Translate this page into:
Seasonal activity of Culicoides bahrainensis Boorman, 1989 (Diptera: Ceratopogonidae) in Saud Arabia
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Very little information is available on Culicoides (Diptera: Ceratopogonidae) fauna of Saudi Arabia. During the period March 2004–February 2006, a country-wide survey was conducted to study the distribution of Culicoides or biting midges in Saudi Arabia using light traps. During the study, 2308 specimens of Culicoides bahrainensis Boorman, 1989 were collected for the first time in Saudi Arabia from Al Hassa and Al Dammam on the Arabian Gulf, and from Jeddaha on the Red Sea. This distribution suggests that C. bahrainensis requires high humidity for its survival and development. This observation was further supported by the positive significant correlation between the number of Culicoides collected and the relative humidity. The seasonal activity of C. bahrainensis showed two seasonal peaks, a higher peak in April and a lower peak in November where the temperature varied between 22.4 and 29.3 °C and the relative humidity varied between 34% and 66%. No Culicoides was collected during the period July–August where the temperature varied between 33 and 38 °C and the relative humidity varied between 24% and 54%.
Keywords
Seasonal activity
Culicoides bahrainensis
Saudi Arabia
1 Introduction
The biting midges or Culicoides Latreille (Diptera: Ceratopogonidae) are very tiny haematophagous insects measuring from 1 to 3 mm in size. More than 1400 species have been identified, and they occur on all large landmasses, with the exception of Antarctica and New Zealand, ranging from the tropics to tundra and from sea level to 4000 m above sea level (Mellor et al., 2000).
The role of Culicoides in disease transmission has been reviewed by many workers (Du Toit, 1944; Kettle, 1965; Linley et al., 1983; Lane, 1983; Boorman, 1989; Lane and Crosskey, 1993; Mellor et al., 2000). More than 50 viruses have been isolated from Culicoides species, and the most important of these are African Horse Sickness Virus (AHSV), Blue Tongue Virus (BTV), Akabane Virus (AKAV) and Bovine Ephemeral Fever Virus (BEFV). Some species of Culicoides cause considerable nuisance through their bites (Linley et al., 1983).
Despite reports of severe outbreaks of arboviral diseases in domestic animals in Saudi Arabia (Anderson et al., 1989; Abu El Zein et al., 1998a,b), very little effort has been made to study the potential vectors of these diseases such as Culicoides.
Few reports are available on the distribution and seasonal activity of Culicoides in Saudi Arabia (Lane, 1983; Boorman, 1989; Abu El Zein et al., 2002; Hilali et al., 2003; Boorman and Harten, 2003; Alahmed and Kheir, 2005). More recent information on distribution, seasonal activity and vectorial capacity of Culicoides species in Saudi Arabia is required to assess the economic losses due to these serious haematophagous pests. During a recent study of distribution of Culicoides in Saudi Arabia, Alahmed et al. (2010) encountered Culicoides bahrainensis for the first time in Saudi Arabia in the Eastern and the Western Regions, but no studies on its seasonal abundance are available. In this study, an attempt was made to study the seasonal activity of C. bahrainensis in Saudi Arabia.
2 Materials and methods
2.1 Study area
Saudi Arabia is a vast country with an area of about 2.25 million km2. It lies in the southwestern part of Asia, between lat. 15° 44′ N–32° 9′ N and long. 34° 24′ E–55° 39′ E (Fig. 1). The kingdom is an extremely arid area, except the southwestern region and some coastal zones. The mountains in the south and the west, plateaus in the north and the center, sand dunes and desert in the east and some scattered valleys characterize the topography of the Kingdom (Brown, 1968; Mohammedein, 2001).
Collection sites of Culicoides in Saudi Arabia.
Most of the Eastern Region of Saudi Arabia is a desert region, which includes the part of the Kingdom located along the Arabian Gulf between Sultanate of Oman in the south and Kuwait in the north. The region is separated from the interior by the sand dunes known as Al Dahna, and is bordered to the south by Al Rub Al Khali or the Empty Quarter desert (Fig. 1).
The western coastal escarpment can be considered as two mountain ranges separated by a gap at the vicinity of Makka Al Mukarrama. The northern range seldom exceeds 2100 m above sea level, and the elevation gradually decreases towards the south to about 600 m around Makka Al Mukarrama, with some coastal plains. The climate is hot dry in summer and warm, slightly humid in winter. The vegetative cover is very poor in the study areas, except for some coastal zones.
2.2 Collection and identification of Culicoides bahrainensis
During the period March 2004–February 2006, a country-wide survey for collection of Culicoides by light traps was conducted in Saudi Arabia (Fig. 1), and C. bahrainensis was reported for the first time in Saudi Arabia from Al Dammam, Al Hassa and Jeddaha (Alahmed et al., 2010). C. bahrainensis were collected by one Center for Disease Control (CDC) miniature light trap and one standard New Jersey (NJ) light trap (Bioquip Company, Gardena, CA, 90248-3602, USA) from Al Dammam, Al Hassa and Jeddaha (Fig. 1). The CDC and the NJ light traps were attached to a battery that supplies power, and installed permanently near suitable breeding sites of Culicoides such as near animal farms with aquatic habitats, human habitations and animal housings. The farms were surrounded by plantation. The light traps were operated once every 2 weeks from sunset to sunrise the following day throughout the study period. The collected adult Culicoides were preserved into 70% ethyl alcohol in glass vials with screw caps, labeled and sent to the Entomology Laboratory, College of Food and Agricultural Sciences, King Saud University, Riyadh. The collected Culicoides spp. were identified by Dr. Art Borkent, Department of Entomology, Royal British Columbia Museum for Natural History, British Columbia, Canada, according to the method described by Borkent and Bissett (1990). The meteorological data of the study areas was obtained from National Centre for Meteorology and Ecology, Riyadh, Saudi Arabia.
For statistical analysis, comparison of two means (t-test) to compare the efficiency of CDC and NJ light traps, and the correlation coefficient (r) between relative humidity and the number of Culicoides were calculated (SAS, 2001).
3 Results
The seasonal activity of C. bahrainensis in the three collection sites showed two distinct peaks, a higher peak in April (after the end of the rainy season), and a lower peak in November (after the end of the dry season), while no Culicoides was collected during the dry season (July–August). In Al Dammam (Fig. 2), 872 specimens of C. bahrainensis were collected, and the highest number was collected during May, when the mean monthly temperature was 31.5 °C and mean monthly relative humidity was 29%. In June, the population density started to decline, and no Culicoides was collected during July, when the mean monthly temperature was 36.4 °C and the mean monthly relative humidity was 27%. In September, after the end of the dry season, the population density started to increase and a peak was attained in November when the mean monthly temperature was 22.4 °C and mean monthly relative humidity was 55%. In December the population density started to decline because of the onset of winter season and reached the minimum in February, when the mean monthly temperature was 18.9 °C and the mean monthly relative humidity was 56%. In March, the population density started to rebuild and a peak was attained in May. Similarly, in Al Hassa (Fig. 3), the activity of Culicoides was lowest during the period July–August when the temperature was 37–38 °C and relative humidity was 24–25%. In September, the population size started to increase because of the improvement of temperature and humidity and a peak was attained in November when the mean monthly temperature was 22.5 °C and relative humidity was 54%. In December and January, the number of Culicoides collected decreased because of low temperatures during winter (15–18 °C). In March, Culicoides population started to rebuild, and a peak was attained in April when the temperature was 28 °C and relative humidity was 40%. In June, the activity of Culicoides started to decrease because of the onset of the dry season. In Jeddaha (Fig. 4), a higher peak of activity was attained in April, when the temperature was 29 °C and relative humidity was 64%; and a lower peak in November when the temperature was 25.8 °C and relative humidity was 66%. During the period July–August, the activity of Culicoides was lowest, where the temperature was 33 °C and relative humidity was 54–66%.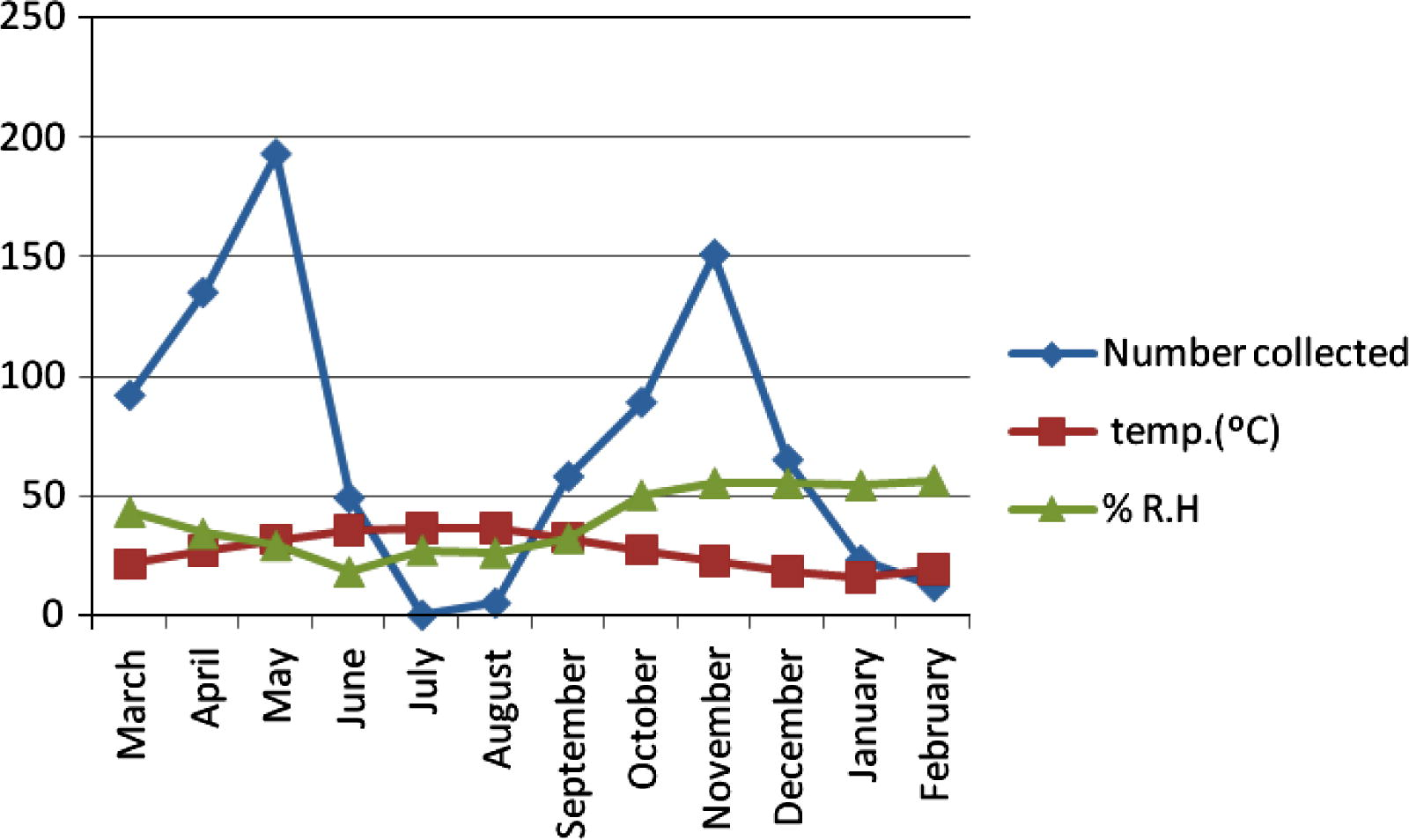
Seasonal activity of Culicoides bahrainensis in Al Dammam Region.
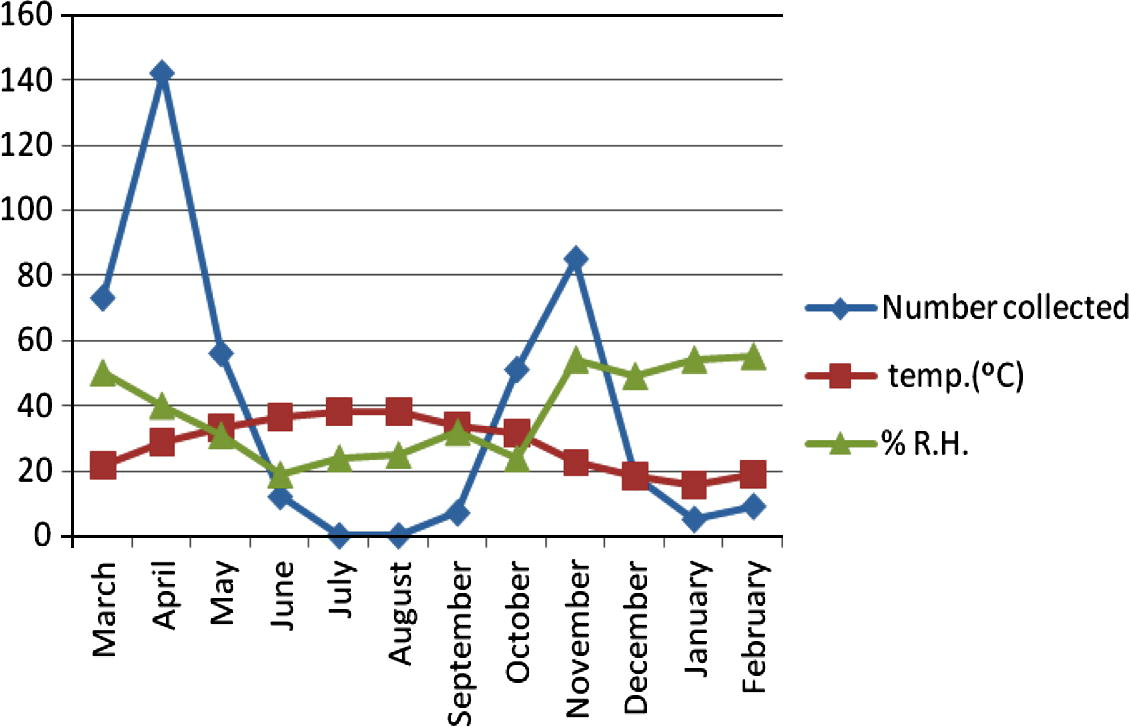
Seasonal activity of Culicoides bahrainensis in Al Hassa Region.
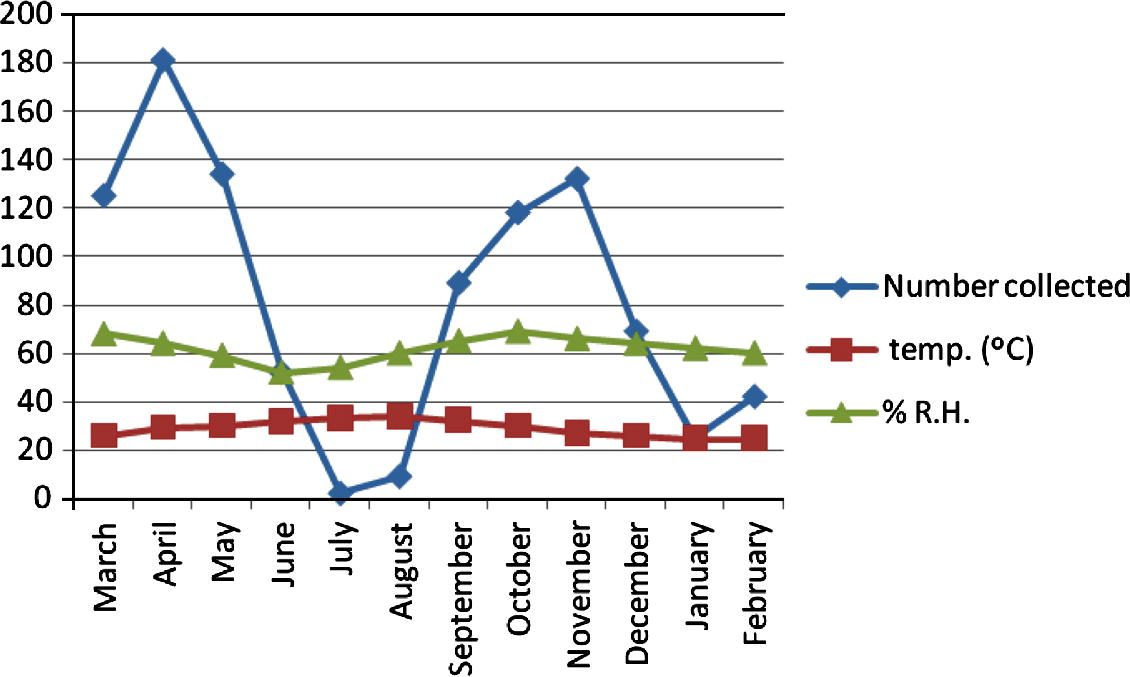
Seasonal activity of Culicoides bahrainensis in Jeddaha Region.
In this study, 872 specimens of C. bahrainensis were collected from Al Dammam by the light traps (Table 1). Out of these, 427 (48.96%) were collected by CDC light trap, and 445 (51.04%) by NJ light trap. Similarly, 458 specimens were collected by the light traps from Al Hassa (Table 2). Out of these, 237 (51.75%) were collected by CDC light trap and 221 (48.25%) by NJ light trap. In Jeddaha (Table 3), the light traps collected 978 specimens of C. bahrainensis, 505 (51.64%) were collected by CDC light trap and 473 (48.36%) by NJ light traps. In the three collection sites, there was no significant differences in the mean catches of CDC and NJ light traps (P > 0.05 for the three collection sites). The results have shown a significant positive correlation between the mean monthly catches of C. bahrainensis collected and the mean monthly relative humidity in Al Dammam, Al Hassa and Jeddaha (r = 0.88; r = 0.76 and r = 0.71 respectively; P < 0.05; P < 0.05 and P < 0.05 respectively).
Date
Mean monthly
Number of Culicoides collected
Rainfall (mm)
% R.H.
Temperature (°C)
CDC trap
NJ trap
Total
March 2005
11.1
43
21.4
38
54
92
April 2005
3.4
34
26.9
66
69
135
May 2005
0
29
31.5
102
91
193
June 2005
0
18
35.2
21
28
49
July 2005
0
27
36.4
0
0
0
August 2005
0
26
36.2
1
4
5
September 2005
0
32
32.4
26
32
58
October 2005
0
50
27.2
43
46
89
November 2005
7.9
55
22.4
81
70
151
December 2005
0
55
18.1
31
34
65
January 2006
2.3
54
15.5
11
12
23
February 2006
6
56
18.9
7
5
12
Total
427 (48.96%)
445 (51.04%)
872 (100%)
Date
Mean monthly
Number of Culicoides collected
Rainfall (mm)
% R.H.
Temperature (°C)
CDC trap
NJ trap
Total
March 2005
9.3
50
21.7
35
38
73
April 2005
0
40
28.5
81
61
142
May 2005
0
31
32.9
31
25
56
June 2005
0
19
36.5
8
4
12
July 2005
0
24
38
0
0
0
August 2005
0
25
37.8
0
0
0
September 2005
0
32
33.9
2
5
7
October 2005
0
24
31.6
23
28
51
November 2005
10.8
54
22.5
41
44
85
December 2005
0
49
18.4
7
11
18
January 2006
0
54
15.6
3
2
5
February 2006
4.7
55
18.8
6
3
9
Total
237 (51.75%)
221 (48.25%)
458 (100%)
Date
Mean monthly
Number of Culicoides collected
Rainfall (mm)
% R.H.
Temperature (°C)
CDC trap
NJ trap
Total
March 2005
0
68
25.6
59
66
125
April 2005
45
64
29.3
103
78
181
May 2005
0
59
30
71
63
134
June 2005
0
52
31.7
24
28
52
July 2005
0
54
33
0
2
2
August 2005
0
60
33.8
3
6
9
September 2005
0
65
32.1
48
41
89
October 2005
0
69
29.9
64
54
118
November 2005
0
66
26.9
72
60
132
December 2005
1
64
25.8
31
38
69
January 2006
0
62
24.5
11
14
25
February 2006
0
60
24.8
19
23
42
Total
505 (51.64%)
473 (48.36%)
978 (100%)
4 Discussion
The distribution of C. bahrainensis in Saudi Arabia is restricted to Al Hassa and Al Dammam on the Arabian Gulf, and Jeddaha on the Red Sea (Alahmed et al., 2010). This distribution suggests that C. bahrainensis requires high humidity for its survival and development, since it was collected from near Arabian Gulf and Red Sea only. This observation is further supported by the positive significant correlation between the number of C. bahrainensis collected and the relative humidity in the three collection sites.
In this study, two peaks of seasonal activity were observed for C. bahrainensis, a higher peak in May (after the rainy season) and a lower peak in November (after the dry season). In fact, the prevailing climatic conditions influence the seasonal activity of Culicoides (Summer, 2009). Cooler conditions inhibit development of immature stages of Culicoides species, while high temperature may adversely affect adult survival (Hunt et al., 1989). Within favourable limits, the development of immature stages of Culicoides species is directly related to temperature (Akey et al., 1978; Mullen and Rutz, 1983; Bishop et al., 1996; Mellor et al., 2000). In fact, when the temperature is suitable, relative humidity can influence the abundance and seasonal activity of biting Culicoides species, through its effects on the availability of breeding sites. Further studies on the effect of temperature and humidity on the distribution of C. bahrainensis in Saudi Arabia are required.
Changes in the distribution and abundance of Culicoides are likely to be amongst the most important and immediate effects of climate change (Mellor, 1996; Summer, 2009). This is particularly worrying in case of insects that transmit pathogens or parasites to humans and livestock, since it is likely to affect the prevalence of insect-borne diseases. The role of C. bahrainensis in disease transmission requires further investigation. Climatic factors play a major role in the distribution and vectorial capacity of Culicoides population (Wittman and Baylis, 2000). Low temperatures tend to be more significant than high temperatures as determinants of distribution (Gates, 1993). Wind speed and direction can also affect Culicoides distribution, through their influence on the passive dispersal of the adults. Due to their small size, Culicoides can be easily dispersed by wind.
In this study, few numbers of C. bahrainensis were collected by the light traps, particularly in Al Hassa oasis. Despite the arid conditions of Saudi Arabia, the ecological conditions in the oases, which could be manifested by the presence of stagnant water, boggy lands, irrigated canal with cultivated margins along with summer climatic conditions were expected to support the breeding sites of Culicoides in large numbers (Nevill and Neville, 1995). However this did not seem to match the low number of Culicoides caught during this study. This could have been due to unforeseen adverse conditions, such as the existence of predators preying on Culicoides larvae, or presence of residual insecticides or other toxic chemicals in the collection sites.
Similar pattern of seasonal activity was shown by C. bahrainensis in the three study areas, and this may be due to the similar climatic conditions in these collection sites. On the other hand, different seasonal activity patterns exist with regard to Culicoides imicola (Diptera: Ceratopogonidae). In Morocco, and winter rainfall regions of South Africa, C. imicola showed a pronounced peak of activity at the end of summer and it was absent during winter (Baylis and Rawlings, 1998; Mellor et al., 2000). In contrast, the annual peak of activity in Nigeria occurred shortly after the rainy season and during winter (Mellor et al., 2000). In Sudan and summer rainfall regions of South Africa, the peak activity of C. imicola occurred towards the end of the hot rainy seasons (Mellor et al., 2000). These differences in activity pattern of C. imicola might be due to the effect of the weather, larval development, adult survival and dispersal (Nevill et al., 1988; Mellor et al., 2000).
During the identification of C. bahrainensis, significant morphological variations were observed within this species (Borkent, personal communication), and this may be due to the fact that this species is conspecific with C. badooshensis and C. kurensis which are more broadly distributed throughout much of western Palaearctic Region (Boorman, 1989). Another possibility for these morphological variations is that there may be more species in this species group that are not described in this study. More research is needed to better understand the taxonomy of this species.
The results of this study have shown that there were no significant differences between the number of biting midges caught either by CDC or NJ light traps in the three collection sites, suggesting that the two light traps can be used for collection of biting midges. It has been found that the height of the light trap may have an effect on the catch of Culicoides, since Braverman and Linely (1993) showed that C. circumscriptus, C. cataneli and C. imicola were caught in greater numbers in the higher than in lower light traps, while more females of C. schultzi group were caught in lower than higher traps. In fact, the species collected more frequently in higher traps may be more prone to carriage for long distances by air currents and therefore are more likely to be important as dispersal vectors. In this study few numbers of C. bahrainensis were collected, this might be due to some limitations in the light traps. Further studies on the effect of the height of the trap and the attractiveness of light on the catch of Culicoides are required.
Acknowledgement
I would like to thank Dr. Art Borkent, Royal Museum for Natural History, British Colombia, Canada, for identification of Culicoides specimens and valuable comments on Culicoides fauna of Saudi Arabia. Thanks are also extended to King Abdul Aziz City for Science and Technology, Riyadh, for financial support.
References
- A study on Bluetongue virus infection in Saudi Arabia using sentinel ruminants. Onderstepoort J. Vet. Res.. 1998;65:243-251.
- [Google Scholar]
- Study of Akabane infection in Saudi Arabia by use of sentinel ruminants. J. Comp. Pathol.. 1998;119:473-474.
- [Google Scholar]
- Seasonal abundance of four Culicoides spp. (Diptera: Ceratopogonidae) at Al Ahsa Oasis, Eastern Province, Saudi Arabia. Onderstepoort J. Vet. Res.. 2002;69(2):115-122.
- [Google Scholar]
- Effects of rearing temperature and larval density on longevity, size and fecundity of the biting gnats Culicoides varipennis. Ann. Entomol. Soc. Am.. 1978;71:411-418.
- [Google Scholar]
- Seasonal activity of some haematophagous insects in Riyadh Region, Saudi Arabia. J Saudi Soc. Agric. Sci.. 2005;4(2):95-104.
- [Google Scholar]
- Distribution of Culicoides Latreille (Diptera: Ceratopogonidae) In Saudi Arabia. J. Entomol.. 2010;7(4):227-234.
- [Google Scholar]
- Modeling the distribution and abundance of Culicoides imicola in Morocco and Iberia using climatic data and satellite imagery. In: Mellor P.S., Baylis M., Hamblin C., Calisher C.H., Mertens P.P.C., eds. African Horse Sickness. In: Mellor P.S., Baylis M., Hamblin C., Calisher C.H., Mertens P.P.C., eds. Archives of Virology. Vol vol. 14. Wien, New York: Springer; 1998. p. :137-153. (Supplement)
- [Google Scholar]
- Effects of temperature regimes on the development, survival and emergence of Culicoides brevitarsis (Diptera: Ceratopogonidae) Aust. J. Entomol.. 1996;11:361-367.
- [Google Scholar]
- Culicoides (Diptera: Ceratopogonidae) of Arabian Peninsula with notes on their medical and veterinary importance. Fauna of Saudi Arabia. 1989;10:160-224.
- [Google Scholar]
- Some Ceratopogonidae (Insecta: Diptera) from Arabian Peninsula, with particular reference to the Republic of Yemen. Fauna of Arabia. 2003;19:427-462.
- [Google Scholar]
- A revision of the Holarctic species of Serromyia Meign (Diptera: Ceratopogonidae) Syst. Entomol.. 1990;15:153-217.
- [Google Scholar]
- Effect of light trap height on catch of Culicoides (Diptera: Ceratopogonidae) in Israel. J. Med. Entomol.. 1993;30(6):1060-1063.
- [Google Scholar]
- Desert Biology. Vol vol. 1. New York, USA: Academic Press; 1968.
- The transmission of blue tongue and African horse sickness by Culicoides. Onderstepoort J. Vet. Res. Anim. Husbandry. 1944;19:7-16.
- [Google Scholar]
- Climate Change and its Biological Consequences. Sunderland: Sinauer Associates; 1993.
- Culicoides midges (Diptera: Ceratopogonidae) in some localities of Saudi Arabia and their veterinary significance. Veterinarski Arhiv. 2003;73(5):285-294.
- [Google Scholar]
- Environmental factors affecting mortality of adult Culicoides varipennis (Diptera: Ceratopogonidae) in the laboratory. J. Am. Mosq. Control Assoc.. 1989;5:387-391.
- [Google Scholar]
- Biting Ceratopogonidae as vector of human and animal diseases. Acta Tropica. 1965;22:356-362.
- [Google Scholar]
- Insects of Saudi Arabia: Culicoides (Diptera: Ceratopogonidae) of Saudi Arabia and their potential veterinary importance. Fauna of Saudi Arabia. 1983;5:529-544.
- [Google Scholar]
- Culicoides (Diptera: Ceratopogonidae) In: Lane R.P., Crosskey R.W., eds. Medical Insects and Archnids. Dept. of Entomology, The Natural History Museum, London, UK: Chapman and Hall; 1993.
- [Google Scholar]
- Biting midges (Diptera: Ceratopogonidae) and Human health. J. Med. Entomol.. 1983;20:347-364.
- [Google Scholar]
- Culicoides biting midges: their role as arbovirus vectors. Annu. Rev. Entomol.. 2000;45(1):307-340.
- [Google Scholar]
- Kingdom of Saudi Arabia: A Geographical Study (in Arabic). Riyadh, Saudi Arabia: Al Khereji Printing Press; 2001.
- Development of immature Culicoides varipennis (Diptera: Ceratopogonidae) at constant laboratory temperature. Ann. Entomol. Soc. Am.. 1983;76:747-751.
- [Google Scholar]
- A survey of Culicoides (Diptera: Ceratopogonidae) of Umlalazi Natural Reserve in Zululand, South Africa, with notes on two species biting man. Onderstepoort J. Vet. Res.. 1995;62:51-58.
- [Google Scholar]
- Culicoides species associated with livestock in the Stellenbosch area of the Western Cape Province, South Africa. Onderstepoort J. Vet. Res.. 1988;55:101-106.
- [Google Scholar]
- SAS System for Windows. Cary, NC, USA: SAS Institute Inc.; 2001. pp. 27512–8000
- Climate change: effects on Culicoides transmitted viruses and implications for the UK. Vet. J.. 2000;160:107-117.
- [Google Scholar]







