Seasonal monitoring of River through heavy metal bioaccumulation and histopathological alterations in selected fish organs
⁎Corresponding author. arif143@yahoo.com (Tayyaba Sultana)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Heavy metal discharge is the primary cause of freshwater pollution, harming fish health. This research was planned to calculate the seasonal variations in metal (Pb, Cu, and Ni) bioaccumulation in different tissues of Gills, Liver, Kidney, and Muscle from Cirrhinus mrigala sampled from River Chenab from an area of high-intensity pollution near Thatta Muhammad Shah. The results revealed significant (P ≤ 0.5) variations in metals bioaccumulation in different seasons. The lowest level of selected metals was observed in winter, whereas the maximum level was noted in the summer. The concentration of selected heavy metals differed seasonally. During winter Pb concentration distribution in descending order was observed in liver (0.823 ± 0.009 ppm) > kidney(0.817 ± 0.011 ppm) > muscle(0.758 ± 0.009 ppm) > gills(0.742 ± 0.011 ppm). Whereas, in summer it was noted as liver (1.726 ± 0.066 ppm) > gills (1.633 ± 0.060 ppm) > kidney (1.575 ± 0.051 ppm) > muscle (1.532 ± 0.050 ppm). While during winter, the bioaccumulation of Cu in organs was noted with descending order of kidney (1.88 ± 0.038) > liver(1.75 ± 0.037) > gills (1.67 ± 0.043) > muscle (1.45 ± 0.031)ppm, while in summer, it was gills (2.85 ± 0.071) > kidney (2.84 ± 0.082) > liver (2.83 ± 0.068) > muscle (2.72 ± 0.065) ppm. In winter, the Ni bioaccumulation with descending order was in the kidney (7.83 ± 0.139) > liver (7.68 ± 0.159) > muscle (7.63 ± 0.162) > gills (7.61 ± 0.129)ppm and in a season of summer, it was detected as in kidney (8.71 ± 0.129) > muscle (8.59 ± 0.131) > liver (8.56 ± 0.141) > gills (8.56 ± 0.131) ppm. All studied heavy metals were found to be above the WHO permissible limits. Histopathological evaluation of fish tissues has been considered an important indicator of environmental pollution monitoring. Histology of kidney and liver tissues showed congestion, necrosis, dilation in Bowman capsule space, while liver showed necrosis, cytoplasmic vacuolation, and sinusoid dilation. This study revealed that the Chenab River is being polluted due to the untreated discharge of industrial effluents and sewage runoff from the city, and these heavy metals from anthropogenic pollution load have adverse effects on the fish population in the river system.
Keywords
Histopathology
Organs
Pollution
Heavy metals
Cirrhinus mrigala
1 Introduction
Aquatic life is at a rigorous threat due to continuously growing freshwater pollution (Alinnor and Obiji, 2010), increasing daily (Vander et al., 2003). Freshwater contamination is the primary cause of heavy metal accumulation in the various organs of freshwater fish (Al-Attar, 2005; Afshan et al., 2014). The introduction of toxic domestic wastes into water bodies decreases biological oxygen demand to the fatal level. Untreated industrial wastewater release highly toxic chemicals and metallic salts into rivers, causing the demise of aquatic life even at low concentrations of these toxicants (Shaheen and Jabeen, 2015; Sultana et al., 2017; Zaqoot et al., 2017).
Pb, Ni and Cu are toxic heavy metals and enter freshwater bodies through erosion and leaching of soil, combustion of gasoline, industrial wastes, mining, and wastewater treatment plants (Department of Water Affairs and Forestry, 1996; ATSDR, 1997). Cu is the most toxic metal for aquatic organisms and ecosystems. However, Cu concentrations that exceed 20 µg/g can be harmful (ATSDR, 1997). A higher level of heavy metals in wastewater is the leading cause of lethal histological alterations in vital tissues of fish species due to their tendency of bioaccumulation (Gupta et al., 2017). The kidney and liver were selected as target organs for detecting metal accumulation due to their involvement in removing toxic metabolites' detoxification processes (Tepe et al., 2008). Fish are sensitive organisms to the changes in their environment, including pollution, and act as model animals to reflect such changes (Nikalje et al., 2012). Fish histopathology is used as a biomonitoring tool of aquatic pollution that gives information on the health risk of chemicals/pollutants to humans through fish consumption with cellular alterations and damage (Marchand, 2010). This study was planned to detect a seasonal effect on alteration in heavy metals bioaccumulation in various organs (kidney, liver, muscle, and gills) of Cirrhinus mrigala and evaluate the toxic effects of the metals through histopathological alterations.
2 Materials and methods
2.1 Animal collection and study site
Twenty fish samples were collected from the polluted river with an average total length of 32 cm and a wet weight of 1258 g. Fish was collected downstream to the entrance of Chakbandi Main Drain to River Chenab near Thatta Muhammad Shah (31° 32′ 57′ N, 72° 32′ 6′E), which is a highly contaminated area of the River Chenab (Hussain et al., 2016). The representative samples were collected from (November 2015 to October 2017). For further experimental work, the collected fish specimens were kept in polythene bags filled with oxygen and transported to the fisheries research laboratory, Department of Zoology, Government College University, Faisalabad. Farmed fish was used as control.
2.2 Heavy metals analysis
Water samples were collected in polystyrene water bottles (1.5-liter capacity). Water samples from the area of fish harvest were analyzed for heavy metal concentrations (Pb, Cu, and Ni). Samples were preserved with 55% HNO3 and stored at 4 °C in the refrigerator (Hussain et al., 2016). Metals were detected by Atomic Absorption Spectrophotometer (Aurora-A-I1200) following the protocol adapted by Mahboob et al. (2014).
2.3 Digestion of water sample
took 100 ml of preserved water sample in a conical flask. Heated on a hot plate till the volume reached 20 ml. Cool down it for some time and then added 5 ml of 55% HNO3 and 10 ml of 70 % HClO4. Heated this mixture until yellow fumes were replaced by white fumes under the fume hood and set them at room temperature by adding up to 100 ml distilled water. Filtered this mixture using Whatman filter paper pore size 0.45 µm, and the filtrate was analyzed for selected heavy metals.
2.4 Digestion of fish tissue samples
One gram of fresh sample tissue was weighed and taken into a glass beaker and digested with hydrogen peroxide (>35% H2O2) and nitric acid (65% HNO3) in a glass beaker and placed beaker on a hotplate under fumes hood until yellowish fumes changed into white. Cooled the sample at room temperature, added distilled water up to 100 ml, and filtered the sample with Whatman filter paper with pore size 0.45 µm. The filtrate was analyzed for Pb, Cu, and Ni concentrations according to APHA (1998) on an Atomic Absorption Spectrophotometer (Authman et al., 2015).
2.5 Histopathology
Fish specimens were recorded for morphometric measurements. Liver and kidney tissues were obtained following the method adopted by Sulatna et al. (2016). The tissue specimens were cut into about 5 mm thick pieces and were fixed in sera (60% ethanol + 10% acetic acid + 30% formalin) for 3 to 4 h at room temperature (Jabeen and Chaudhry, 2013). Fixed tissue sections were dehydrated at room temperature through ascending grades of ethanol and cleared with xylene, embedded with melted paraffin. Microtome (Histo-line MR 2258) was used for 3 µm thick tissue section cutting. Tissue slice ribbon from the water surface was picked on the slide coated with albumen/glycerin as an adhesive material and was then kept in an oven at 37 °C and then transferred into xylene thrice times each for three minutes. Slides were immersed in descending ethanol grade each for 1–2 min, and later these were stained for 3–5 min in hematoxylin. Mounting was done in Canada balsam; it was observed with a light microscope and photomicrographed. Histopathological changes were observed by comparing with recent literature and control.
2.6 Statistical analysis
The one-way ANOVA analyzed results for heavy metals. The results represent mean along with standard error. The variance was considered significant at P < 0.05. DMR test was used to compare the means. Statistical analyses were performed through SPSS-9.
3 Results and discussion
The maximum mean value of Pb (0.759 ± 0.015) in drain water was observed during winter while it was minimum (0.505 ± 0.005) during summer, whereas during spring and autumn it was (0.631 ± 0.009) and (0.645 ± 0.009), respectively. In river water, the maximum mean value of Pb (0.765 ± 0.043) was observed during winter, whereas it was observed minimum (0.340 ± 0.015) during autumn while during spring and summer it was (0.568 ± 0.015) and autumn 0.620 ± 0.035 ppm (Table 1). The maximum mean value of Cu (1.282 ± 0.021) in drain water was observed during winter while it was minimum (1.219 ± 0.036) during spring, whereas during summer 1.485 ± 0.034 and autumn, 1.419 ± 0.024 values were observed. In river water, a maximum mean value of Cu (0.576 ± 0.038) was observed during winter, whereas it was observed a minimum of 0.381 ± 0.014 during autumn while during spring and summer it was (0.384 ± 0.021) and 0.448 ± 0.020 ppm. The maximum mean value of Ni (1.666 ± 0.027) in drain water was observed during spring while it was minimum (1.143 ± 0.020) during autumn, whereas during winter and summer it was (1.225 ± 0.021) and (1.272 ± 0.013), respectively. In river water, a maximum mean value of Ni (0.490 ± 0.018) was observed during spring, and it was observed minimum (0.318 ± 0.011) during summer while during winter and autumn it was (0.407 ± 0.017) and autumn 0.367 ± 0.018 ppm (Table 1).
| Metal | Organ | Season | Mean | |||
|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | |||
| Pb | Gills | 0.742 ± 0.011 | 1.147 ± 0.046 | 1.633 ± 0.060 | 0.893 ± 0.024 | 1.103 ± 0.024CD |
| Kidney | 0.817 ± 0.011 | 1.152 ± 0.036 | 1.575 ± 0.051 | 0.986 ± 0.026 | 1.132 ± 0.021ABC | |
| Liver | 0.823 ± 0.009 | 1.197 ± 0.040 | 1.726 ± 0.066 | 1.012 ± 0.024 | 1.190 ± 0.025AB | |
| Muscle | 0.758 ± 0.009 | 1.111 ± 0.044 | 1.532 ± 0.050 | 0.878 ± 0.020 | 1.070 ± 0.021CDE | |
| Cu | Gills | 1.67 ± 0.043 | 2.14 ± 0.053 | 2.85 ± 0.071 | 2.31 ± 0.059 | 2.24 ± 0.033BCD |
| Kidney | 1.88 ± 0.038 | 2.27 ± 0.056 | 2.84 ± 0.082 | 2.47 ± 0.061 | 2.36 ± 0.034B | |
| Liver | 1.75 ± 0.037 | 2.22 ± 0.044 | 2.83 ± 0.068 | 2.03 ± 0.068 | 2.21 ± 0.032CD | |
| Muscle | 1.45 ± 0.031 | 1.92 ± 0.047 | 2.72 ± 0.065 | 2.02 ± 0.060 | 2.03 ± 0.032EF | |
| Ni | Gills | 7.61 ± 0.129 | 7.59 ± 0.129 | 8.56 ± 0.131 | 8.00 ± 0.137 | 7.94 ± 0.067BC |
| Kidney | 7.83 ± 0.139 | 8.02 ± 0.134 | 8.71 ± 0.129 | 8.16 ± 0.130 | 8.18 ± 0.068AB | |
| Liver | 7.68 ± 0.159 | 7.73 ± 0.163 | 8.56 ± 0.141 | 8.18 ± 0.142 | 8.04 ± 0.077BC | |
| Muscle | 7.63 ± 0.162 | 7.80 ± 0.129 | 8.59 ± 0.131 | 8.01 ± 0.145 | 8.01 ± 0.072BC | |
Means (capital letters) sharing similar letter in a row or in a column are statistically non-significant (P > 0.05). WHO permissible limits for Pb: 0.025 μg/g, Cu: 3.5 μg/g and Ni: 0.035 μg/g.
The selected metals concentrations were detected in selected organs of freshwater major carp Cirrhinus mrigala sampled from different sites of The Chenab River in different seasons of the year. The concentration of selected heavy metals differed seasonally. In winter, the concentration of Pb was recorded in gills (0.742 ± 0.011 ppm), kidney (0.817 ± 0.011 ppm), liver (0.823 ± 0.009 ppm), and muscle (0.758 ± 0.009 ppm) while during winter their concentration distribution in descending order was in liver > kidney > muscle > gills. The descending order of Pb concentration in summer was as liver (1.726 ± 0.066 ppm) > gills (1.633 ± 0.060 ppm) > kidney (1.575 ± 0.051 ppm) > muscle (1.532 ± 0.050 ppm). The highest level of Pb was observed in summer, and the minimum level was recorded in winter. All the organs showed non-significant (p > 0.05) differences in summer while significant (p < 0.05) among winter, spring, and autumn. In winter Cu concentration in the descending order was found in the kidney (1.88 ± 0.038) > liver(1.75 ± 0.037) > gills (1.67 ± 0.043) > muscle (1.45 ± 0.031)ppm. Whereas, in summer, the Cu concentration was observed in descending order in gills (2.85 ± 0.071) > kidney (2.84 ± 0.082) > liver (2.83 ± 0.068) > muscle (2.72 ± 0.065) ppm. During winter Cu concentration in kidneys and gills showed significant (p < 0.05) variation (Table 1). Liver and muscle also showed a significant difference between winter and spring. In winter, Ni concentrations were also observed, and the order of concentration was kidney (7.83 ± 0.139) > liver (7.68 ± 0.159) > muscle (7.63 ± 0.162) > gills (7.61 ± 0.129)ppm. In summer season gills, the concentration descending order was in the kidney (8.71 ± 0.129) > muscle (8.59 ± 0.131) > liver (8.56 ± 0.141) > gills (8.56 ± 0.131) ppm. Ni was in low concentration in winter and highest in the summer season. The Ni concentration was different non-significantly (p > 0.05) among all the selected organs and seasons (Table 1). In the present investigation, the liver of fish collected from the upstream river site exhibited a normal structure with hepatocytes collected in the winter season. Fish collected from downstream selected sites of River Chenab revealed hepatocytes degeneration and vacuolar degeneration in the spring season. Blood cell infiltration and cytoplasmic vacuolation were observed during autumn and summer seasons necrosis and dilation in sinusoids in hepatic tissues (Table 2; Fig. 1). Kidney photomicrographs showed normal renal corpuscles; glomerulus, and tubules, epithelial cells with prominent and round nuclei that characterize the proximal segment were observed during the winter and spring season. The experimental fish (Labeo rohita) specimens were collected downstream after receiving drain water, which showed more damage due to more pollution (Fig. 2). In case of the pathology of fish gills collected from upstream and downstream of River Chenab in different seasons. These results showed various changes in the histology of fish. During the winter season, gill lamellae were normal. Various gill lesions were observed during spring collection of fish gills, Primary and secondary lamellae congestion, and hypertrophy. During the autumn season, secondary lamellae breakage, shortening, and fusion of secondary lamellae were found. Damaged epithelium, lamellar hyperplasia) and several primary gill lamellae were clubbed with the accumulation of many inflammatory cells, especially at the base of the primary gill lamellae were observed during the summer season (Table 2; Fig. 3). The fish samples collected from the polluted (downstream) site of River Chenab and histological analysis revealed more damage like fragmentation in muscle fibers was observed during the spring season, congestion and edema were observed during autumn while during summer season necrosis and degenerated and splitter elements were observed as shown in (Table 2; Fig. 4).
| Parameters of kidney/Gills damage | Cirrhinus mrigala | Parameters of liver/Muscle damage | Cirrhinus mrigala |
|---|---|---|---|
| Necrosis | +++ | Cytoplasmic disorganization | +++ |
| Tubular hemorrhage | +++ | Cytoplasmic vacuolation | +++ |
| Congestion | +++ | Dilation of sinusoids | +++ |
| Fusion of lamellae | +++ | Necrosis | +++ |
| Lamellar breakage | +++ | Congestion | +++ |
| Lamellar hyperplasia | +++ | Edema | +++ |
| Clubbed lamellae | +++ | Degenerated and splitted elements | +++ |
| Cellular inflammation | +++ |
(+++) Severe
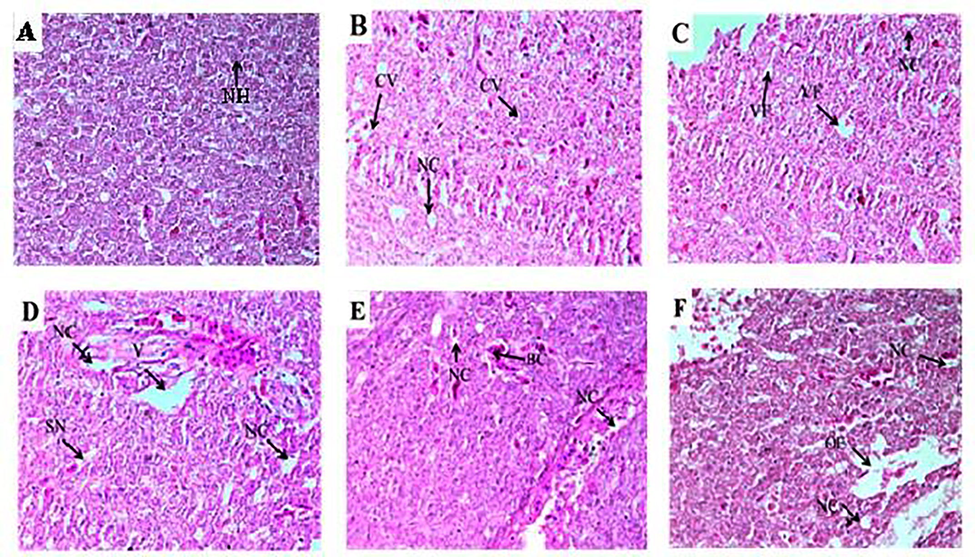
- Control tissue Photomicrographs of the liver Cirrhinus mrigala showing normal hepatocytes (NH) (A). Histological abnormalities like cytoplasmic vacuolation (CV), Necrosis (NC), Oedema (OE) and Sinusoid dilation (SD) (B-F). Histological photomicrograph of C. mrigala sampled from the Chenab River polluted sites (H and E staining, 40x).
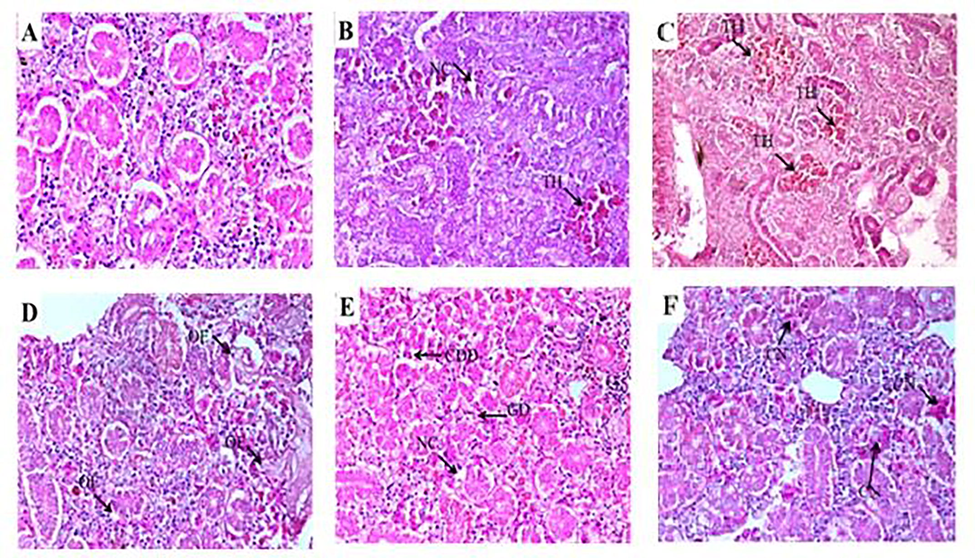
- Photomicrographs of control kidney tissue of Cirrhinus mrigala showing typical structures (A). Fish tissues collected from selected sites of River Chenab showed necrosis and tubular hemorrhage, edema, damaged glomerulus, and collecting duct damage and congestion (B-F). Congestion (CN) of blood cells, Dilation (DL) in Bowman capsule space were observed during autumn in upstream and downstream as shown in (Fig. 4BC&D). Necrosis (NC), Degeneration of glomerulus (DG) and Tubular Hemorrhage (TH) (H and E staining 40x).
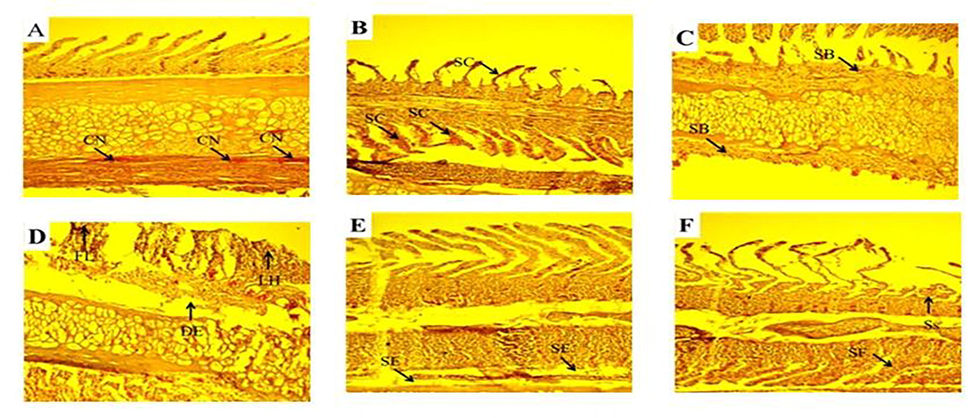
- Photomicrographs of gill tissues of Cirrhinus mrigala showing various gill lesions were observed during spring collection of fish gills, Primary and secondary lamellae congestion (SC) and hypertrophy (HY). During autumn season secondary lamellae breakage (SB), shortening and fusion of sec. lamellae (SS, SF), Congestion (CN). Damaged epithelium (DE), lamellar hyperplasia (LH) and several primary gill lamellae were clubbed (CB) with the accumulation of many inflammatory cells especially at the base of the primary gill lamellae were observed during summer season.
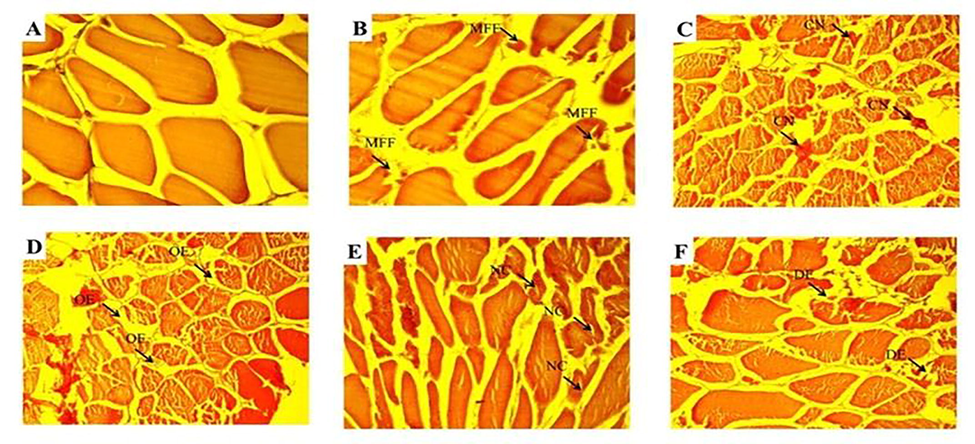
- The photomicrograph of muscle tissues showed normal structure of muscle bundle; muscle fiber and distinct nuclei were clearly observed collected from control and upstream sites during winter season (A). The fish samples collected from polluted (downstream) site of River Chenab and histological analysis revealed more damage like fragmentation in muscle fibers (MFF) was observed during spring season (B), congestion (CN) and edema (OE) were observed during autumn (D) while during summer season necrosis (NC) and degenerated and splitted elements (DE) were observed as shown in (E&F).
The present study revealed that kidneys, gills, muscles, and liver have higher levels of selected metals. The highest concentrations load of these selected metals was observed in summer in all the desired organs. Iqbal and Shah also described similar findings (Iqbal and Shah, 2014); in summer, they also observed maximum levels of the metals and minimum levels in the winter season in Cyprinus carpio from Rawal Lake. This study revealed maximum concentrations levels of metals in the liver, kidney and the lowest in the muscle. The findings of this study for heavy metal accumulation in fish were in line with Mohammadi et al. (2011) and Başyiğit and Tekin-Özan, (2013). The liver and kidneys perform detoxification and remove toxic substances from the bloodstream (Kent, 1998). The liver is a vital organ in vertebrates and has a significant metabolism (Liu et al., 2012). The accumulation of metals in the liver might be due to the more capacity to react with the oxygen carboxylate, amino group, and nitrogen in the metallothionein protein, whose level is highest (Al-Yousuf et al., 2000). The higher level of heavy metals in the fish body in summer may be due to the increased physiological motion of fish during summer (Canpolat and Çalta 2003). The freshwater contamination with a wide range of toxicants has become a matter of great concern to aquatic life and public water supplies (Sultana et al., 2017; Jamdade and Gawande, 2017). The liver histopathological alterations included cytoplasmic vacuolation, cytoplasmic disorganization, hepatic necrosis, edema, blood cells congestion, and dilation of the sinusoid. These outcomes were in line with the results of Bantu et al. (2017) for Labeo rohita. Faheem et al. (2016) stated that such tissue alterations for the liver include congestion of the central vein, hepatocytes necrosis, inflammation, and tissue degeneration. Bhuyan et al. (2014) described such kinds of tissue alterations in the liver of Oreochromis mossambicus. Liebel et al. (2013) reported similar modifications in the histology of the liver. These findings were substantiated by Paul et al. (2014), who observed distended sinusoids with pyknotic nuclei, necrosis, degeneration of blood vessels, degradation of hepatocytes, and vacuolation of cells. Rana et al. (2017) reported similar histological changes in the liver (Catla catla, Labeo rohita, and Cirrhinus mrigala). In this study, kidney sections of experimental fish showed edema, damaged glomerulus, collecting duct damage, tubular hemorrhage, necrosis in renal tubular cells, and blood congestion. The present study's findings upheld with Latif et al. (2012) described anomalies in the kidney such as the occlusion in the tubular lumen, necrosis and degeneration of the glomerulus tissue. Similar results were reported by Mohammed (2009). Udotong (2015) also reported similar findings in tilapia fish exposed to some of the heavy metals (Fe, Pb, and Cu), and our results are also in agreement with Abalaka (2015) too. Current study findings were similar to Mustafa et al. (2017) and Rana et al. (2017) in major carps.
Heavy metals like Cu, Ni, and Pb were examined in organs like the liver, kidney, gills, and muscles of the control fish enduring in the natural water system. The affected fish have a hazardous health impact on his ultimate consumer. Histopathological changes in vital organs of Cirrhinus mrigala are valuable tools to estimate the effects of xenobiotic toxicity in water. The conclusions of the present research are supportive for assessing ecotoxicological effects on freshwater pollutants that ultimately approach humans via the food chain (fish consumption). Anthropogenic actions are the leading cause of disproportion in the food chain. Aquatic loss and imbalance can be reduced by using advanced and more efficient technologies producing less or none of the heavy metal pollution.
4 Conclusions
The Chakbandi Main Drain delivers a substantial amount of contaminants to the River Chenab. Water and fish collected from the river showed a significant amount of heavy metal contamination. Histopathology of fish showed colossal tissue degradation in liver, kidney, gills, and muscle.
Acknowledgments
The authors (PI & Co-PI) extend their sincere appreciation to the HEC, Pakistan for the research support through NRPU Project No. 20-3416/NRPU/R&D/HEC/14/194. The authors (KAG) express their sincere appreciation to the Researchers Supporting Project number (RSP-2021/48), King Saud University, Riyadh, Saudi Arabia.
References
- Heavy metals bioaccumulation and histopathological changes in Auchenoglanis occidentalis fish from Tiga dam, Nigeria. J. Environ. Health Sci. Eng.. 2015;13:1-8.
- [Google Scholar]
- Effect of different heavy metal pollution on fish. Res. J. Chem. Environ. Sci.. 2014;2(2):35-40.
- [Google Scholar]
- Changes in haematological parameters of the fish, Oreochromis niloticus treated with sublethal concentrations of cadmium. Pak. J. Bio. Sci.. 2005;8(3):421-434.
- [Google Scholar]
- Assessment of trace metal composition in fish samples from Nworie River. Pak. J. Nutr.. 2010;9(1):81-85.
- [Google Scholar]
- Trace elements in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Tot. Environ.. 2000;256(2–3):87-94.
- [Google Scholar]
- Standard Methods for the Examination of Water and Waste Water. American Public Health Association; 1998. p. :874.
- ATSDR, 1997. Toxicological Profile for Nickel. (Final Report). NTIS Accession No. PB98-101199. Atlanta, G. A: Agency for Toxic Substances and Disease Registry. 293 pp.
- Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aqu. Res. Dev.. 2015;6(4):328-338.
- [Google Scholar]
- The histological investigation of gill and liver of freshwater fish, Labeo rohita after lethal and sublethal exposures to Dimethate. Innov. Int. J. Med. Pharm. Sci.. 2017;2(2)
- [Google Scholar]
- Concentrations of some heavy metals in water, sediment, and tissues of pikeperch (Sander lucioperca) from Karataş Lake related to physicochemical parameters, fish size and seasons. Poll. J. Environ. Stud.. 2013;22(3):633-644.
- [Google Scholar]
- Qualitative and quantitative analysis of fish tissues of Oreochromis mossambicus collected from Kedilam River, Cuddalore, Tamilnado, India. Int. J. App. Sci. Biotech.. 2014;2(2):135-141.
- [Google Scholar]
- Heavy metals in some tissues and organs of Capoetaca poetaumbla (Heckel, 1843) fish species in relation to body size, age, sex and seasons. Fresen. Environ. Bull.. 2003;12(9):961-966.
- [Google Scholar]
- Department of Water Affairs and Forestry (DWAF), 1996. South African Water Quality Guidelines. Volume 7: Aquatic Ecosystems. Department of Water Affairs and Forestry. Pretoria, South Africa.
- Histopathological effects of bisphenol on liver, kidneys and gills of Indian major carp, Catla catla (Hamilton, 822) JAPS. 2016;26(2):514-522.
- [Google Scholar]
- Risk assessment of heavy water pollution in middle stretch of river Ganga: an introspection. Int. Res. J. Environ. Sci.. 2017;6(2):62-71.
- [Google Scholar]
- Variation in genotoxic susceptibility and biomarker responses in Cirrhinus mrigala and Catla catla from different ecological niches of the Chenab River. Environ. Sci. Pollut. Res.. 2016;23(14):14589-14599.
- [Google Scholar]
- Study of seasonal variations and health risk assessment of heavy metals in Cyprinus carpio from Rawal Lake, Pakistan. Environ. Monit. Assess.. 2014;186(4):2025-2037.
- [Google Scholar]
- Metal uptake and histological changes in Gills and Liver of Oreochromis mossambicus inhabiting Indus River. Pak. J. Zool.. 2013;45(1):9-18.
- [Google Scholar]
- Basic Toxicology. New York: John Wiley Sons Inc; 1998. p. :1-402.
- Effect of cadmium chloride and ascorbic acid exposure on the vital organs of freshwater Cyprinid Labeo rohita. Afr. J. Biotech.. 2012;11(33):8398-8403.
- [Google Scholar]
- Fish histopathology as biomarker to evaluate water quality. Ecotoxicol. Environ. Cont.. 2013;8(2):9-15.
- [Google Scholar]
- Metal accumulation in the tissues of grass carps (Ctenopharyngodon idellus) from fresh water around a copper mine in Southeast China. Environ. Monit. Assess.. 2012;184(7):4289-4299.
- [Google Scholar]
- Tissue metal distribution and risk assessment for important fish species from Saudi Arabia. Bull. Environ. Cont. Toxicol.. 2014;92(1):61-66.
- [Google Scholar]
- Histological assessment of selected organs as biomonitoring tool to assess the health status of Clariasgarie pinus in two dams Rietvlei Nature Reserve. South Africa: Department of Zoology. University of Johannesburg; 2010. PhD Thesis
- Determination of heavy metals in two barbs, Barbus grypus and Barbus zanthopterus in Karoon and Dez Rivers, Khoozestan, Iran. J. Environ. Cont. Toxicol.. 2011;87:158-162.
- [Google Scholar]
- Histopathological studies on Tilapia zilli and Solea vulgaris from Lake Qarun, Egypt. J. Marin. Sci.. 2009;1:29-39.
- [Google Scholar]
- Histopathological alterations in gills, liver and kidney of common Carp, Cyprinus carpio L. exposed to lead acetate. Adv. Ani. Veter. Sci.. 2017;5(9):371-376.
- [Google Scholar]
- Histopathological changes in gills of a freshwater major carp Labeo rohita after acute and chronic exposure to textile mill effluent (tme) Int. J. Environ. Sci.. 2012;3:108-118.
- [Google Scholar]
- Copper and Cadmium induced histopathological alterations in liver of Heteropneustes fossilis (Bloch) at varying water pH. Int. J. Fisher. Aqu. Stud.. 2014;1(5):38-42.
- [Google Scholar]
- Histopathology of liver and kidneys for the assessment of genotoxicity in Indian major carps treated with domestic waste and industrial effluents of Chakbandi drain water. IJB. 2017;11(6):178-185.
- [Google Scholar]
- Effect of various doses of Cr (VI) on survival and growth of Cyprinuscarpio. Pak. J. Zool.. 2015;47(4):913-919.
- [Google Scholar]
- Fish scales as a non-lethal tool of the toxicity of wastewater from the River Chenab. Environ. Sci. Pollut. Res.. 2017;24(3):2464-2475.
- [Google Scholar]
- Assessment of heavy metals in two commercial fish species of four Turkish seas. Environ. Monit. Assess.. 2008;146(1-3):277-284.
- [Google Scholar]
- Histopathological changes in liver and muscle of tilapia fish from QIRE exposed to concentrations of heavy metals. IJBBAFBE. 2015;9:659-662.
- [Google Scholar]
- Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharm.. 2003;13(2):57-149.
- [Google Scholar]
- Baseline concentration of heavy metals in fish collected from Gaza fishing harbor in the Mediterranean Sea along Gaza Coast, Palestine. Turk. J. Fisher. Aqu. Sci.. 2017;17:101-110.
- [Google Scholar]







