Translate this page into:
Seasonal food composition of a burrowing asp, Atractaspis engaddensis Haas, 1950 from natural habitats of an arid Arabian desert
⁎Corresponding author. msadoon@ksu.edu.sa (Mohammed Khalid Al-Sadoon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The study was conducted to record seasonal variation in the diet composition of the burrowing asp, Atractaspis engaddensis. A total of 65 snake specimens (35 males and 30 females) were collected from Al-Qassim Region of Saudi Arabia. The captured individuals were weighed, measured and dissected to record the stomach content. The study showed that this carnivorous snake is nocturnal and feeds on rodents (75%), lizards 15% (Geckos and worm lizards), small snakes (7%) and unidentified materials (3%) independent of its sex. This finding is attributed to the intensive spread of rodents in the study area contributes in the preference of such type of food. With regard to the monthly and seasonal behavior of feeding, A. engaddensis was more active forager in terms of gut contents in May and November followed by June and September. The percentage of total amount of diet (g) in the stomach of A. engaddensis was highest in spring followed by autumn and summer. The activity of this snake was found to be decreased or ceased during winter months (December to February) owing to the decrease of temperature.
Keywords
Atractaspis engaddensis
Diet
Seasonal
Nocturnal
Arid desert
Saudi Arabia
1 Introduction
An interesting aspect about the feeding ecology of snakes is to know their prey selection (Greene, 1997). Limited studies have been undertaken about general biology of atractaspidids (Spawls et al., 2002) in general, however, many authors have reported their defensive behavior (Golani and Kochva, 1988), ecology (Greene, 1977; Boycott, 1995), feeding mechanics (Deufel and Cundall, 2003) and diets (Akani et al., 2001; Gower et al., 2004). Still basic dietary information for many snake species are missing (Mushinskhy, 1987). To understand the natural history of any species, feeding ecology makes the base for exploring its social behavior (Macdonald, 1983; Kruuk, 1995). Diet and behavior of seeking food may differ not only between animals of different sex but of different age groups as well (Begg et al., 2003). Animals might show seasonal variations in activity and feeding habits to fulfil their nutritional requirements (Gedir and Hudson, 2000).
There are many reports regarding feeding habits of reptiles (Stamps and Tanaka, 1981; Diaz and Carrascal, 1990; Castro et al., 1991; Loumbourdis and Hailey, 1991; Gilet al., 1994; Ballingeret al., 1995). Although feeding behaviour of many reptilian species of Saudi Arabia has been documented (Mandaviile, 1967; Al-ogily and Hussain, 1983; Al-Sadoon and Al-Otaibi, 2014; Al-Sadoon et al., 1999; 2016), however, the reports on the natural history of Atractaspis engaddensis are limited (Gasperetti, 1988).
About 65 species of Actractaspididae snakes are broadly distributed through Africa, with a restricted penetration into the regions of adjacent Middle East (Branch, 1988, 1998; Underwood and Kochva, 1993). The nocturnal burrowing asp, Atractaspis engaddensis belonging to family Atractaspididae is a fossorial venomous snake found in western Jordan, eastern and southern Israel and the Sinai Peninsula (Egypt). This species has also been recorded from the central and western region of Saudi Arabia (Al-Sadoon, 1989). This snake prefers dry bushland and olive groves, mostly found in loose soil, avoids sand and highly arid situations. Although, rarely encountered due to its fossorial habitat (Al-Oran and Amr, 1995), this species is common throughout their range (Force, 1935; Tennant, 1984).
Since there is a dearth of detailed information on the diet of this burrowing asp, this paper provides description of feeding ecology of the A. engaddensis in Al-Qassim Region of Saudi Arabia, and investigates how frequency of feeding change through the active season and differences in gut weights between males and females.
2 Materials and methods
All animals were handled as per the guidelines set for the Animal care and Ethics by King Saud University (KSU), Riyadh, Kingdom of Saudi Arabia. Snake specimens were collected during October 2015 to September 2016 from the study area, Al-Qassim Region (25°48′23″N 42°52′24″E) of Saudi Arabia which comprises different arid sandy habitats. A total of 65 individuals (35 males and 30 females) of A. engaddensis were collected during the study period. The study area was visited twice every month. The snakes were found through active search in the study area looking for their tracks, followed on foot to reach them in their burrows until the snakes were in sight. The captured individuals were placed at reptilian laboratory in Zoology Department, King Saud University at a temperature of 23–28 °C with free access to water.
Ecological field data of the collected specimens including distribution, their morphological characteristics and diet were recorded. For each snake snout vent length (SVL), tail length (TL), head length (HL), head width (HW) and body mass (BM) were recoded. SVL and body mass were measured from live or recently sacrificed individuals, whereas head length and head width were taken after preservation. The length from the medial and dorsal point above the jaw articulation to the tip of the snout was considered Head length. Head width was measured at the point of articulation of the mandible and quadrate bones. The sex of the snakes was determined by the observation of their gonads during dissection or by the presence or absence of hemipenes. All individuals were weighed and measured before dissection. The snakes were humanely euthanized by freezing at −2° for 24 h (Al-Sadoonet al., 2016). After dissection, seasonal diet composition analysis was carried out based on the collection in four different seasons: spring, summer, autumn and winter. The stomach removed from the dissected specimens were stored in 70% alcohol. After incision of the stomach, the contents were weight and the composition of each stomach was studied qualitatively using dissecting microscope. The number of each food item found in the stomach of dissected animals were identified and grouped as rodents, lizards, snakes and unidentified items to calculate their percent of occurrence.
One-way analysis of variance (ANOVA) was applied to specify any difference in the number of prey items and in the mean volume of the prey consumed per snake in various seasons.
3 Results and discussion
A total of 65 captures, adult males (SVL = 51.15 ± 5.2 cm, range = 37.5–63.4 cm, n = 35) and adult females (SVL = 54.24 ± 4.6 cm, range = 39.5–61.2 cm, n = 30), of A. engaddensis were analysed. Out of 65 snakes, 58 showed stomach contents. There were significant sexual differences (P < 0.05) in morphometric variables like snout vent length and total body mass of both sexes (Table 1).
Snout-vent length (cm)
Tail length (cm)
Head length (mm)
Head width (mm)
Body mass (g)
Sex
M
F
M
F
M
F
M
F
M
F
Sample size
35
30
35
30
35
30
35
30
35
30
Mean
51.15
54.24
3.12
3.14
14.91
14.94
9.85
9.98
53.92
56.24
Standard deviation
5.2
4.6
0.5
0.5
1.7
1.6
1.3
1.5
11.4
12.8
Minimum
37.5
39.5
2.1
2.3
10.2
7.4
7.2
6.3
43.2
39.5
Maximum
63.4
61.2
3.9
3.7
18.1
12.5
12.3
12.4
73.4
71.2
Prey items visually observed in each stomach were classified into 4 food types. Prey items included small rodents (75%) belonging to genus Gerbillus and Mus, lizards (15%) belonging Geckos (Stenodactylus slevinii, Bunopus tuberculatus) and worm lizard (Diplometopon zarudnyi) and snakes (7%) belonging to the Genus Leptotyphlops (Fig. 1). The remaining 3% of the stomach content were described as unidentified materials since they were completely digested.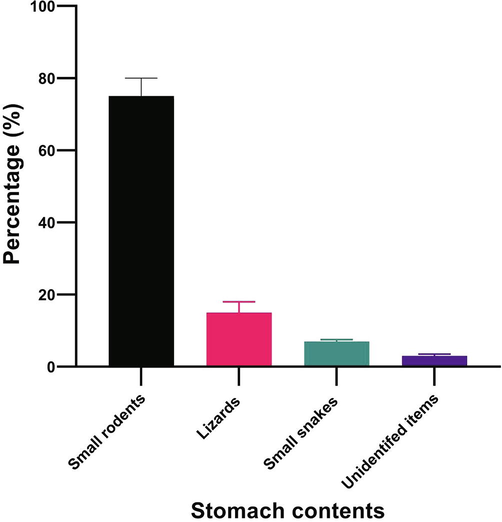
Percentage of various food categories found in the stomach of A. engaddensis.
Analysis of prey items identified of this species indicated the presence of rodents, lizards, small snakes suggesting the species to be carnivorous and may relate to the availability of these prey items. The findings are in agreement with earlier reports on different snake species such as, Cerastes c. gasperettii (Al-Sadoon et al., 2016); Eryx jayakari (Al-Sadoon and Al-Otaibi, 2014); Crotalus atrox (Reynolds et al., 1982) and Crotalus cottlinensis (Avila-Villegaset al., 2007). Presence of Geckos and worm lizard which comprised 15% of the total prey items in their stomach content is in agreement with the findings of Al-Sadoonet al. (2016) on the diet of the horned viper, Cerastes cerastes gasperettii whereas this is in contrast to the diet of Sand Boa, Eryx jayakari where lizards constitute 50% of the total diet (Al-Sadoon and Al-Otaibi, 2014).
Seasonal diet composition in terms of percentage show significant variation (Fig. 2). The snakes were active during spring with more preys in the stomach (35%), they reduced to the lowest proportion during the summer (32%), and then showed slight increase in terms of frequency of occurrence in autumn (33%). No snakes were caught in winter except in the month of December (n = 7) whose stomachs were empty.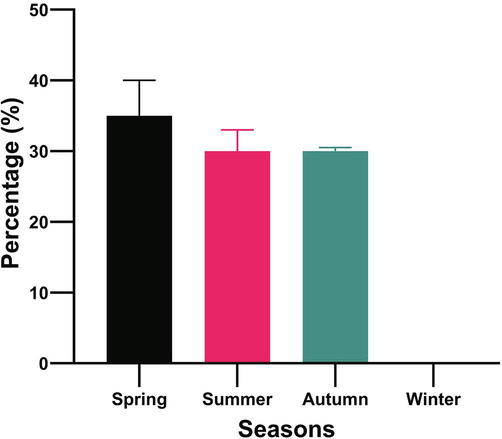
Percentage of total amount of diet (g) in the stomach of A. engaddensis during different seasons of the year.
Regarding the seasonal diet habits, percentage of the prey items was highest in spring. This might be attributed to the increase in availability of the rodents and lizards in the study area during this season. This is similar to the finding of Al-Sadoon and Al-Otaibi (2014) on the diet of the E. jayakari. Lowest percent of the stomach content during July may be because of intervening mating period during which interest in feeding is lost. However, feeding was rationally high during Autumn, and the feeding curves reached the peak in November for both males and females, although, the snake number was witnessed to decline during these months in both sexes, before disappearing altogether by December. The activity of snake and small reptiles increases during the spring season due to reoccurrence of warmer climatic conditions and a subsequent increase in prey availability. They reemerged in March where trend of feeding curves showed slight increase over March and April and reached the second peak in May in preparation for breeding season. This finding is similar to several studies done on different species of reptiles (Al-Sadoon et al., 1999; 2016), which showed a similar seasonal cycle of feeding.
The total weight of the gut was evaluated on a monthly basis between the two sexes. When males and females were compared, overall significant difference (p < 0.05) was observed between the sexes in terms of occurrence of prey items in their stomachs. Comparisons among groups showed that the gut biomass ingested in males contained more prey than females (Fig. 3). Peak values were observed in May and November in both males and females (Fig. 3). These findings are in contrast with those of Al-Sadoon et al. (2016) who found no significant differences between the diets of adult males and females in Cerastes cerastes gasperettii.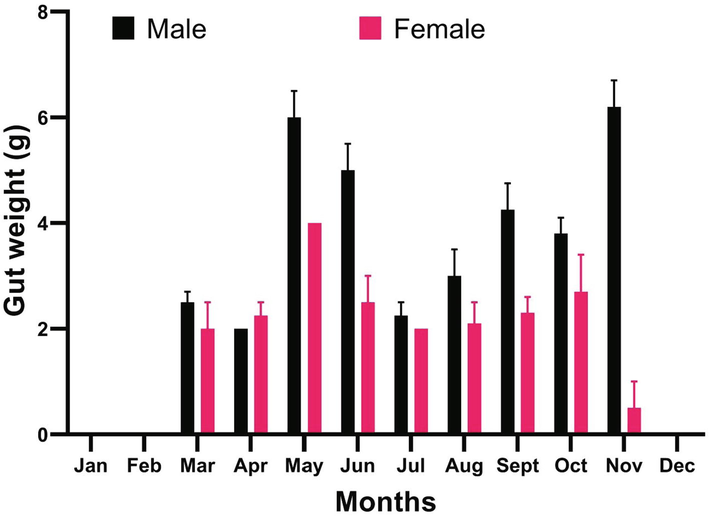
Variation in the amount of diet (g) in stomach of A. engaddensis for males and females on monthly basis.
As regard to the daily and seasonal behavior of feeding, the results showed that this species is nocturnal and has unimodal activity cycle. This activity continues during the period from May to September during which the temperature tends to elevate. Its activity begins at dusk and continues until dawn. The activity of this snake was found to be decreased or ceased during winter season owing to the decreased temperature. Small reptiles and snakes also depict poor activities with a general inactivity in cold winters (Begg et al., 2003). The snake, during day hours, is only found underneath woods, stones and burrows.
In conclusion, this work is the first attempt to investigate the diet composition of burrowing asp by analyzing its stomach contents from Al-Qasim, Saudi Arabia. This snake does not show much seasonal variation in diet. It is evident that male and female do not show much difference in diet and foraging behavior, however, males consume more prey than females can be attributed to meet their energetic demands.
Acknowledgements
The authors would like to express their sincere appreciation to the Deanship of scientific Research at the King Saud University, Riyadh, Saudi Arabia for funding this Research Group Project No. RGP-289.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The case of rainforest stiletto snakes (genus Atractaspis) in southern Nigeria. Evidence of diverging foraging strategies in grossly sympatric snakes with homogeneous body architecture? Ethol. Ecol. Evol.. 2001;13:89-94.
- [Google Scholar]
- Studies on the ecology of the Egyptian spiny-tailed lizard, Uromastix aegyptius (Forskål, 1775) in the Riyadh region, Saudi Arabia. J. Col. Sci. King Saud Uni.. 1983;14:341-351.
- [Google Scholar]
- First record of the Mole Viper, Atractaspis microlepidota engaddensis, from Jordan. Zool. Middle East.. 1995;11:47-49.
- [Google Scholar]
- Survey of the reptilian fauna of the kingdom of Saudi Arabia I-The snake fauna of the central region. J. King Saud Univ. A. Sci.. 1989;1:53-69.
- [Google Scholar]
- Food and feeding habits of the sand fish lizard Scincus mitranus. Saudi J. Bio. Sci.. 1999;6:91-101.
- [Google Scholar]
- Ecology of the Sand Boa, Eryx jayakari in Riyadh region of Saudi Arabia. Saudi J. Bio. Sci.. 2014;21:391-393.
- [Google Scholar]
- Diet of the Worm Lizard, Diplometopon zarudnyi (Nikolsky, 1907), in Riyadh province, Saudi Arabia (Reptilia: Trogonophidae) Zool. Middle East.. 2016;62:227-230.
- [Google Scholar]
- Feeding ecology of the endemic rattleless rattlesnake, Crotalus catalinensis, of Santa Catalina Island, Gulf of California. Mexico. Copeia.. 2007;1:80-84.
- [Google Scholar]
- Ecological observation of the lizard, Xenosaurus grandis in Cuautalapan, Veracurz. Mexico. Biotropica.. 1995;27:128-132.
- [Google Scholar]
- Sexual and seasonal variation in the diet and foraging behavior of a sexually dimorphic carnivore, the honey badger (Mellivora capensis) J. Zool. Lond.. 2003;260:301-316.
- [Google Scholar]
- Notes on the distribution and ecology of Amblyodipsas concolor (A. Smith, 1849) in Swaziland (Serpentes: Colubridae) Afr. J. Ecol.. 1995;33:417-419.
- [Google Scholar]
- Field Guide to the Snakes and Other Reptiles of Southern Africa. Cape Town: Struik Publishers; 1988.
- A Field Guide to the Snakes and Other Reptiles of Southern Africa (revised ed.). Cape Town: Struik Publishers; 1998.
- J. Herpetol.. 1991;25:127-129. Diet of the racerunner Callopistes palluma in north-central Chile
- Zoology.. 2003;106:43-61. Feeding in Atractaspis (Serpentes: Atractaspididae): a study in conflicting functional constraints
- Prey size and food selection of Psammodromus algirus (Lacertidae) in central Spain. J. Herpetol.. 1990;24:342-347.
- [Google Scholar]
- A local study of the opisthogylph snake Tantilla gracilis Baird and Girard. Pap. Mich. Acad. Sci. Arts Lett.. 1935;20:645-659.
- [Google Scholar]
- Seasonal foraging behavioural compensation in reproductive wapiti hinds (Cervus elaphus canadensis) Appl. Anim. Behav. Sci.. 2000;67:137-150.
- [Google Scholar]
- Seasonal variation in diet composition and prey selection in the Mediterranean gecko Tarentola mauritanica. Isr. J. Zool.. 1994;40:61-74.
- [Google Scholar]
- Striking and other offensive and defensive behavior patterns in Atractaspis engaddensis (Ophidia, Atractaspididae) Copeia 1988:792-797.
- [Google Scholar]
- The caecilian amphibian Scolecomorphus kirkii Boulenger as prey of the burrowing asp, Atractaspis aterrima Gunther: trophic relationships of fossorial vertebrates. Afr. J. Ecol.. 2004;42:83-87.
- [Google Scholar]
- Behavior, ecology, and the adaptive zone of African mole vipers (Atractaspis) Herp. Rev.. 1977;8(3 Suppl.):9.
- [Google Scholar]
- Snakes: The Evolution of Mystery in Nature. USA: University of California Press; 1997.
- Wild otters: predation and populations. New York: Oxford University Press; 1995.
- Food consumption of the lizard Agama stellio stellio. J. Arid Environ.. 1991;21:353-356.
- [Google Scholar]
- The hooded Malpolon Moilensis reuss and notes on other snakes of North-Eastern Arabia. J. Bombay Nat. Hist. Soc.. 1967;64:115-117.
- [Google Scholar]
- Foraging ecology. In: Seigel R.A., Collins J.T., Novak S.S., eds. Snakes: Ecology and Evolutionary Biology. New York: Macmillan; 1987. p. :302-334.
- [Google Scholar]
- Use of a mammalian resource by a Chihuahuan snake community. In: Scott N.J., ed. Herpetological Communities. Washington D.C.: U.S. Dept. Interior; 1982. p. :99-118.
- [Google Scholar]
- A Field Guide to the Reptiles of East Africa. London: Academic Press; 2002.
- The relationship between selectivity and food abundance of a juvenile lizard. Ecology. 1981;62:1079-1092.
- [Google Scholar]
- The Snakes of Texas. Austin: Texas Monthly Press; 1984.
- On the affinities of the burrowing asps Atractaspis (Serpentes, Atractaspididae) Zool. J. Linn. Soc.. 1993;107:3-64.
- [Google Scholar]







