Translate this page into:
Screening of the normal bacterial flora in the gut of Aedes aegypti Mosquito in Saudi Arabia
⁎Corresponding author. rabdelgaber.c@ksu.edu.sa (Reem Alajmi) ralajmi@ksu.edu.sa (Reem Alajmi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aedes aegypti (Culicidae), the mosquito that causes yellow fever, is regarded as a significant vector for many disease agents. The current study sought to learn more about mosquito mid-gut bacteria and their impacts. Mosquito specimens were gathered in Jeddah city (Saudi Arabia), and their gut bacterial flora was then analyzed. The collected mosquitoes exhibit all the characteristic features of A. aegypti, according to a microscopic study. Utilizing the partial mitochondrial cytochrome oxidase (COI) gene analysis, molecular testing established the identity of A. aegypti as a close relative of previously recognized Aedes species submitted in the GenBank, particularly those from Kenya. Unfortunately, based on the results of RT-PCR, none of the Aedes mosquitoes under study had dengue virus (DENV) present. On nutrient agar plates, the mid-gut bacteria were isolated. A total of 34 Gram-positive bacteria were isolated and identified at the molecular level using the 16S rRNA gene and were divided into the two genera Bacillus and Lysinibacillus within the family Bacillaceae. To our knowledge, this study is the first to examine the normal existence of bacterial flora in the gut of DENV-free mosquitos in Saudi Arabia. It is advised to conduct more research to determine how these bacteria affect the transmission of harmful pathogens carried by mosquitoes. Additionally, further research into the antibacterial and anticancer activities of metabolites extracted from the mid-gut bacteria may help in the development of unique drugs.
Keywords
Aedes aegypti
Microbiota
Molecular analysis
Gram stain
1 Introduction
Since they are carriers of numerous pathogenic agents that cause numerous infectious diseases that can be fatal, such as malaria, yellow fever, dengue fever, Zika, and many other serious illnesses that may infect humans and other vertebrates, mosquitoes have been regarded as one of the most annoying arthropods to humans over the past century (Resh and Cardé, 2009; Kraemer et al., 2019). Because they bite, other mosquito species are seen to be annoying because of their bites (Mullen and Durden, 2009). Around the world, there are roughly 3000 species and subspecies of mosquito.
Due to its wide range and impact on public health, Aedes aegypti (A. aegypti) (Diptera, Culicidae) is a well-known mosquito (Bhatt et al., 2013; Gómez et al., 2022). As the carrier of numerous arboviruses, such as Dengue, Chikungunya, Yellow fever, and Zika virus (Brady et al., 2012; Powell, 2018; Weetman et al., 2018), it is well known for its significance in medicine. Adult females are the ones who spread pathogenic agents and require blood meals for the deposition of their eggs (Ponlawat and Harrington, 2005). According to the World Health Organization (WHO, 2009), it is one of the most rapidly spreading mosquito-borne viral diseases globally.
One of the most important pathogenic agents transmitted by A. aegypti is DENV which causes dengue disease which is the highest mortality threat due to viral infection in more than half of the world’s population (Goswami et al., 2012; Betanzos-Reyes et al., 2018; Gabiane et al., 2022). In several Saudi cities, dengue fever is regarded as a severe public health issue. Aedes mosquito bites are the primary method of human-to-human transmission of the DENV (Guha-Sapir and Schimmer, 2005). Although millions of dollars are spent on vector control programs, Aedes aegypti, the DENV vector, has not yet been successfully eradicated (Reiner et al., 2016; Caragata et al., 2019; Hossain et al., 2022).
Recently, researchers concentrated on learning more about the gut microbiome of mosquitoes, which is crucial to their survival as it participates in numerous physiological and metabolic processes such as blood digestion, egg development, and fecundity (Fouda et al., 2001). They acknowledged the significance of the microbiome in viral development and the mosquito susceptibility to a particular infection, which can also be employed in managing vector competence (Muturi et al., 2017). However, research on the stomach microbiome of the common A. aegypti mosquito is scarce. The current study’s goal is to discover the bacterial strains found in the mid-gut of mosquitoes.
2 Materials and methods
2.1 Collection of mosquitoes
All samples have been received from King Abdulaziz City for Science and Technology which were collected by black hole traps from different residential areas in Jeddah city during the spread of DENV cases in September and October of 2021. The morphological characteristics were used to identify the mosquitoes using the keys previously described by Darsie (1999), Becker et al. (2020a,b), and Alzahrani et al. (2021). The specimens were preserved in 95% ethanol at 4 °C and used for the molecular identification of mosquitoes.
2.2 Molecular identification of mosquitoes
Following the manufacturer's recommendations, DNA was extracted using the QIAamp DNA Blood and tissues extraction kit (Qiagen, Germany). NanoDrop ND-1000 Spectrophotometer (Thermofisher Scientific Inc, USA) was used to calculate the quantity and purity of DNA. PCR was carried out to amplify the mitochondrial Cytochrome c oxidase subunit I (COI) gene using the primer set of COI-LC01490 F (5′-GGTCAACAAATCCATAAAGATATTGG-3′) and/COI-HC02198 R (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al., 1994) with the recommended thermocycling profile. All PCR products were tested on a 1.2% agarose gel (Sigma-Aldrich, Missouri, USA) in 1 × TAE buffer and post-stained with ethidium bromide against the GeneRuler 100 bp (Fermentas, Lithuania) and then examined using the image analysis system. The amplified PCR products were sent to Macrogen Inc.’s DNA sequencing unit (Seoul, South Korea). Using NCBI’s BLAST program, sequences were found based on the partial COI gene. Using MEGA version 7.0, a phylogenetic tree was constructed based on the Maximum Likelihood method with 1000 bootstrap replicates and the appropriate substitution models.
2.3 DENV detection using reverse-transcription PCR (RT-PCR)
Following the protocol’s instructions, viral RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Germany). RT- PCR was used for detecting and typing DENV by using the universal primers of D1_F (5′-TCAATATGCTGAAACGCGCGAGAAACCG-3′) and D2_R (5′-TTGCACCAACAGTCAATGTCTTCAGGTTC-3′) (Ranjan et al., 2016) with the recommended thermocycling profile. Then, a further serotype-specific PCR combining primer D1 with one of the following internal primers: TS1, TS2, TS3, or TS4 mentioned previously in Gupta et al. (2012) for typing. The authority of public health in Saudi Arabia generously provided the positive samples (cDNA of DENV) after receiving KACST (IRB#20006) ethical approval. Gel electrophoresis has been performed as mentioned above.
2.4 Bacterial flora screening
A total of 50 mosquito specimens were dried and then ground while adding 150 μl of sterilized PBS. Subsequently, the supernatant fluid was transferred into pre-prepared Petri dishes containing nutrient agar medium (Thermo Scientific, Oxoid, USA) as non-selective media and then incubated for 24 hrs at 33 °C. The growing colonies were picked up and streaked individually over NA plates (Sanders, 2012) and then incubated for 24 hrs at 37 °C. After that, the second round of purification was made following incubation at 37 °C for 24 hrs. On the next day, the single colonies were inoculated in labeled tubes containing 5 ml Nutrient broth using a sterile loop, and then tubes were incubated at 37 °C for 24 hrs.
2.5 Gram stain
The culture was spread with an inoculation loop to a thin film on a microscope slide and left to dry then fixed with heat by passing it over the flame 2–3 times. The staining process was started with a Crystal violet stain and then poured off with dist·H2O. After that, gram iodine solution was used to cover the smear for 1 min followed by rinsing with dist·H2O. A few drops of decolorizer (ethanol 95%) are added followed by rinsing with dist·H2O. Smear was counterstained with Safranin solution for 1 min then washed off with dist·H2O. The slide was examined under an Olympus B × 61 microscope (Tokyo, Japan).
2.6 Molecular identification of bacterial flora
After 24 hrs of bacteria-broth incubation, the broth was divided into two equal volumes to extract the bacterial DNA of Gram-positive and negative separately. Genomic DNA was extracted using the QIAamp DNA Blood and tissues extraction kit (Qiagen, Germany) using the recommended protocol. A different step in DNA extraction for Gram-positive bacteria is the need to lysis the cell wall, so it must be ensured that ethanol has not been added to AL Buffer. PCR was performed to amplify the partial 16S rRNA gene from all the bacterial isolates by using primer set 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 149R (5′-GGTTACCTTGTTACGACTT-3′) (De Lillo et al., 2006) with the recommended thermocycling profile. Gel electrophoresis has been performed as described previously. PCR products were sent to the DNA sequencing unit in Macrogen Inc. (Seoul, South Korea). Sequences were identified, and the phylogenetic tree was constructed using MEGA ver. 7.0 based on the Neighbor-Joining method with 1000 bootstrap replicates under appropriate substitution models.
3 Results
3.1 Morphological species identification
The targeted mosquito is the small to medium-sized Aedes aegypti (Fig. 1), which has a distinctive silvery-white scaling pattern on its head, scutum, legs, and abdomen. The distinctive pattern of the species is formed by the lyre-shaped pattern of white scales on the scutum. A pair of membranous wings with narrow scales on all the veins and toothed claws of the fore- and mid-tarsi. Piercing and sucking proboscis is long dark scaled, the palps are shorter than proboscis in females while they are the same length in males. Sparsely hairy antennae in females but the males have plumose antennae.
Micrograph of the adult of Aedes aegypti. (A) Whole body. (B) Female head. (C) Male head.
3.2 Molecular identification of mosquito species
The partial sequences of the COI gene were amplified via PCR reaction. PCR products were deposited in GenBank under the accession numbers OQ023868, OQ023869, OQ023969, OQ024016, OQ024046, OQ024067, OQ024185, and OQ024190. One family (Culicidae) was identified for the recovered mosquito species. All the GenBank entries that matched our COI sequence under the highly stringent criteria (99.71–99.13% identity, 100% query coverage, and E-value 0.0) were assigned to the species of Aedes. In ML analysis (Fig. 2), the taxa of Aedes were grouped in a distinct clade with high bootstrap values of 100. Dendrograms confirmed the association of our specimen with the Aedes group, with special reference to the previously deposited sequences in the GenBank for A. aegypti (MW255873, MW255872 MH538701, MW255845, and KU186990) that were collected from Kenya.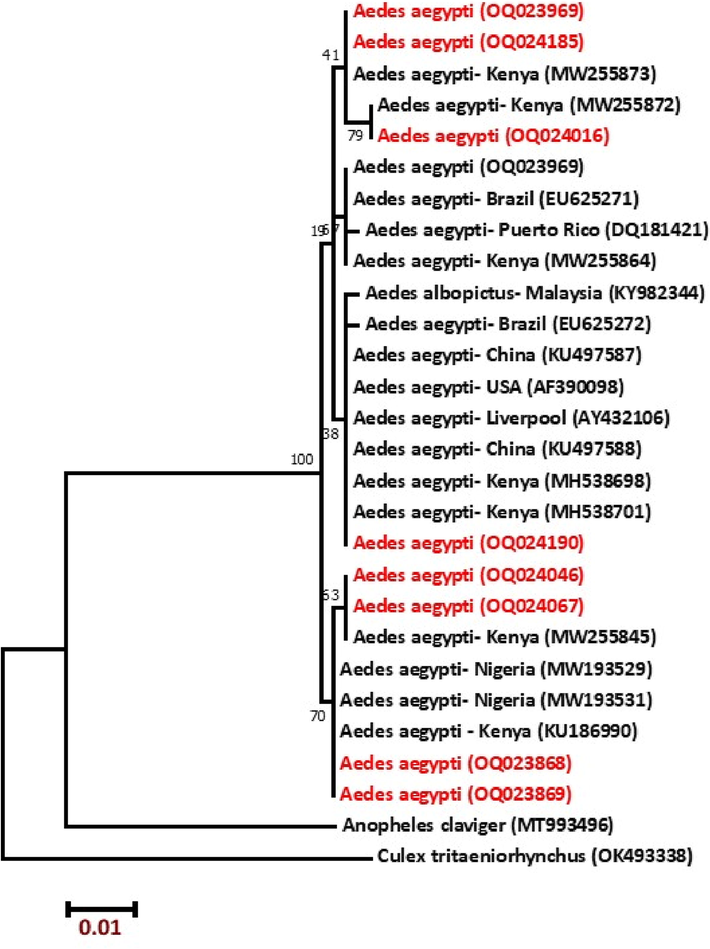
Molecular phylogenetic analysis of mt COI gene of mosquito specimens by Maximum Likelihood method based on the Tamura-Nei model. The tree with the highest log likelihood (−1147.40) is shown.
3.3 DENV detection
All examined mosquito specimens had failed to demonstrate the presence of DENV in comparison to the positive control which contains the DENV, all samples were considered negative for dengue (Fig. 3).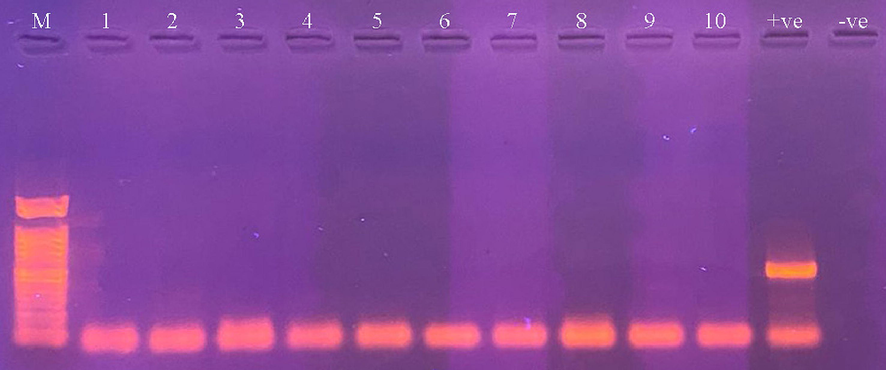
Agarose gel showing PCR products for the detection of DENV in different mosquito samples (Lanes 1–10). M: DNA marker, +ve: positive control, and −ve: negative control.
3.4 Bacterial flora mosquitoes
After 24 hrs of incubation, different morphological colonies appeared (Fig. 4). 34 isolates were classified according to their reactivity to the Gram stain, where the Gram-positive group was detected as shown in Table 1, and appeared in violet under the microscope. The partial 16S rRNA gene was targeted and amplified using PCR to identify the bacterial flora (Fig. 5). PCR products for nine Gram-positive bacteria were isolated and deposited in the GenBank database under the accession numbers OQ071618–OQ071627. One family (Bacillaceae) was identified for all bacterial flora isolated in this study. All sequences were matched with a dataset on GenBank with seven species embedded within the genus Bacillus and two sequences in the genus Lysinibacillus. In ML analyses (Fig. 6), the taxa of Bacillus and Lysinibacillus were closely related to each other with high bootstrap values.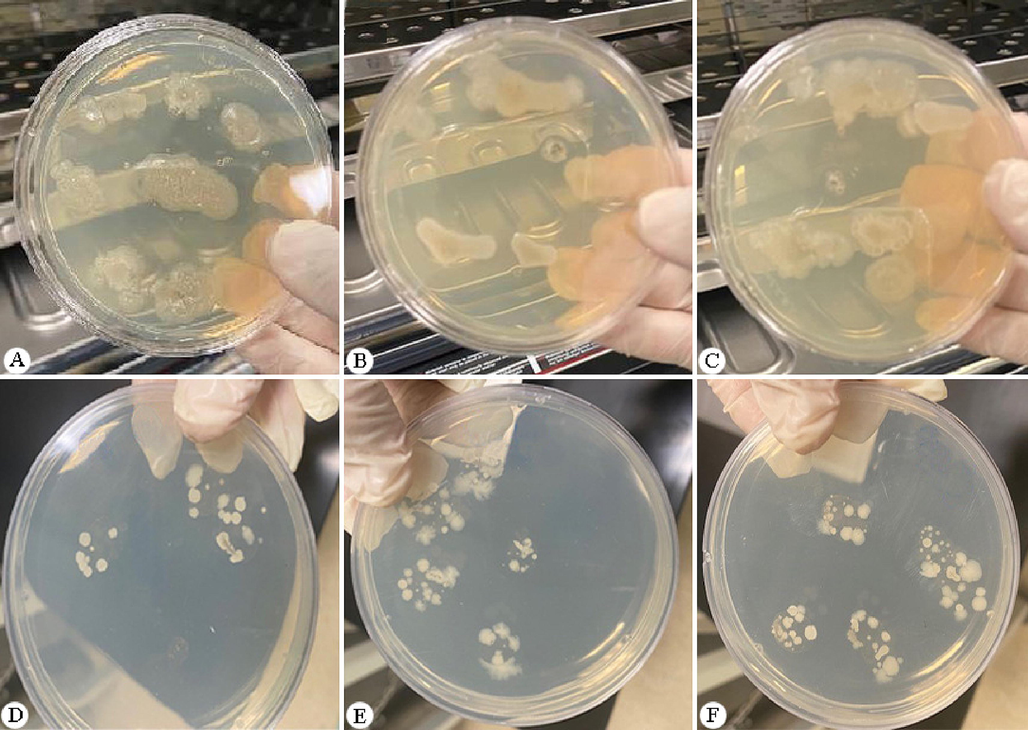
Colonies of mid-gut bacteria isolated from mosquitoes on NA plates.
Bacterial strain
Family
Accession numbers
Gram-positive/negative
Bacillus amyloliquefaciens
Bacillaceae
OQ071618
Gram-positive
Bacillus australimaris
Bacillaceae
OQ071625
Gram-positive
Bacillus paramycoides
Bacillaceae
OQ071621
Gram-positive
Bacillus cereus
Bacillaceae
OQ071622
Gram-positive
Bacillus aryabhattai
Bacillaceae
OQ071620
Gram-positive
Lysinibacillus macroides
Bacillaceae
OQ071627
Gram-positive
Lysinibacillus xylanilyticus
Bacillaceae
OQ071626
Gram-positive
Bacillus vallismortis
Bacillaceae
OQ071624
Gram-positive
Bacillus velezensis
Bacillaceae
OQ071624
Gram-positive
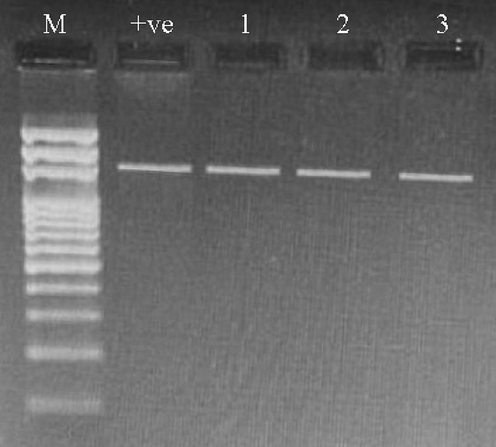
Agarose gel after loading the PCR products of 16S rRNA gene (Lanes 1–3). M: DNA marker, + ve positive control.

Neighbor-Joining tree of the 16 s rRNA gene for bacterial flora. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown above the branches.
4 Discussion
Mosquitoes are tiny arthropod organisms that have destroyed global health since ancient times (Mullen and Durden, 2009). According to Pise et al. (2022), mosquitoes are considered a severe hazard because they propagate deadly diseases that kill billions of people each year. One of the most important species that contributed to tampering with global health scales is A. aegypti which is reported as a vector of many arboviruses diseases such as Dengue, Chikungunya, Yellow fever, and Zika virus (Resh and Cardé, 2009; Brady et al., 2012; Bhatt et al., 2013; Sofi et al., 2022).
In this study, both morphological and molecular techniques were used to identify the collected mosquito species to obtain our aim which is screening the bacterial normal flora present in A. aegypti gut. Based on the morphological characteristics of the gathered mosquitoes which include small to the medium-sized dark body with unique silvery white scaling patterns of body parts, pair of membranous wings, piercing and sucking proboscis where the palps are shorter than proboscis in females, and sparsely hairy antennae in females, all of these features proved that the collected species related to A. aegypti. The same morphological characteristics were described by previous studies by Darsie (1999), Becker et al. (2020a,b), and Alzahrani et al. (2021).
Moreover, molecular analysis based on the partial COI gene sequences confirms the morphological identification of A. aegypti specimens. This is inconsistent with Kumar et al. (2007) and Alajmi et al. (2021) reported that the COI gene is considered an effective taxonomic gene used to identify insects in general and insects of complex species. All obtained sequences were identical and showed 99.71–99.13% identity with different strains of the A. aegypti in the GenBank which was collected from Kenya, which is compatible with Ali et al. (2016) proved that A. aegypti entered Saudi Arabia through African pilgrims and continued to circulate in the western region of Saudi Arabia. Genetic diversity within closely related mosquito species is also recorded herein, this agreed with El-Badry et al. (2016), Khater et al. (2021), and Noureldin et al. (2022) showed the same point between local mosquito species and their phylogeny.
Reverse Transcription PCR testing on all mosquitoes used in this investigation produced negative DENV results. This result is consistent with the most recent statistical report announced by the Ministry of Health (MOH) in Saudi Arabia (2019), which indicated a decline in the number of dengue cases recorded (Melebari et al., 2021). However, previous studies reported that the emergence of dengue fever disease in Saudi Arabia synchronized with the presence of A. aegypti mosquitoes, as it has been the vector of the disease since 1994 in the Jeddah region (Fakeeh and Zaki, 2001; Brady et al., 2012; Alhaeli et al., 2016; Al Sheikh et al., 2017; Organji et al., 2017). The negative PCR results of DENV in the collected mosquitoes could be attributed to two factors, firstly is the strict control measures launched by the Saudi MOH, and secondly is the lockdown all over the Kingdom during Corona epidemic may be a factor in decreasing the spreading of Dengue, which is consistent with Lemey et al. (2020) and Khan et al. (2022) as they fail to collect mosquitoes infected with Dengue during the coronavirus disease 2019 (COVID-19) pandemic period.
Gut microbiota is represented as the most famous biota that has been demonstrated in several insect species (Dillon and Dillon, 2004). Our findings based on culture-dependent assays to screen the microbiota associated with A. aegypti's gut showed that all bacterial isolates from collected mosquitoes were Gram-positive, belonging to the Bacillaceae family. According to Valiente Moro et al. (2013), mosquito gut microbiota is predominantly obtained through vertical transition or environmental acquisition. Based on the partial sequencing of the 16S rRNA gene, which has already been reported from the midgut of Aedes as well as other mosquito species (Pidiyar et al., 2004; Rani et al., 2009; Yadav et al., 2015), the most prevalent bacterial genera in our data were the Bacillus rather than Lysinibacillus. This is consistent with the findings of De Lillo et al. (2006), Sogin et al., (2006), and Geng et al. (2022), who indicated that 16S rRNA gene sequencing technology is the most widely used approach for detecting insect gut microbiota.
Moreover, this study indicates that the microbial community associated with A. aegypti samples is characterized by a limited bacterial diversity which agreed with Zouache et al. (2011) reported few genera in A. aegypti either by culturing or by PCR-based methods. In agreement with our study, previous studies have been conducted to manipulate gut microbiota to control the spreading of many diseases (Moreira et al., 2009; Osei-Poku et al., 2012). This confirms our finding for the negative results of DENV in the examined mosquitos. According to Dong et al. (2009), Rodrigues et al. (2010), Apte-Deshpande et al. (2014), and Thongsripong et al. (2018), the mosquito’s susceptibility to several pathogenic taxa is influenced by its microbiome. Additionally, they might prevent viruses like DENV and chikungunya from growing in mosquito vectors (Joyce et al., 2011; Ranasinghe et al., 2021).
The limitation of this study is the use of nutrient agar to isolate the mid-gut bacteria which is biased since some bacteria require specific culture media and others can not be cultivated. The use of the metagenomics approach in future studies will give a complete profile of the mid-gut microbiome.
5 Conclusion
In this study, the gut microbiota structure of A. aegypti mosquitoes collected in Jeddah city, Saudi Arabia, is described for the first time. Moreover, the gut microbiota’s functions of A. aegypti remains poorly understood and more studies are still needed to understand the interaction of midgut microbiota and their role in A. aegypti susceptibility to infection with pathogenic agents, particularly DENV.
Acknowledgments
This study was supported by the Researchers Supporting Project (RSP2023R99), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Serotypes of dengue viruses circulating in Jazan region, Saudi Arabia. J. Egypt. Soc. Parasitol.. 2017;47(2):235-246.
- [Google Scholar]
- Molecular identification of Campanulotes bidentatus Scopoli, 1763 (Phthiraptera, Philopteridae) infecting the domestic pigeon Columba livia from Saudi Arabia. Saudi J. Biol. Sci.. 2021;28(4):2613-2617.
- [Google Scholar]
- The epidemiology of Dengue fever in Saudi Arabia: A systematic review. J. Infect. Public Health. 2016;9(2):117-124.
- [Google Scholar]
- Phylogenetic analysis of Aedes aegypti based on mitochondrial ND4 gene sequences in Almadinah, Saudi Arabia. Iran. J. Biotechnol.. 2016;14(2):58-62.
- [Google Scholar]
- Distribution of mosquitoes and the first record of Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae) in the Riyadh Province, Kingdom of Saudi Arabia. Inter. J. Mosq. Res.. 2021;8(3):34-43.
- [Google Scholar]
- Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. IJMR. 2014;139(5):762.
- [Google Scholar]
- Chapter 6: Key to female mosquitoes. Published in the book: Mosquitoes: identification, ecology and control. Springer International Publishing; 2020. p. :103-122. ISSN 2509-6753
- Chapter 6: Key to male mosquitoes. Published in the book: Mosquitoes: identification, ecology and control. Springer International Publishing; 2020. p. :123-141. ISSN 2509-6753
- Association of dengue fever with Aedes spp. abundance and climatological effects. Salud Pública Méx.. 2018;60(1):12-20.
- [Google Scholar]
- Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLOS Negl. Trop. Dis.. 2012;6(8):e1760.
- [Google Scholar]
- Pathogen blocking in Wolbachia-infected Aedes aegypti is not affected by Zika and dengue virus co-infection. PLoS Negl. Trop. Dis.. 2019;13(5):e0007443.
- [Google Scholar]
- Description of the pupa of Aedes cretinus Edwards, a key to the pupae of the Albopictus subgroup, subgenus Stegomyia Theobald, genus Aedes Meigen, and characters to separate the European Stegomyia species (Diptera: Culicidae) Proc. Entomol. Soc. Wash.. 1999;101(3):614-618.
- [Google Scholar]
- Novel subgingival bacterial phylotypes detected using multiple universal polymerase chain reaction primer sets. Oral Microbiol. Immunol.. 2006;21(1):61-68.
- [Google Scholar]
- The gut bacteria of insects: nonpathogenic interactions. Ann. Rev. Entomol.. 2004;49(1):71-92.
- [Google Scholar]
- Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog.. 2009;5(5):e1000423.
- [Google Scholar]
- Phylogenetic analysis of Aedes aegypti based on mitochondrial ND4 gene sequences in Almadinah, Saudi Arabia. Iran. J. Biotechnol.. 2016;14(2):58.
- [Google Scholar]
- Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994–1999. Am. J. Trop. Med. Hyg.. 2001;65(6):764-767.
- [Google Scholar]
- DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol.. 1994;3:294-299.
- [Google Scholar]
- Effect of midgut bacteria of Culex pipiens L. on digestion and reproduction. J. Egypt. Soc. Parasitol.. 2001;31(3):767-780.
- [Google Scholar]
- Aedes mosquitoes in the emerging threat of urban yellow fever transmission. Rev. Med. Virol.. 2022;32(4):e2333.
- [Google Scholar]
- 16S rRNA Gene Sequencing Reveals Specific Gut Microbes Common to Medicinal Insects. Front. Microbiol.. 2022;13:892767
- [Google Scholar]
- Aedes aegypti and Ae. Albopictus microbiome/virome: new strategies for controlling arboviral transmission? Parasit Vectors. 2022;15:287.
- [Google Scholar]
- Two cases of dengue meningitis: A rare first presentation. J. Infec. Dev. Countries.. 2012;6:208-211.
- [Google Scholar]
- Dengue fever: new paradigms for a changing epidemiology. Emerg. Themes Epidemiol.. 2005;2(1):1.
- [Google Scholar]
- Circulation of dengue virus-1 (DENV-1) serotype in Delhi, during 2010–11 after Dengue virus-3 (DENV-3) predominance: a single centre hospital-based study. J. Vector Borne Dis.. 2012;49(2):82-85.
- [Google Scholar]
- Aedes larva detection using ensemble learning to prevent Dengue endemic. Biomedinformatics. 2022;2:405-423.
- [Google Scholar]
- Interactions between La Crosse virus and bacteria isolated from the digestive tract of Aedes albopictus (Diptera: Culicidae) J. Med. Entomol.. 2011;48(2):389-394.
- [Google Scholar]
- Co-existence of a pandemic (SARS-CoV-2) and an epidemic (Dengue virus) at some focal points in Southeast Asia: Pathogenic importance, preparedness, and strategy of tackling. Lancet Regional Health-Southeast Asia. 2022;4
- [Google Scholar]
- Molecular Phylogenetics and Population Genetics of the Dengue Vector Aedes aegypti From the Arabian Peninsula. J. Med. Entomol.. 2021;58(6):2161-2176.
- [Google Scholar]
- Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol.. 2019;4:854-863.
- [Google Scholar]
- DNA barcodes can distinguish species of Indian mosquitoes (Diptera: Culicidae) J. Med. Entomol.. 2007;44(1):01-07.
- [Google Scholar]
- Accommodating individual travel history and unsampled diversity in Bayesian phylogeographic inference of SARS-CoV-2. Nat. Commun.. 2020;11(1):1-14.
- [Google Scholar]
- The epidemiology and incidence of dengue in Makkak, Saudi Arabia, during 2017–2019. Saudi Med. J.. 2021;42(1):1173-1179.
- [Google Scholar]
- A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268-1278.
- [Google Scholar]
- Medical and veterinary entomology. 2009;Vol. 2:637.
- Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl. Trop. Dis.. 2017;11:e0005377.
- [Google Scholar]
- DNA Barcoding of Potential Mosquito Disease Vectors (Diptera, Culicidae) in Jazan Region. Saudi Arabia. Pathogens. 2022;11(5):486.
- [Google Scholar]
- Circulation of Dengue Virus Serotypes in the City of Makkah, Saudi Arabia, as Determined by Reverse Transcription Polymerase Chain Reaction. Can. J. Infect. Dis. Med. Microbiol.. 2017;2017:1646701.
- [Google Scholar]
- Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol. Ecol.. 2012;21(20):5138-5150.
- [Google Scholar]
- Studies on cultured and uncultured microbiota of wild Culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am. J. Trop. Med. Hyg.. 2004;70(6):597-603.
- [Google Scholar]
- Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol.. 2005;42:844-849.
- [Google Scholar]
- Mosquito-Borne Human Viral Diseases: Why Aedes aegypti? Am. J. Trop. Med. Hyg.. 2018;98(6):1563-1565.
- [Google Scholar]
- Diversity of midgut bacteria in larvae and females of Aedes aegypti and Aedes albopictus from Gampaha District, Sri Lanka. Parasit. Vectors. 2021;14(1):1-11.
- [Google Scholar]
- Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol.. 2009;9(1):1-22.
- [Google Scholar]
- High Seroprevalence of Dengue Virus Infection in Blood Donors From Delhi: A Single Centre Study. J. Clin. Diagn. Res.. 2016;10(10):DC08-DC10.
- [Google Scholar]
- Quantifying the epidemiological impact of vector control on dengue. PLoS Negl. Trop. Dis.. 2016;10(5):e0004588.
- [Google Scholar]
- Resh, V.H., Cardé, R.T., 2009. Ommochromes, in Encyclopedia of Insects. (2nd ed.). Berlington, MA, p. 625.
- Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329(5997):1353-1355.
- [Google Scholar]
- Larvicidal activity of Artemisia absinthium extracts with special reference to inhibition of detoxifying enzymes in larvae of Aedes aegypti L. J. King Saud Univ. Sci.. 2022;34(7):102248
- [Google Scholar]
- Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl. Acad. Sci. USA. 2006;103:12115-12120.
- [Google Scholar]
- Mosquito vector-associated microbiota: Metabarcoding bacteria and eukaryotic symbionts across habitat types in Thailand endemic for dengue and other arthropod-borne diseases. Ecol. Evol.. 2018;8(2):1352-1368.
- [Google Scholar]
- Diversity of culturable bacteria including Pantoea in wild mosquito Aedes albopictus. BMC Microbiol.. 2013;13(1):1-11.
- [Google Scholar]
- Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health. 2018;15:220.
- [Google Scholar]
- World Health Organization (WHO), 2009. Dengue and severe dengue. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
- Molecular characterization of midgut microbiota of Aedes albopictus and Aedes aegypti from Arunachal Pradesh, India. Parasit. Vectors. 2015;8(1):1-8.
- [Google Scholar]
- Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol. Ecol.. 2011;75(3):377-389.
- [Google Scholar]
Appendix A
Appendix A
Supplementary material
The following are the Supplementary material to this article:







