Translate this page into:
Seasonal variations of colony activities linked to morphometric and glands characterizations of hybrid Carniolan honey bee (Apis mellifera carnica Pollmann) workers
⁎Corresponding author. elkazafi.taha@agr.kfs.edu.eg (El-Kazafy A. Taha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The activity and productivity of the honey bee colony depend upon many factors operating simultaneously and reacts to some environmental conditions. We aimed to study the morphometric characteristics and glands development of nurse and forager of hybrid Carniolan honey bee (Apis mellifera carnica Pollmann) workers in relation to activities of the colony during the different seasons.
Methods
The study was conducted during spring, summer, fall, then winter in 2020/2021. Ten colonies of hybrid Carniolan honey bees, every colony with about 14,000 bees, were utilizedz. The seasonal variations of foraging activity, hoarded pollen area, and sealed brood area were determined. Also, the body weight, some morphometric characteristics, and development of glands of nurses and foragers bees were measured.
Results and conclusions
The highest numbers of foragers and pollen foragers/colony/min., stored pollen area, and worker sealed brood area were recorded in May. Measurements of nurses reared during spring were larger than nurses and foragers of other seasons for body weight, mandibular and hypopharyngeal glands (HPGs), length and width of the 2nd wax mirror. The highest proboscis and corbicula lengths were found in foragers reared during spring, without significant differences between worker castes. It can be concluded that the performance of the colony, morphometric characteristics, and development of glands were dependent on the time of the year. Beekeepers in Kafrelsheikh province and similar environmental condition areas should provide the colonies with pollen substitutes or supplements during April, June, and October to February to maintain the strength of the colonies.
Keywords
Brood
Foragers
Hypopharyngeal glands
Mandibular glands
Nurses
Wax glands
1 Introduction
The honey bee (Apis mellifera L.) colony shows two systems in the division of labor: the first one (temporal polyethism) occurs in active seasons during spring and summer, and the second (generalist workers) occurs in the winter. During the active seasons, there are four castes (Johnson, 2010): (1) cell cleaners during the first 4-days of the worker age (Johnson, 2010), (2) nurses from 4 to 12 days of age (Seeley, 1995), (3) middle-aged bees from ages 12–21 days (Johnson, 2008), and (4) foragers from age 21 days (Seeley, 1995). The nurses care and feed the queen, act as messenger bees by spreading the pheromones of the queen through the nest (Free, 1987), and feed royal jelly to the queen and brood (Kamakura, 2011; Ali et al., 2019). The foragers gather the four sources required for the colony: nectar, pollen, water, and propolis (Seeley, 1995).
The secretory vesicles of the HPGs develop in 3-days old workers, whereas top production happens in about 6-days. After that, the acini volume and the number of secretory vesicles decline, and resulting in no visible vesicles once the worker age reaches at 3-weeks (Deseyn and Billen, 2005; Ahmad et al., 2021). The HPG cells of wintertime workers have numerous secretory vesicles which are possibly accumulated till spring (Deseyn and Billen, 2005; Richter et al., 2016). The development and activity of the HPGs of honey bee workers are variable and tend to depend on the age of the worker (Ali et al., 2019), nectar and pollen flora (Hrassnigg and Crailsheim, 1998), and rearing season (Ali et al., 2019; Shakeel et al., 2020). Also, the protein level in the diet (DeGrandi-Hoffman et al., 2010; Shawer and Mousa, 2016) and the species/subspecies of honey bee (Ali et al., 2019; Shakeel et al., 2020) have been reported as effective factors in the development of the HPGs.
The activity and productivity of the honey bee colony depend upon many factors operating simultaneously and reacts to some environmental conditions. Colony productivity has been correlated with worker body size (Kolmes and Sam, 1989). The worker body weight is persuaded by various factors for example the availability of nectar and pollen flora, rearing season (Ivanov and Spasov, 1990), age of comb (Taha et al., 2021b). In addition, the race of the honey bee was recorded as an important factor to influence the weight of a worker (Al-Kahtani and Taha, 2021).
The impact of the season on the foraging activity, collecting and depositing pollen, and colony growth has been reported (Taha, 2015a–c; Taha and Al-Kahtani, 2019; Taha et al., 2019; Al-Kahtani and Taha, 2020a; Shawer et al., 2021; Taha et al., 2021b). Also, the nutritive value of bee pollen has been influenced by the harvest season (Al-Kahtani and Taha, 2020a; Al-Kahtani et al., 2020; Al-Kahtani et al., 2021). Very few data are available on nurse and forager bees and their relationship with colony productivity during different seasons. Therefore, the present study aimed to investigate the seasonal variations of the colony activities in relation to the morphometric characteristics and development of glands of nurse and forager workers.
2 Materials and methods
2.1 Experimental site
Research experiments were accomplished at the apiary of the Faculty of Agriculture, Kafrelsheikh University, Kafrelsheikh, Egypt, during springtime (March–May), summertime (June–August), fall (September–November), and wintertime (December–February) in 2020/2021. Kafrelsheikh is located at longitude 30° 56′45″E, latitude 31° 6′42″N, and an altitude of 17 m above sea level.
2.2 Activities of the colony
Ten colonies (14,000 bees on seven combs for each) of hybrid Carniolan honey bees (A. m. carnica) ruled by newly naturally insmeinated sister queens were made equal for brood (5 brood combs), stored food, and bee population. All colonies were controlled for Varroa infestation before the beginning of the experiment. In visual observation, the foraging activity was estimated by counting the numbers of foragers and pollen foragers at 8:00, 10:00, 12:00, 14:00, and 16:00 hrs twice a week using a tally counter, and the peaks of foraging activity were recorded. The areas (inches2) of stored pollen, worker sealed brood were calculated at 12 days intervals by means of an unoccupied standard frame separated into inches2.
2.3 Body weight and some morphometric characteristics
The nurse workers amassed from the brood nest area, and the forager workers collected from the flight board from the same colony. Fifty workers of each group were used to determine the fresh body weight (mg) by means of an electrical balance. The worker was dissected to separate the proboscis and the right hind leg. The proboscis and corbicula lengths were measured using the Scan Photo technique, according to Al-Aw et al. (2012). An HP scanner at a high resolution of 1200 dpi was connected with a personal computer, and the samples were scanned. After taken the image, it was saved on the computer and measured using a Photoshop software program (Adobe Photoshop CS5).
2.4 Glands development
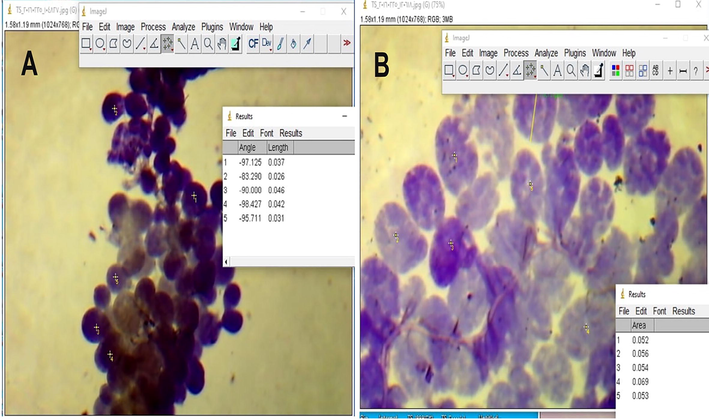
Fifty workers of each group were used to estimate the areas of the mandibular gland and HPG acini and the 2nd wax mirror length and width. The head of the worker was dissected to remove the glands. The gland was put on a slide, and an image was taken using a high magnification microscope containing a digital camera connecting with a computer. The area was measured using an Image J 1.46 program for geometric morphometric analysis. The area was calculated after an inter-scale measuring, which 400 pixels equal 1 mm. The 2nd wax mirror was removed from the 5th abdominal sternite. The maximum length and width of the mirror were measured using an Image J software program after an inter-scale measuring, which 250-pixels equal 1 mm.
2.5 Statistical analysis
The variances between nurses and foragers through spring, summer, autumn, and winter were examined by the two-way analysis of variance (ANOVA). The normalcy in statistics was confirmed by the Shapiro-Wilk normality test, that revealed the normal distribution of the data. So, the evaluation was accomplished on the original statistics. The ANOVA was applied to evaluate changes between the nurses and foragers during the spells tested via the PROC GLM function in SAS version 9.1 (SAS, 2003). Duncan’s Multiple Range Test (Duncan, 1955) was employed for comparison of treatment means.
3 Results
3.1 Activities of the colony
Data illustrated in Figs. 1–3 showed that the highest average numbers of foragers, pollen foragers/colony/min., stored pollen area, and worker sealed brood area (66.20 ± 3.45 workers, 29.00 ± 2.67 workers, 256.10 ± 9.15 inches2, and 1218.7 ± 16.53 inches2, respectively) were obtained in May. The lowest values of the previous parameters (23.20 ± 2.56 workers, 9.20 ± 1.48 workers, 71.82 ± 6.14 inches2, and 283.50 ± 9.47 inches2, respectively) were recorded in December.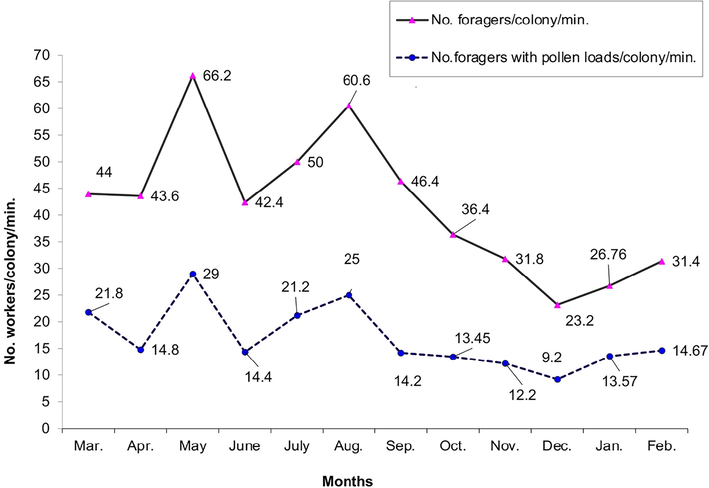
Monthly fluctuation of the foraging activity in Kafrelshiekh Province during 2020/2021.
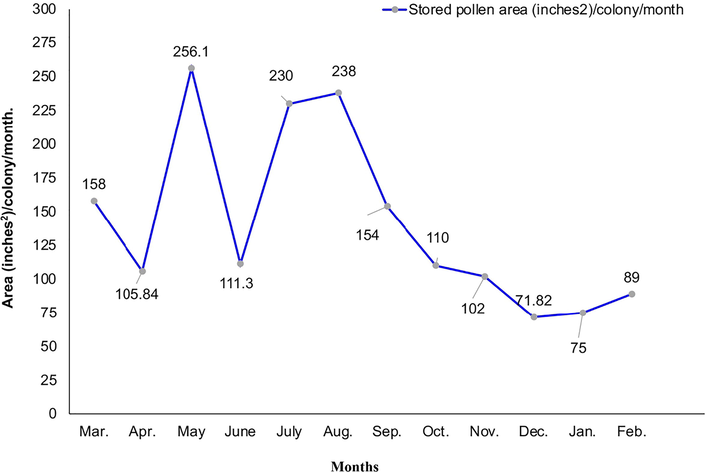
Monthly fluctuation of storing pollen in Kafrelshiekh Province during 2020/2021.
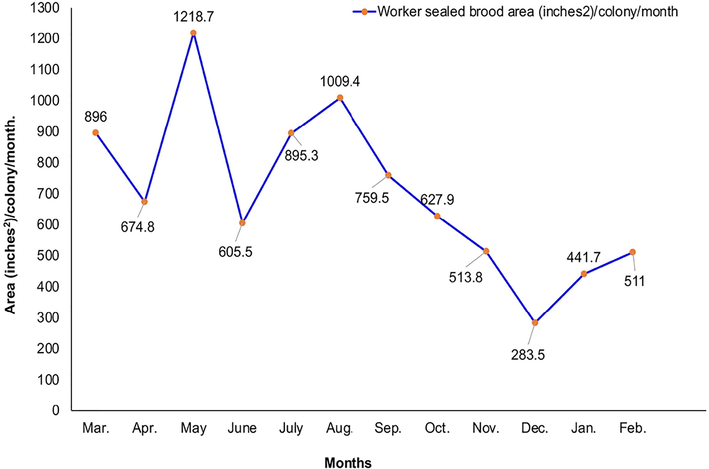
Monthly fluctuation of worker brood rearing in Kafrelshiekh Province during 2020/2021.
3.2 Body weight, some morphometric characteristics, and glands development
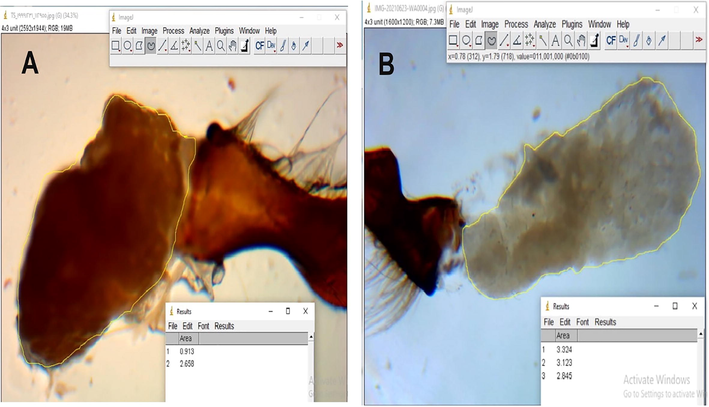
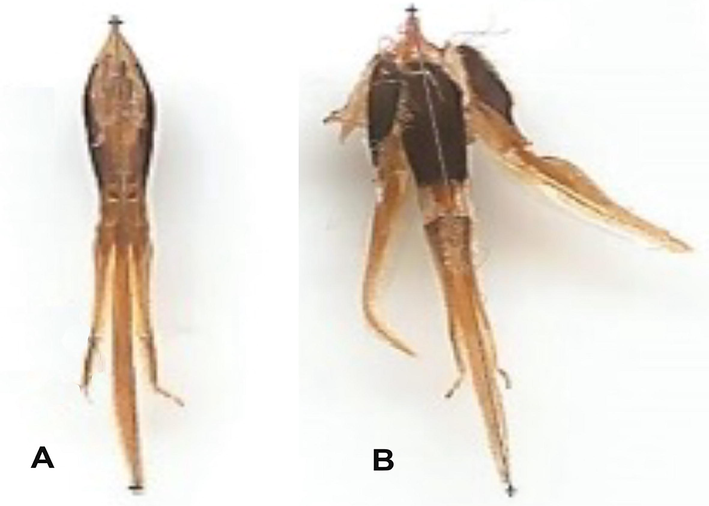
Data presented in Tables (1 and 2) showed that the body weight, corbicula length (Fig. 4), areas of the mandibular gland (Fig. 5) and acini (Fig. 6), and the 2nd wax mirror length and width (Fig. 7) of nurses and foragers were significantly (P < 0.01) varied and depended on the rearing season. No significant differences were found in proboscis length (Fig. 8) either between worker castes or among seasons. The highest values of body weight (110.87 mg), area of the mandibular gland (2.43 mm2), acini area (0.68 mm2), length (1.90 mm), and width (2.50 mm) of the 2nd wax mirror were found in the nurses reared during spring. The tallest lengths of the proboscis (6.09 mm) and corbicula (3.81 mm) were obtained from foragers reared during spring. The lowest values of body weight (65.5 mg), area of the mandibular gland (1.35 mm2), acini area (0.045 mm2), length (1.35 mm) and width (1.45 mm) of the 2nd wax mirror were obtained from the foragers reared during autumn. The shortest lengths of the proboscis (6.06 mm) and corbicula (2.81 mm) were obtained from the nurses reared during autumn. The values of the body weight, corbicula length, areas of the mandibular gland and acini, and the 2nd wax mirror length and width of nurses and foragers could be arranged in the following descending order: spring > summer > winter > autumn (Tables 1 and 2). Quantities are the average ± standard deviation. The averages of every factor afterwards the different letters are substantially distinctive at the 0.01 level. ** indicate P < 0.01, NS indicates P > 0.05. Quantities are the mean ± standard deviation. The averages of every factor afterwards the different letters are are substantially distinctive at the 0.01 level.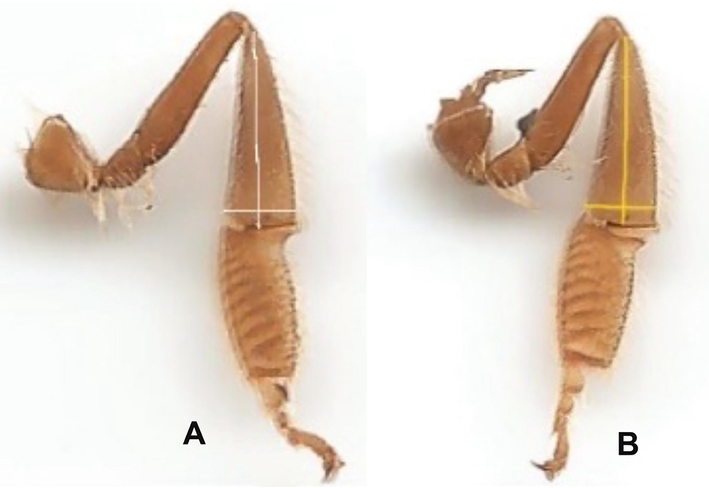
Corbicula length and width in forager (A) and nurse (B) workers.
Mandibular gland area in forager (A) and nurse (B) workers.
Acini area in forager (A) and nurse (B) workers.
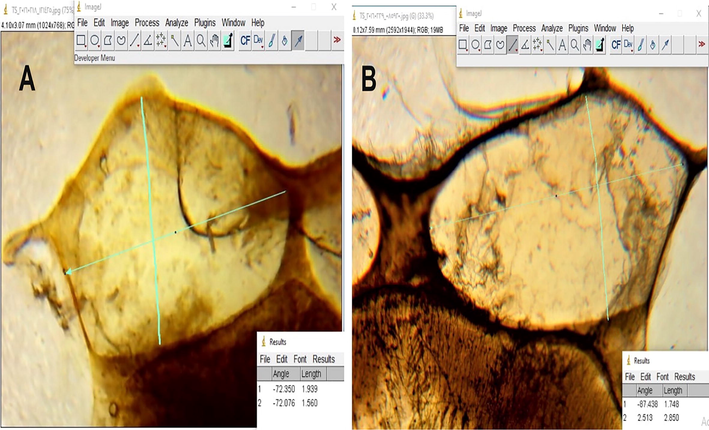
Second wax mirror length and width in forager (A) and nurse (B) workers.
Proboscis length in forager (A) and nurse (B) workers.
Season
Body weight
Proboscis length
Corbicula length
Nurse
Forager
Nurse
Forager
Nurse
Forager
Spring
110.87 ± 2.47a
79.35 ± 2.49c
6.08 ± 0.08
6.09 ± 0.12
3.77 ± 0.13 a
3.81 ± 0.08 a
Summer
110.10 ± 4.67a
79.15 ± 3.45c
6.08 ± 0.08
6.09 ± 0.11
3.77 ± 0.24a
3.78 ± 0.31a
Autumn
96.20 ± 5.43b
65.50 ± 4.64e
6.06 ± 0.05
6.08 ± 0.10
2.81 ± 0.13b
2.83 ± 0.14b
Winter
100.11 ± 5.45b
70.46 ± 4.23 d
6.07 ± 0.16
6.08 ± 0.11
2.85 ± 0.10b
2.87 ± 0.11b
Significance
**
NS
**
Season
Mandibular gland area
Acini area
2nd wax mirror
Length
Width
Nurse
Forager
Nurse
Forager
Nurse
Forager
Nurse
Forager
Spring
2.43 ± 0.06a
1.86 ± 0.05b
0.068 ± 0.01a
0.057 ± 0.01b
1.90 ± 0.09a
1.69 ± 0.11b
2.50 ± 0.09a
2.16 ± 0.10c
Summer
1.78 ± 0.06b
1.60 ± 0.13c
0.064 ± 0.01ab
0.055 ± 0.01b
1.86 ± 0.07a
1.63 ± 0.12c
2.31 ± 0.11b
2.15 ± 0.10c
Autumn
1.43 ± 0.23de
1.35 ± 0.15e
0.055 ± 0.01b
0.045 ± 0.01c
1.43 ± 0.15 d
1.35 ± 0.24e
1.79 ± 0.10 e
1.65 ± 0.08f
Winter
1.55 ± 0.10 cd
1.49 ± 0.24de
0.058 ± 0.01b
0.045 ± 0.01c
1.62 ± 0.07c
1.43 ± 0.05d
1.91 ± 0.10d
1.79 ± 0.06 e
Significance
**
**
**
**
4 Discussion
The searching for nectar and pollen is persistent all over the year. In the present study, the max numbers of foragers and pollen foragers were documented during May [the flow spell of Egyptian clover (Trifolium alexandrinum L.)], trailed by August when cotton (Gossypium spp.), sunflower (Helianthus annuus L.), maize (Zea mays L.), and some Cucurbitaceae plants were bloomed. A gradual decrease occurred after August until it reached the lowest values in December and then raised during January and February. The decrease of flight activity during September–December resulted from a shortage of pollen and nectar flora. These findings are in harmony with the outcomes of Taha et al. (2019, 2021a,b) in Egypt, who found the highest numbers of foragers and pollen foragers during May, afterward July and August. In contrast, Taha and Al-Kahtani (2019) in Saudi Arabia have found the highest numbers of foragers during September and October [the sidr, Ziziphus spp. flow period (Taha et al., 2021a)]. The rate of foraging activity is affected by the obtainability of bee plants, colony population range, and weather conditions (Taha, 2014).
The prevalent area of stored pollen and worker sealed brood were recorded during May, after that July and August. A significant reduction happened from September to December, then came back to rise during January and February. These outcomes are relatively similar to those of attained by Taha et al. (2021b) in Egypt. The major peak of stored pollen in Saudi Arabia (Taha, 2014; Taha, 2015a; Taha and Al-Kahtani, 2019) and India (Sattigi and Lingappa, 1993) was found in March. Conversely, the maximum area of stored pollen has been noted through October in Ethiopia (Wakjira et al., 2020) and during April in Himachal Pradesh, India (Brar, 2018). The amount of stored pollen in the combs influenced by the rate of pollen collection and utilization (Taha and Al-Kahtani, 2019).
Three peaks of worker sealed brood area were observed analogous to the apexes of stored pollen. The first occurred in March, the second and the greatest occurred in May, and the third occurred in August. The brood-rearing was reliant on on the quantity of gathered pollen, which is subject to the availability of pollen flora and the rate of foragers (Taha, 2014). Relatively similar results have been obtained by Taha et al. (2021b). A significant decrease in worker brood-rearing occurred from October to December resulted from the sharp shortage of pollen supply. In contrast, a high rate of brood-rearing in Sudan has recorded in November (Musa et al., 1989).
Except for proboscis and corbicula lengths, all the tested parameters were substantially (P < 0.01) influenced by the worker castes. The proboscis length did not affect either by season or worker castes. Simultaneously, the corbicula length was affected by the season but did not affect by the worker castes. Al-Kahtani and Taha (2014, 2021) discovered important positive correlations among the proboscis, corbicula lengths, and body weight.
The nurses were significantly heavier than the foragers, but the lengths of the proboscis and corbicula were insignificantly taller in the foragers. The body weight of the honey bee worker could be used as an indicator of morphometric characteristics (Al-Kahtani and Taha, 2021). The body weight and morphometric features associated to the colony productivity, including proboscis and corbicula lengths, could predict the colony yield (Mostajeran et al., 2006). Honey production was surely linked to proboscis length, that one is clearly correlated with the brood area (Mostajeran et al., 2006). It is known that Apis mellifera carnica, which has a large body size with a larger proboscis, wings, and corbicula (Al-Kahtani and Taha, 2021), produces greater honey yield than A. m. jemenitica (Taha and Al-Kahtani, 2019).
The body weight, proboscis and corbicula lengths of the nurses and foragers were substantially (P < 0.01) shaped by the rearing season. In this study, all workers were obtained from one queen, and all of them were reared in the same colony, so the differences among the body size of workers in the same caste should be due to the rearing season. Spring workers showed the highest values of body weight, proboscis and corbicula lengths due to the abundance of nectar and pollen resources. Body size has reportedly correlated with the lengths of proboscis and corbicula, and other morphometric characterizations related to colony productivity (Al-Kahtani and Taha, 2021).
The importance of the mandibular and HPGs is due to the secretion of royal jelly (Al-Kahtani and Taha, 2020b,c). In our study, the mandibular, hypopharyngeal, and wax glands were significantly (P < 0.01) more developed in the nurses than the foragers. Similar results have been reported by Shawer and Mousa (2016), Aliet al. (2019), and Shakeel et al. (2020). They found that the development of the mandibular gland and HPG acini have been affected by worker age. Feeding the queen and brood on a royal jelly by the nurses (Ali et al., 2.019; Shakeel et al., 2020) explains the superiority of the mandibular gland and HPG acini of the nurses compared to those in foragers. The area of the mandibular gland has clearly correlated with the secretion of royal jelly (Peters et al., 2010). In this study, the length and width of the 2nd wax mirror in nurses were significantly exceeded those in the foragers. In contrast, Shawer and Mousa (2016) found that wax glands have not affected by the age of the worker. According to Johnson (2010), the middle-aged bees spend more time on comb building, and by the end of the third week of age, switch to foragers. This explains why the wax glands are more developed in nurses than the foragers. Simultaneously, the mandibular, hypopharyngeal, and wax glands in spring workers were more developed than those in other seasons. The development of the glands was related to the abundance of nectar and pollen sources (Ali et al., 2019; Shakeel et al., 2020).
Under the environmental conditions of Kafrelsheikh province, the colonies showed the best performance in spring due to the presence of workers with good morphometric characterizations and developed glands related to the colony productivity, which itself related to the colony performance that resulted from the abundance of nectar and pollen flora. Based on the low amount of stored pollen during April, June, and October to February, beekeepers should provide the colonies with pollen substitutes or supplements during these periods.
5 Conclusion
The present study established that the foraging activity, collecting and storing pollen, and brood-rearing were extensively relied on the time of the year. The values of body weight, areas of the mandibular gland and HPG acini, and 2nd wax mirror length and width in nurses were higher than in foragers. The best performance of the colony occurred in spring. Therefore, beekeepers in Kafrelsheikh province and similar environmental conditions regions should provide the colonies with pollen substitutes or supplements during April, June, and October to February to maintain the strength of the colonies.
Acknowledgments
The authors extend their appreciation to Taif University for funding the current work by Taif University Researchers Supporting Project number (TURSP – 2020/142), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel insight into the development and function of hypopharyngeal glands in honey bees. Front. Physiol.. 2021;11:1853.
- [Google Scholar]
- Measuring the morphological characters of honey bee (Apis mellifera L.) using a simple semi-automatic technique. J. Amer. Sci.. 2012;8:558-564.
- [Google Scholar]
- Effect of season and behavioral activity on the hypopharyngeal glands of three honey bee Apis mellifera L. races under stressful climatic conditions of central Saudi Arabia. J. Hymenoptera Res.. 2019;68:85-101.
- [Google Scholar]
- Morphometric studies on dwarf honey bee Apis florea F. workers in Saudi Arabia. J. Apic. Sci.. 2014;58:127-134.
- [Google Scholar]
- Seasonal variations in nutritional composition of honey bee pollen loads. J. Kansas Entomol. Soc.. 2020;93:105-112.
- [Google Scholar]
- Effect of harvest time on royal jelly yield and chemical composition. J. Kansas Entomol. Soc.. 2020;93:132-139.
- [Google Scholar]
- Post grafting time significantly influences royal jelly yield and content of macro and trace elements. PLoS One. 2020;15
- [Google Scholar]
- Morphometric study of the Yemeni (Apis mellifera jemenitica) and Carniolan (A. m. carnica) honey bee workers in Saudi Arabia. Plos One. 2021;16:0247262.
- [Google Scholar]
- Effect of harvest season on the nutritional value of bee pollen protein. Plos One. 2020;15
- [Google Scholar]
- Harvest season significantly influences the fatty acid composition of bee-pollen. Biology. 2021;10:482.
- [Google Scholar]
- Brar, A.S.; Sharma; H.K.; Rana, K., 2018. Colony strength and food reserves of Apis mellifera L. under stationary and migratory beekeeping in Himachal Pradesh India. J. Entomol. Zool. Stud. 6, 1156–1159
- The effect of diet on protein concentration, hypopharyengeal gland development and virus load in worker honey bees (Apis mellifera L.) J. Insect Physiol.. 2010;56:1184-1191.
- [Google Scholar]
- Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae) Apidologie. 2005;36:49-57.
- [Google Scholar]
- Pheromones of Social Bees. Londan UK: Chapman and Hall; 1987.
- The influence of brood on the pollen consumption of worker bees (Apis mellifera L.) J. Insect Physiol.. 1998;44:393-404.
- [Google Scholar]
- Study of some physiological parameters characterizing productivity and winter resistance of honey bee. Zhivotnovudni Nauki. 1990;26:67-73.
- [Google Scholar]
- Within-nest temporal polyethism in the honey bee. Behav. Ecol. Sociobiol.. 2008;62:777-784.
- [Google Scholar]
- Division of labor in honey bees: form, function, and proximate mechanisms. Behav. Ecol. Sociobiol.. 2010;64:305-316.
- [Google Scholar]
- Relationships between sizes of morphological features in worker honeybees (Apis mellifera) J. N. Y. Entomol. Soc.. 1989;99:684-690.
- [Google Scholar]
- Analysis of colony and morphological characteristics in honey bees (Apis mellifera meda) Pakistan J. Biol. Sci.. 2006;9:2685-2688.
- [Google Scholar]
- Musa, F.H.; Abdalla, M.R.; El-Sarag, M.A., 1989. Studies on feeding colonies of honey bees in Sudan. Proc. 4th Inter. Conf. Apis in Tropic Climate, Cairo, Egypt, 6–10 Nov. 1988– London Uk; Inter. Bee Res. Assoc. 27–28.
- Effect of primer pheromones and pollen diet on the food producing glands of worker honey bees Apis mellifera L. J. Insect Physiol.. 2010;56:132-137.
- [Google Scholar]
- Secretory cells in honeybee hypopharyngeal gland: polarized organization and age-dependent dynamics of plasma membrane. Cell Tissue Res.. 2016;366:163-174.
- [Google Scholar]
- SAS, 2003. Institute SAS/STAT User’s Guide release 9.1. SAS Institute Inc, Cary, NC 27513.
- Foraging activities of Indian honey bee Apis Cerana Fabr. Under Dhrwad Conditions Karnataka. J. Agric. Sci.. 1993;6:352-354.
- [Google Scholar]
- Seeley, T.D., 1995. The wisdom of the hive. Harvard University Press, Cambridge.
- Seasonal impact and comparative analysis of hypopharyngeal glands in worker and forager honey bees of two different species: Apis mellifera and A. cerana. Fresenius Environ. Bull.. 2020;29:9024-9030.
- [Google Scholar]
- Effect of brewer yeast diet on the biological activities and the development of mandibular, hypopharyngeal, and wax glands of honey bees, Apis mellifera L. (Hymenoptera, Apidae) Bull. Entomol. Soc. Egpt. Econ. Ser.. 2016;42:13-20.
- [Google Scholar]
- Shawer, D.M.B.; Rakha, O.M.; Taha, E-K.A.; AL-Kahtani, S.N. and Elnabawy, S.M., 2021. The impact of caging the queens during the flow season on some biological activities of honey bee colonies. Saudi J. Biol. Sci. 28(4): 2975–2979.
- Seasonal variation of foraging activity, pollen collection and growth of honey bee colonies in Al-Ahsa, Saudi Arabia. Bull. Entomol. Soc. Egypt. 2014;91:163-175.
- [Google Scholar]
- A study on nectar and pollen sources for honey bee Apis mellifera L. in Al-Ahsa, Saudi Arabia. J. Entomol. Zool. Stud.. 2015;3:272-277.
- [Google Scholar]
- Chemical composition and amounts of mineral elements in honey bee-collected pollen in relation to botanical origin. J. Apic. Sci.. 2015;59:75-81.
- [Google Scholar]
- The impact of feeding certain pollen substitutes on maintaining the strength and productivity of honey bee colonies (Apis mellifera L.) Bull. Entomol. Soc. Egpt. Econ. Ser.. 2015;41:63-74.
- [Google Scholar]
- Comparison of the activity and productivity of Carniolan (Apis mellifera carnica Pollmann) and Yemeni (Apis mellifera jemenitica Ruttner) subspecies under environmental conditions of the Al-Ahsa oasis of eastern Saudi Arabia. Saudi J. Biol. Sci.. 2019;26:681-687.
- [Google Scholar]
- Nectar and pollen resources for honey bees in Kafrelsheikh, northern Egypt. Saudi J. Biol. Sci.. 2019;26:890-896.
- [Google Scholar]
- Taha, E-K.A., Al-K.ahtani, S.N. and Taha, R., 2021a. Comparison of physicochemical characteristics of sidr (Ziziphus spp.) honey produced by Apis florea F. and Apis mellifera L. J. Apic. Res. 60, 470–477.
- Comb age significantly influences the productivity of the honey bee (Apis mellifera) colony. J. King Saud Univ. – Sci.. 2021;33
- [Google Scholar]
- Two-queen colonies in central highland conditions of Ethiopia increase population size and honey yield. Bee World. 2020;97:109-113.
- [Google Scholar]







