous published paper Shah et al. (2018)).
2.3 Experiment 1
Surface sterilization of seeds was carried out with 0.5% sodium hypochlorite for 10 mins. Sterilized seeds were subjected to nano-priming with 40 (T1), 60 (T2) and 80 (T3) ppm TiO2 solution for 24 h at 27 °C and for normal (S0) and some salinity stressed seeds were exposed to hydro-priming for 24 h. Aquarium pump was used to provide oxygen to seeds during priming hours.weight weight/volume ratio was maintained 1:5 as prescribed by Farooq et al., 2013. Following this, seeds were subjected to vigorous washing with distilled water and then drying at 25 °C to retain their original weight approximately under shade. Seed germination papers measuring 10 × 15 cm were double-layered rolled and placed in plastic having dimensions (20 × 14 cm) for subsequent sowing of thirty seeds for each replication. These were kept for 7 days at 25 °C in the growth chamber, exhibiting 60% relative humidity, 16 h day length and 250 µmol m−2 s−1 light intensity. For S1, T1, T2 and T3 seed germination papers were moistened with 30 ml Hoagland’s solution (Hoagland, 1920), confirming immersion of seeds in solution and with paper change every 2 days. These experiments were carried out with five replications per treatment, for one plastic bag each treatment.
2.4 Experiment 2
Sand about 100 g per pot was sterilized after passing through a 2 mm mesh, put in plastic pots (volume 150 ml). Sterilized sand was loaded with 10% (V:W) Hoagland nutrient solution containing 200mMNacl as final concentration. Following the surface sterilization of seeds were subjected to priming with 60 ppm TiO2 solution for 24 h at 27 °C. Concomitant with this, another set of seeds was dipped in aerated water for 24 h to compare them with former treatment while as seed set which was not immersed was treated as control. For evaluation, seedlings were harvested following 12 days and washed carefully in tap water and were dried with filter paper. Plants were classified into two parts: each part having three replicates for each treatment and 10 replications for seedlings. Following 12 days, seedlings were harvested, carefully rinsed in tap water, and surface blotted with filter paper. Plants were classified in two parts: one part at 105 °C for half an hour then oven-dried at 80 °C up to constant dry weight and potassium and sodium content for the assessment of biochemical index another portion was used.
2.5 Measurement techniques
2.5.1 Determination of seed germination and seedling vigor
After the commencement of the experiment, seed germination was observed daily up to the 7th day. Seed was considered as germinated after radical emergence of 2 mm. 4th day after seed plantation, germination energy (GE, %) was recorded, it is on the 4th day after sowing the number of seedlings emerged (Farooq et al., 2006). Plants were harvested on the 7th day for the assessment of root and shoot length (cm) and fresh and dry weight of seedlings (mg plant−1). For each replication, we have randomly selected five seedlings, and sample data were selected from their average values. The growth and development attributes were estimated towards the end of the experiment including germination percentage (GP), seedling vegetation index (VI) and mean emergence time (MET) based on the below cited formula (Ellis & Roberts, 1981):
VI = (mean root length + mean shoot length) × Germination percentage (%) (Abdul-Baki and Anderson, 1973). Where n is the total number of germinated seeds during the experiment, N is the total number of planted seeds, and D is the number of days from the day to emergence.
2.5.2 Determination of physiological characteristics of seedlings
Maize leaf samples were estimated for their RWC (relative water content) following the protocol of (Abbasi et al., 2015a), sodium and potassium content was determined following Ghosh et al., 2011. Proline content was determined using Bates et al. (1973), superoxide dismutase activities (SOD, Ug−1FW−1) and malonaldehyde (MDA, µmolg−1FW−1) were estimated according to the procedure adopted by Maia et al. (2010), and using Zhang et al. (2013) methodology catalase (CAT, Ug−1FW−1 min−1) was determined. Through Folin- Ciocalteu reagent method, total phenolic contents, according to Nadernejad et al. (2013) were estimated. According to Nadernejad et al. (2013), activities of phenylalanine ammonia-lyase (PAL, Ug−1FW−1h−1) were assessed.
2.6 Statistical analyses
In all the experiments, Statistics SPSS 17.0 software was used to find analysis of variance (ANOVA). Duncan’s multiple range test was employed for comparison among the treatments, and significance was evaluated at 0.05 level. The data are presented as mean in the table while Microsoft Excel 2016 was used for making graphs.
3 Results
3.1 Nano-priming enhances seed germination and seedling growth under salinity
In comparison to S0 (no priming), S1 (seed priming with 60ppmTiO2) significantly reduced GE, GP, VI, but improved MET at P < 0.05. Thus 60 ppm TiO2 priming (nano-priming) was the best treatment that effects different growth-related parameters, including GE, GP, VI and MET in maize (Table 1). Different letters in the same column indicate a significant difference among treatments according to Duncańs multiple range test at 0.05 level. The values of the brackets are the reduction or promotion over S0. S0 = normal; S1 = salt stress; T1 = seed priming with 40 ppm TiO2 solution; T2 = seed priming with 60 ppm TiO2 solution; T3 = seed priming with 80 ppm TiO2 solution; GE = germination energy; GP = germination percentage; MET = mean emergence time; VI = seedling vigor index.
Treatments
GE(%)
GP(%)
MET(days)
VI
S0
87.00a
96.67a
4.34c
15.11a
S1
58.89d
83.33c
4.73a
5.47e
T1
70.00c
88.89bc
4.56b
7.39d
T2
81.11ab
94.44ab
4.38c
9.56b
T3
76.67b
90.00b
4.51b
8.65c
Salinity stress significantly reduced shoot length (SL), root length (RL), fresh seedling weight (SFW), and seedling dry weight (SDW) of maize hybrid; CS-20 at P > 0.05 (Table 2). Both nano-priming and hydro-priming increased RL, SH, SFW and SDW under normal and salinity stress conditions (Table 2). A significant difference for all above-mentioned parameters in control and TiO2 treated plants was observed. Nano-priming is more effective on RL, SL, SFW and SDW than hydro-priming under normal and salinity stress conditions. While Nano-priming and hydro-priming were equally effective for RL and SL but showed significant differences for RWF and SFW under normal conditions. However, there was a remarkable difference between nano- and hydro-priming for all parameters under stress conditions. Different letters in the same column indicate a significant difference among treatments according to Duncańs multiple range test at 0.05 level. The values of the brackets are the promotion over control. Control = seed unprimed; Hydro-priming = seed priming with water; Nanopriming = seed priming with TiO2 solution; SL = shoot length; RL = root length; FW = fresh weight; DW = dry weight.
Treatments
SL(cm)
RL(cm)
FW(mg−1plant−1)
DW(mg−1plant−1)
Control
6.86b
9.03b
405.7b
39.8b
Hydro-priming
754ab
9.54ab
436.3b
43.9db
Normal
Nanopriming
7.32a
10.05a
466.8a
48.1a
Salinity
Control
Hydro-priming1.67e
2.02d4.91e
6.31d144.9e
192.9d14.9e
20.3d
Nanopriming
2.49c
7.65c
241.81c
25.2c
3.2 Nano-priming reduces the MEL and MDA content under salinity
salinity stress leads to a significant increase in membrane electrolyte leakage (MEL) of maize hybrid, CS-20 at P > 0.05 (Table 3). Both nano-priming and hydro-priming reduced MEL under normal and salinity stress conditions (Table 3). A significant difference for all above mentioned parameter in control and TiO2 treated plants was observed. Nano-priming is more effective on MEL than hydro-priming under normal and salinity stress conditions. Similarly, salinity stress also significantly augmented malondialdehyde (MDA) levels of leaves (Table 3). However, both nano-priming and hydro-priming reduced MDA under normal and salinity stress conditions. This effect was comparable with that of MEL. Different letters in the same column indicate a significant difference among treatments according to Duncańs multiple range test at 0.05 level. The values of the brackets are the reduction or promotion over control. Control = seed unprimed; Hydropriming = seed priming with water; Nanopriming = seed priming with 60 ppm TiO2 solution; RWC = relative water content; EL = electrolyte leakage; MDA = malondialdehyde; TP = total phenolic.
Treatments
RWC(%)
EL(%)
MDA(µmolg−1FW−1)
Proline(mg−1FW−1)
TP(mg−1FW−1)
Control
89.20b
13.50d
7.97d
54.90e
43.57e
Hydro-priming
90.35ab
12.70d
7.39d
59.62de
48.22de
Normal Nanopriming
91.76a
10.83e
6.61e
64.76d
53.09d
Control
70.35e
28.51a
15.11a
87.97c
72.97c
Hydro-priming
73.83d
20.82b
11.80b
105.25b
86.58b
Salinity Nanopriming
76.09c
17.27c
8.95c
119.58a
100.58a
3.3 Nano-priming enhances relative water content, proline and total phenolics content
Salinity stress significantly reduced the relative water content of leaf (RWC) of maize hybrid; CS-20 at P > 0.05 (Table 2). Both nano-priming and hydro-priming increased RWC of the leaf under normal and salinity stress conditions (Table 3). A significant difference for above mentioned parameter in control and TiO2 treated plants. Nano-priming is more effective for RWC of leaf than hydro-priming under salinity stress conditions. Furthermore, salinity stress also significantly reduced proline content (PC) and total phenolics content (TPC) of maize hybrid; CS-20 at P > 0.05 (Table 2). There was significant disparity for all PC and TPC in control and TiO2 treated plants. Also. However, there was a remarkable difference between nano- and hydro-priming for these parameters as nanopriming exhibited better enhancement in proline and total phenolic contents over hydro-priming under stress condition (Table 3).
3.4 Effect of seed priming on Na+ and K+ content
Salinity stress leads to a significant decline in potassium ion (K+) concentration in leaves of maize hybrid; CS-20 at P > 0.05 (Table 2). Significant difference for K+ concentration in control and TiO2 treated plants was observed. Nano-priming is more effective in K+ concentration than hydro-priming under normal and salinity stress conditions (Table 4). While Nano-priming and hydro-priming were equally effective for RL and SL but showed significant differences for RWF and SFW under normal conditions. However, there was a remarkable difference between nano- and hydro-priming for all parameters under stress conditions. Nevertheless, salinity stress significantly improved sodium ion (Na+) concentration in leaves of maize hybrid; CS-20 at P > 0.05 (Table 2). Nanopriming and hydro-priming reduced Na+ content, but Nano-priming is more effective for Na+ concentration of leaf than hydro-priming as exhibited more significant decline in Na+ concentration of leaf under salinity stress condition. On the other hand, salinity stress significantly reduced the K+/Na+ ratio while nano-priming significantly improved the K+/Na+ ratio at P > 0.05. Different letters in the same column indicate a significant difference among treatments according to Duncańs multiple range test at 0.05 level. Control = seed unprimed; Hydro-priming = seed priming with water; Nanopriming = seed priming with 60 ppm TiO2 solution.
Treatments
K+ (%)
Na+ (%)
K+/Na + ratio
Control
1.76c
0.59d
2.98b
Normal
Hydro-priming
1.88b
0.62d
3.06ab
Nanopriming
2.24a
0.69c
3.24a
Control
0.76f
0.96a
0.79e
Salinity
Hydro-priming
0.90e
0.78b
1.14d
Nanopriming
1.31d
0.71c
1.84c
3.5 Effect of seed priming on SOD, CAT and PAL activities
Salinity stress leads to a significant increase in sodium oxide dismutase (SOD) activity of maize hybrid; CS-20 leaves at P > 0.05. Both nano-priming and hydro-priming augmented SOD activity under normal and salinity stress conditions. There was significant difference for SOD activity in control and TiO2 treated plants. Nano-priming is more effective than hydro-priming for SOD activity under salinity stress conditions (Fig. 4A). While Nano-priming and hydro-priming were equally effective for RL and SL but showed significant differences for RWF and SFW under normal conditions. However, there was a remarkable difference between nano- and hydro-priming for all parameters under stress conditions. Nanopriming and hydro-priming also improved phenylalanine ammonia lyase rates, and this impact was comparable to that of catalase (CAT) activity (Fig. 4B). salinity stress also significantly increased phenylalanin ammonia lyase (PAL) activity in leaves of maize hybrid under normal and salinity stress conditions (Fig. 4C).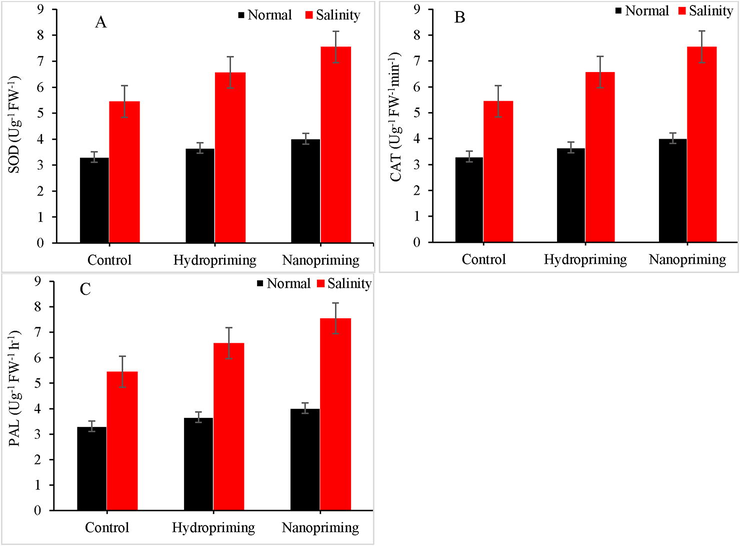
Effect of nanopriming on the activity of (A) SOD, (B) CAT and (C) PAL in maize under saline and normal condition.
4 Discussion
Critical stages for assembly and establishment of crop plants are seed germination and early seedling growth. Seed germination and growth of seedling were adversely affected by salinity stress due to osmotic and ion toxicity (Parida and Das, 2005). The present study showed germination energy, germination percentage and seedling vigor index were significantly reduced and membrane electrolyte leakage was increased dramatically by saline stress at P > 0.05 (Table 1). The present study clearly showed that salinity stress caused a significant decline in SL, RL, SFW and SDW (Table 2). These results showed agreement with those of (Abbasi et al., 2014, 2015b; Liu et al., 2015), who described that salinity stress resulted in the reduced plant vigor. This decline in plant vigor could be because of the decrease in osmotic potential or ions toxicity (Farooq et al., 2015). Conversely, priming with TiO2 can enhance germination energy, germination percentage and seedling vigor index in maize. It can decline membrane electrolyte leakage, the performance was best when TiO2 was at 60 ppm, and a significant difference between nano-priming and salinity stress was observed. In the interim, priming with TiO2 can enhance RL, SL, SFW and SDW. The present study showed priming with TiO2 has a constructive impact on SG and growth of seedling in maize under salinity stress conditions. This might be due to the quick completion of metabolic activities at the pre-germination stage during the priming process (Farooq et al., 2013; Shah et al., 2019), this preeminence of seed subjected to priming led to augmented SG levels and higher growth levels of the seedling. According to Mahmoodzadeh et al. (2013), TiO2 improved SG (seed germination) and facilitated radicle and plumule growth in seedling of canola crops. Jaberzadeh et al. (2013) described that TiO2 nanoparticles improved wheat plant development and yield-related traits under drought stress.
RWC has been employed as an effective method for calculating plants tolerance to salt stress (Suriya-arunroj et al., 2004). In the current research, salinity stress significantly declined RWC in maize. This verified the already reported results that a decline in RWC of Zea mays under salinity stress (Abbasi et al., 2014). TiO2 nano-particle priming can increase RWC, which exhibited TiO2 might play a significant role in maize water relation under salt stress and maintain the plants water retention capacity to fight against salinity.
Salinity causes the exchange of K+ by Na+ carrying, thus decline K+/Na+ ratios, which results in the disarray of plant’s biochemical reactions. The conservation of greater K+/Na+ ratios is possibly appropriate for the plant’s metabolic development (Sytar et al., 2019). Therefore, greater potassium to sodium ion (K+/Na+) ratio is regarded to be one of the significant biochemical methods of plant or crop salt tolerance (Tahjib-Ul-Arif et al., 2019). TiO2 nano-particle priming can enhance potassium to sodium ion (K+/Na+) ratio (Table 4), thus recommended that TiO2 diminished the toxic impacts of sodium ion (Na+), this was in agreement with the enhancement of RL, SL, SFW and SDW of maize.
The accumulation of ROS, hydroxyl radical (•OH), H2O2 and superoxide anion (O2•¯), in plants is one of the prominent physiological indicators confirming that plant suffers from abiotic stress such as salinity. Higher concentration of ROS is injurious to cell structure and function. REL (Relative electrolyte leakage) allows cell membrane damage to be measured when plants are suffering from salinity stress (Demidchik et al., 2014). The cellular membranes remain intact under salinity stress in saline tolerant plant species. TiO2 nano-particle priming significantly reduced the relative electrolyte leakages of the membrane in maize (Table 3). This verified the defensive effect of TiO2 to encounter membrane damage due to salinity stress. The result is in agreement with earlier reported facts that TiO2 assisted in the membrane maintenance functions (Mahmoodzadeh et al., 2013; Qi et al., 2013).
Furthermore, accretion of malondialdehyde (MDA) in plant tissues as a lipid peroxidation product, under saline environment (Parida and Das, 2005), signifies that salinity induces oxidative damage that leads to injury in membranes. The enhancement in malondialdehyde content and relative membrane electrolyte leakage in maize s exhibited the upsurge of LPO (lipid peroxidation), leading to membrane injury due to salinity stress (Table 3). TiO2 seed priming reduced malondialdehyde (MDA) content, which might be associated with the antioxidant response and membranes defensive role that modulates the plant tolerance to injury (Posmyk et al., 2009).
An antioxidative systems comprising enzymatic and non-enzymatic components play a vital role in neutralizing the adverse effects of reactive oxygen species (ROS). Catalase (CAT) and superoxide dismutase (SOD) plays an imperative role in ROS scavenging, while O2¯ dismutation into O2 and H2O2 was catalyzed by SOD (Foyer et al., 1994). Furthermore, ROS production is mainly hindered by the Non-enzymatic antioxidative system (Mehr et al., 2012; Kishor and Sreenivasulu, 2014; Król et al., 2014; Ahammed et al., 2020). In the present study, CAT and SOD activities in shoots of maize plants were enhanced under salt stress compared to control. This indicated that antioxidant system in maize is upregulated under salinity stress. TiO2 seed priming augmented catalase and superoxide dismutase activities under control and salt stress conditions. Due to TiO2 seed priming, scavenging capacity for ROS was enhanced, and adverse effects of salinity stress were lessened. These findings were further verified by dropdown in relative electrolyte leakage level and MDA content. Numerous studies exhibited that TiO2 nanoparticles encouraged plant antioxidant systems to less oxidative damage by scavenging ROS (Mahmoodzadeh et al., 2013; Mishra et al., 2014), which are in agreement to our work.
Proline being multi-functional molecule has several vital functions, i.e., osmotic potential regulation, scavenging free radicals, maintaining membrane integrity and adaptively response to abiotic stress such as salinity by increasing its concentration and accumulation in plant cells (Kishor and Sreenivasulu, 2014). In the present research, under salinity stress, proline accumulation in maize is higher, which is in agreement with previous reports on this topic. TiO2priming significantly increases proline content in tested maize hybrid (Table 3) under salinity stress, this showed that TiO2 alleviates salinity stress by increasing proline production and by reducing proline degradation (Mehr et al., 2012; Kishor and Sreenivasulu, 2014). In present study, reduction in electrolyte leakage and MDA content proved that proline is involved in regulation of osmotic potential, maintenance of membrane integrity as well as scavenging of free radicals.
Phenolic compounds are also reported to play a vital role in scavenging antioxidants and free radicals and their production is increased under various abiotic stresses (Mehr et al., 2012; Król et al., 2014). In present study, maize hybrid accumulated more phenolic compounds under salinity stress (Table 3), likewise increased production of phenolic compounds was reported in Anethumgraveolesby Mehr et al. (2012) and wheat by Mahboob et al. (2016). Nevertheless, TiO2 seed priming regulates phenolic compounds production in maize hybrid under salinity stress (Table 3), and this finding is in line with previously reported results that in Vigna radiata, exogenous application of TiO2 significantly improved total phenolic content (Qi et al., 2013). Phenylalanine ammonia lyase (PAL) is a key enzyme on the biosynthesis of phenols, which are involved in positively fighting against environmental stresses. Phenylalanine ammonia lyase (PAL) is an indicator of stress conditions and is known as biochemical marker which represent the production of not only protective but also structural compounds (Nadernejad et al., 2013). In the present study, Phenylalanine ammonia lyase activities significantly increased when maize seeds are primed with TiO2 under salinity stress. These findings predicted that protective compound production was induced in maize under salinity stress, just like phenolic content. Therefore, improvement in PAL activity is considered as the key physiological reason for maize resistance to salinity stress.
5 Conclusions
The current study concludes that increased salinity levels lead to impaired seed germination and seedling development in maize. TiO2 (Titanium Dioxide) seed priming modulated the salinity stress in maize by improving the maize crop development thriving in normal growth conditions. TiO2 seed priming mitigates the damage to maize under salt stress by enhancing superoxide dismutase (SOD), catalase (CAT) and phenylalanine ammonia lyase (PAL) activities, relative water content (RWC), proline and total phenolic contents and by declining lipid peroxidation product and membrane relative electrolyte leakage. The seed priming with 60 ppm TiO2 concentration is most effective as compared to other priming treatments. Therefore, seed priming with TiO2 might be an effective alternative strategy to decline the influence of salinity stress in maize.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/236), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul.. 2015;76(1):111-125.
- [Google Scholar]
- Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak. J. Bot.. 2014;46:135-146.
- [Google Scholar]
- Morpho-physiological and micrographic characterization of maize hybrids under NaCl and Cd stress. Plant Growth Regul.. 2015;75(1):115-122.
- [Google Scholar]
- Abdel Latef, A.A., Tran, L.-S.P., 2016. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci., 7. Available from http://dx.doi.org/10.3389/fpls.2016.00243. DOI 10.3389/fpls.2016.00243.
- Abdul-Baki, A.A., Anderson, J.D., 1973. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci., 13, 630. Available from http://dx.doi.org/10.2135/cropsci1973.0011183x001300060013x. DOI 10.2135/cropsci1973.0011183x001300060013x.
- Ahammed GJ, Li Y, et al., 2020. Abscisic Acid and Gibberellins Act Antagonistically to Mediate Epigallocatechin-3-Gallate-Retarded Seed Germination and Early Seedling Growth in Tomato. Journal of Plant Growth Regulation. doi: 10.1007/s00344-020-10089-1.
- Inducing salt tolerance in maize (Zea mays L.) through seed priming with chloride salts: growth and ion transport at early growth stages. Acta Physiol. Plant. 2001;23(4):407-414.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205-207.
- [Google Scholar]
- Demidchik, V., Straltsova, D., Medvedev, S.S., Pozhvanov, G.A., Sokolik, A., Yurin, V., 2014. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot., 65, 1259-1270. Available from http://dx.doi.org/10.1093/jxb/eru004. DOI 10.1093/jxb/eru004.
- An investigation into the possible effects of ripeness and repeated threshing on barley seed longevity under six different storage environments. Annals of Botany. 1981;48(1):93-96.
- [Google Scholar]
- Nutrient homeostasis, metabolism of reserves, and seedling vigor as affected by seed priming in coarse rice. Can. J. Bot.. 2006;84(8):1196-1202.
- [Google Scholar]
- Farooq, M., Hussain, M., Wakeel, A., Siddique, K.H.M., 2015. Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev., 35, 461-481. Available from http://dx.doi.org/10.1007/s13593-015-0287-0. DOI 10.1007/s13593-015-0287-0.
- Farooq, M., Irfan, M., Aziz, T., Ahmad, I., Cheema, S.A., 2013. Seed Priming with Ascorbic Acid Improves Drought Resistance of Wheat. J. Agron. Crop. Sci., 199, 12-22. Available from http://dx.doi.org/10.1111/j.1439-037x.2012.00521.x. DOI 10.1111/j.1439-037x.2012.00521.x.
- Effect of priming with gibberellic acid (GA3) on germination and growth of corn (Zea mays L.) under saline conditions. Int. J. Agric. Crop Sci.. 2012;4:883-885.
- [Google Scholar]
- LEA proteins in higher plants: structure, function, gene expression and regulation. Colloids and surfaces B: Biointerfaces. 2005;45(3–4):131-135.
- [Google Scholar]
- Ibrahim, E.A., 2016. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol., 192, 38-46. Available from http://dx.doi.org/10.1016/j.jplph.2015.12.011. DOI 10.1016/j.jplph.2015.12.011.
- Iqbal, M., Ashraf, M., 2007. Seed Treatment with Auxins Modulates Growth and Ion Partitioning in Salt-stressed Wheat Plants. Journal of Integrative Plant Biology, 49, 1003-1015. Available from http://dx.doi.org/10.1111/j.1672-9072.2007.00488.x. DOI 10.1111/j.1672-9072.2007.00488.x.
- Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2013;41:201-207.
- [Google Scholar]
- Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell Environ.. 2014;37:300-311.
- [Google Scholar]
- Król, A., Amarowicz, R., Weidner, S., 2014. Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol. Plant., 36, 1491-1499. Available from http://dx.doi.org/10.1007/s11738-014-1526-8. DOI 10.1007/s11738-014-1526-8.
- Liu, S., Guo, X., Feng, G., Maimaitiaili, B., Fan, J., He, X., 2015. Indigenous arbuscular mycorrhizal fungi can alleviate salt stress and promote growth of cotton and maize in saline fields. Plant Soil, 398, 195-206. Available from http://dx.doi.org/10.1007/s11104-015-2656-5. DOI 10.1007/s11104-015-2656-5.
- Induction of salt tolerance in wheat (Triticum aestivum L.) seedlings through exogenous application of proline. Pak. J. Bot.. 2016;48:861-867.
- [Google Scholar]
- Mahmoodzadeh, H., Nabavi, M., Kashefi, H., 2013. Effect of nanoscale titanium dioxide particles on the germination and growth of canola (Brassica napus).
- Salt-induced changes in antioxidative enzyme activities in root tissues do not account for the differential salt tolerance of two cowpea cultivars. Braz. J. Plant Physiol.. 2010;22:113-122.
- [Google Scholar]
- Differential response of sugar beet to long-term mild to severe salinity in a soil-pot culture. Agriculture. 2019;9:223.
- [Google Scholar]
- Changes on proline, phenolic compounds and activity of antioxidant enzymes in Anethum graveolens L. under salt stress. Int. J. Agron. Plant Prod.. 2012;3:710-715.
- [Google Scholar]
- Interactions of nanoparticles with plants. In: Emerging Technologies and Management of Crop Stress Tolerance. Elsevier:; 2014. p. :159-180.
- [Google Scholar]
- Munns, R., 2005. Genes and salt tolerance: bringing them together. New Phytol., 167, 645-663. Available from http://dx.doi.org/10.1111/j.1469-8137.2005.01487.x. DOI 10.1111/j.1469-8137.2005.01487.x.
- Munns, R., Tester, M., 2008. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol., 59, 651-681. Available from http://dx.doi.org/10.1146/annurev.arplant.59.032607.092911. DOI 10.1146/annurev.arplant.59.032607.092911.
- Nadernejad, N., Ahmadimoghadam, A., Hossyinifard, J., Poorseyedi, S., 2013. Effect of different rootstocks on PAL activity and phenolic compounds in flowers, leaves, hulls and kernels of three pistachio (Pistacia vera L.) cultivars. Trees, 27, 1681-1689. Available from http://dx.doi.org/10.1007/s00468-013-0915-8. DOI 10.1007/s00468-013-0915-8.
- Parida, A.K., Das, A.B., 2005. Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf., 60, 324-349. Available from http://dx.doi.org/10.1016/j.ecoenv.2004.06.010. DOI 10.1016/j.ecoenv.2004.06.010.
- Antioxidant enzymes activity and phenolic compounds content in red cabbage seedlings exposed to copper stress. Ecotoxicol. Environ. Saf.. 2009;72:596-602.
- [Google Scholar]
- Qi, M., Liu, Y., Li, T., 2013. Nano-TiO2 Improve the Photosynthesis of Tomato Leaves under Mild Heat Stress. Biol. Trace Elem. Res., 156, 323-328. Available from http://dx.doi.org/10.1007/s12011-013-9833-2. DOI 10.1007/s12011-013-9833-2.
- Rameeh, V., 2012. Ions uptake, yield and yield attributes of rapeseed exposed to salinity stress. J. Soil Sci. Plant Nutr., 0-0. Available from http://dx.doi.org/10.4067/s0718-95162012005000037. DOI 10.4067/s0718-95162012005000037.
- Effects of potassium on phenological, physiological and agronomic traits of maize (Zea Mays L.) under high nitrogen nutrition with optimum and reduced irrigation. Appl. Ecol. Environ. Res.. 2018;16:7079-7097.
- [Google Scholar]
- Shah, T., Latif, S., Khan, H., Munsif, F., Nie, L., 2019. Ascorbic Acid Priming Enhances Seed Germination and Seedling Growth of Winter Wheat under Low Temperature Due to Late Sowing in Pakistan. Agronomy, 9, 757. Available from http://dx.doi.org/10.3390/agronomy9110757. DOI 10.3390/agronomy9110757.
- Shah T, Munsif F, D'amato R, Nie L., 2020. Lead toxicity induced phytotoxic impacts on rapeseed and clover can be lowered by biofilm forming lead tolerant bacteria. Chemosphere. 246:125766.
- Relative leaf water content as an efficient method for evaluating rice cultivars for tolerance to salt stress. Sci. Asia. 2004;30:411-415.
- [Google Scholar]
- Phytohormone priming: regulator for heavy metal stress in plants. J. Plant Growth Regul.. 2019;38:339-752.
- [CrossRef] [Google Scholar]
- Zhang, C.F., Hu, J., Lou, J., Zhang, Y., Hu, W.M., 2007. Sand priming in relation to physiological changes in seed germination and seedling growth of waxy maize under high-salt stress. Seed Science and Technology, 35, 733-738. Available from http://dx.doi.org/10.15258/sst.2007.35.3.19. DOI 10.15258/sst.2007.35.3.19.
- Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J. Pineal Res.. 2013;54:15-23.
- [Google Scholar]







