Translate this page into:
Screening of anti-oxidant and anti-bacterial metabolites from brown algae Turbinaria ornata for inhibits the multi-drug resistant P. aeruginosa
⁎Corresponding author. rajivgandhimicro@yahoo.com (Govindan Rajivgandhi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, the anti-oxidant and anti-bacterial activity of marine brown algae Turbinaria ornata (T. ornate) extract was purified by soxhlet apparatus method. The invitro studies of total anti-oxidant activity and DPPH hydroxyl scavenging activity assays were suggested that the T. ornata extract has more anti-oxidant activity. Agar well diffusion and minimum inhibition concentration results were exhibited excellent inhibition effect of T. ornata extract against P. aeruginosa at 300 µg/mL concentration. The time-dependent bacterial inhibition assay was also confirmed the 300 µg/mL concentration at 24 h. Further, the increased death cells and extracellular shape alteration results were observed by confocal laser scanning electron microscope and scanning electron microscope. Therefore, our result was suggested that the T. ornata extract as a promising anti-bacterial agent for multi-drug resistant P. aeruginosa.
Keywords
Marine environment
Brown algae
Anti-oxidant
Minimum inhibition concentration
Intracellular death
Morphological damage
1 Introduction
Among the various sources, marine is an important reservoir for microbes to produce potentially active bioactive metabolites for various infections (Sayin et al., 2020). It has heightened the therapeutic values in biomedical and pharmaceutical industry due to the volume nature, temperature, salt, nutrients and other environmental stresses (Rosaline et al., 2012). Most of the marine plant, algae and microbes are beneficial to human for more works. It has unpredictable atmospheric nature, providing greater biodiversity for microbes and also has the enormous chemical constituents’ producers such as alkenes, flavonoids, terpenes, polyketides and other components (Al-Saif et al., 2014). Recent years, researches are focused on marine environment, especially marine algae research. Among the all sources, marine sources are the dominant source and produced excellent bioactive metabolites. Overall, the 20,000 compounds are reported from algae and also 12,000 compounds are reported from other small members of marine plants, animals and other sources except bacteria (Zheng et al., 2020). Majority of these compounds have more anti-bacterial and anti-cancer activities against various infections (Khotimchenko et al., 2020).
In marine environment, algae are an important reservoir of essential biomolecules that are used in all the fields including agricultural, food, neutraceutical, pharmaceutical and biomedical industries (Ramachandran et al., 2019). Based on the survival and nutrient composition, the marine algae are classified into three major classes, such as red algae (rhodophyta), brown algae (phaeophyta) and green alage (chlorpphyta). Among these classes of algae, the brown algae T. ornata is the most important in the biomedical and biopharmaceutical study (Agrawal et al., 2020). It has the capacity to produce excellent anti-oxidant, anti-microbial, anti-fungal, cytotoxic, larvicidal and anti-cancer activities. In addition, it is used to produce hormone in more industries to various applications and also benefit to human to develop immune responses (Vijayabaskar et al., 2012). Therefore, our research is focused on T. ornata and its extract was performed to detect their anti-oxidant and anti-bacterial properties against multi drug resistant bacteria.
2 Materials and methods
2.1 Collection and extraction of algae extract
The algae samples were initially collected from Madurai Kamaraj University campus, Pudumadm, Ramanathapuram, Tamil nadu, India. The distilled water sterilized algae samples were maintained in dark condition 15 days with ordinary room temperature. Then, 50 g of powdered sample was diluted with 70% concentrated 2.5 L ethanol solvent and poured into Soxhlet apparatus for extraction 100 ℃ and continuous heat until the color of the solvent was changed. The clear phase was collected and maintained in rotary vacuum evaporator until solvent dehydration (Al-Saif et al., 2014). Finally, the active crude extract was collected in a separate 1L volume container and yield of 50 g powder was collected by based on the bellowed formula,
2.2 LC-MS analysis
Major and minor phytochemical compounds of T. ornata extract was identified by LC-MS analysis using electro spray passed ionization mass spectrum (ESIMS) (Yang et al., 2020). The finnegan surveyor auto sampler filled 1 mL T. ornate extract was used to perform the LC-MS analysis. The mass spectra range of the machine was attached with 100–1000 range, and 1:1 ratio of acetonitrile: ammonium acetate was fixed for LC-MS analysis. The ionization mode of 40v with MS was used for LC-MS voltage. The injection range was 1 mL and collection time was 1 mL/min used fraction collector. The lowest and highest temperature of 50 °C and 250 °C was used and capillary filter of size of 25 m × 3 mm with 1: 10 split ratio. Ion spray and capillary voltages were used 3 times as 5.3. kv and 34 kv respectively. The machine was run 3o min and data reductions were performed by Xcalibur 1.4 SRI. The ODS-2 at 250 × 4.6 mm (5 μm) of column was fixed.
2.3 Anti-oxidant activity calculation
1 g of the sample was mixed into 10 mL of methanol solution and taken and 2 mL was taken separately in test tube. The 2 mL of sodium phosphate and ammonium molybdate were taken into same test tube between the 5 min time interval. Both materials were mixed together and held 5 min. Then, 2 mL of sulphuric acid was added into the reaction mixture and put into the water bath at 90 °C for 45 min. After incubation, 2 mL of crude extract was added into the reaction mixture sample tube and maintained at room temperature for 15 min. After 15 min, the result of anti-oxidant changes in the tube was initially detected by visible observation, and followed 600 nm of O.D by UV-spectrometer. In this experiment, the ascorbic acid was used as a control and the test result was compared with ascorbic acid O.D values. The interpretation result was calculated for detection of anti-oxidant activity. The result was suggested that the 1 µg of ascorbic acid is equal to 1 g of T. ornata crude extract (Arumugam et al., 2019).
2.4 Detection of free radical scavenging activity by DPPH assay
Different concentration of T. ornata crude extract, and 100 µL of DPPH together and 5 µL of butylated hydroxyl toluene with DPPH in sterile test tubes were taken separately for test and control samples respectively. Also, the 70% ethanol was used as experiment control and held at 45 min for room temperature. Next, all the tubes were detected for anti-oxidant activity under 600 nm O.D (Rajivgandhi et al., 2020). The formed anti-oxidant activity tube was compared with untreated tube based on the bellowed formula,
Percentage of DPPH scavenged activity = [(ControlOD − TestOD)/(ControlOD) × 100].
2.5 Bacterial inactivation assay
One day old nutrient broth inoculated P. aeruginosa culture was swabbed on muller hinton agar poured plate and followed by cut the 4 wells with 6 mm. Then, 50 µL of crude extract was added each wells and allowed to performed anti-bacterial activity at 1 day with room atmosphere. Next, the zone production by crude extract around the wells was calculated for anti-bacterial effect and consecutively, ceftazidime zone was also calculated for detection of multi drug resistant effect of P. aeruginosa (Arumugam et al., 2019).
2.6 Minimum inhibition concentration assay
Micro broth dilution method of 96-well plate method was used to detect the minimum inhibition concentration of T. ornata extract by spectrophotometer study, followed by Maruthupandy et al. (2018). Shortly, previous day nutrient agar inoculated P. aeruginosa culture was added into the 96-well well plate containing sterile tryptic soy broth and T. ornata with different concentration (50–300 µg/mL) was inoculated into respective wells and followed by incubator maintenance with room atmosphere with 1 day. Then, O.D value of tested wells results were interpreted by control O.D by UV-spectrometer. The interpretation was done by bellowed equation for detect the percentages of inhibition,
% of inhibition = [(ControlOD − TestOD)/(ControlOD) × 100].
2.7 Time differentiation killing assay
Different concentration of T. ornata was proved by time based effect using 6-well plates by microtitre plate followed by recently published article of Olajuyigbe and Afolayan (2012). Shortly, the same one day P. aeruginosa culture was inoculated into 6-well plate of tryptic soy broth containing wells. Then, T. ornata extract with MIC concentration was used to treatment of 6-well plates containing pathogen. In this experiment, 7 different 6-well plate with pathogens and their respective MIC of T. ornata extract was used in this study and result was interpreted by O.D values of UV-spectrometer at 590 nm. The percentages of inhibition based on the time dependent was calculated by mentioned formula.
% of inhibition = [(ControlOD − TestOD)/(ControlOD) × 100].
2.8 Intracellular damage by CLSM
The intracellular damage of MIC treated P. aeruginoas was morphologically viewed by fluorescence microscope using AO/EB as a fluorescence dye (Ramachandran et al., 2020). Briefly, the MIC of T. ornata extract was filled on the surface of pathogen containing cover slip into the 6-well plate. After, the plate with coverslip was carefully kept at incubator overnight for one day. The pellet was received after centrifugation of treated and untreated samples. Then, the pellet was washed by PBS and followed by addition of 1µg/mL of AO/EB mixed dye. After mixing of the dye, the sample was covered by black color box and allowed to bind in inside of the cells for 15 min. After, the cover slip was directly seen in inverted position of fluorescence microscope at 40x magnification. The treated cells were compared with control cells to detect the anti-bacterial effect of T. ornata extract.
2.9 Size and shape differentiation of T. ornata extract
Size and shape of the P. aeruginosa in the presence of MIC treated and untreated samples were monitored under scanning electron microscope using published article methodology of Maruhtupandy et al. (2020). Previous day culture and MIC and culture alone were used for this study in cover slip soaked 6-well plates. Then, the PBS washed cover slip was used to 4% glutaraldehyde fixation, and degraded with ethanol graded series using 40–100% ethanol at 10 min time interval. Complete the time interval, the cover slip culture was washed by n-butanol and washed by PBS after 15 min. Then, the sample was treated by t-butanol with one day at 4 °C for complete fixation. Next day, both the treated and untreated samples were monitored under 40x magnification of scanning electron microscope.
3 Result
3.1 Detection of extraction and chemical composition of extract
Available chemical derivatives were shown in the LC-MS peaks and also interpreted by NSIT Wiley library based on the retention time, occupation area, percentage of retention time and occupation percentages. After complete interpretation, the phenolic, flavonoid, alkaloids, phytochemicals and bioactive compounds were detected for LC-MS result. Totally, 56 peaks of the chemical derivatives were received from the LC-MS analysis (Fig. 1). Based on the previous report, the 15%, 12%, 10% and 25% of the chemical derivatives were identified based on the NIST Wiley library reports (Riad et al., 2020; Lee et al., 2017; Wang et al., 2018). Also, retention time, occupation area and occupation percentages were suggested that the phytochemical compounds and secondary metabolites moieties were mostly available in the samples. It was indicated that the extract has more phenol and flavonoid content, and it may suggested that the extract has excellent anti-oxidant activity. Retention time of 24.60, 20.24, 16.40, 15.30, 10.20, 22.30, 26.45, 14.50, and 18.70 were shown for respective compounds of phenol, p-tert-butyl, dodecane, tetracosanoic acid, methyl ester, 5-eicosene, phenol-nonyl, pentanoic acid, 2-hydroxy-4-methyl-, methyl, 3-tetradecene, isobutyl pentyl ester, and 1,2-benzenedicarboxylic acid. The anti-cancer properties of T. ornata was reported from Delma et al., 2015; Canoy and Bitacura, 2018 reports. Very rich polysaccharides compounds concentration in the T. ornata was agreed by Vijayabaskar et al. (2012). The LC-MS result was supported to our study to detect the phytochemical, anti-oxidant and anti-bacterial studies.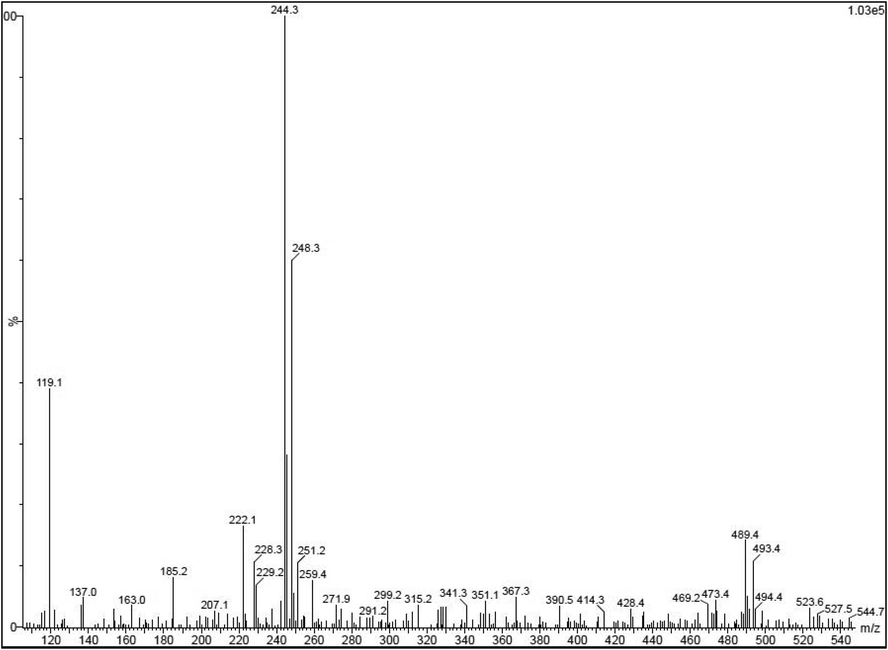
Detection of phytochemical and bioactive derivatives from Turbinaria ornata extract by LC-MS analysis.
3.2 Total anti-oxidant and DPPH scavenging activity
Excellent anti-oxidant activity result was detected in the crude extract of T. ornata extract after interpreted the O.D values of UV-spectrometer. In this experimental study, T. ornata was more higher compared to gallic acid and rutin. Also, both the results of respective controls were nearly close to extract and proved that the expected anti-oxidant result was very high for T. ornata (Fig. 2a). At the concentration of 250 μg/mL concentration, the total anti-oxidant activity was very high and DPPH scavenging assay was also very close to total anti-oxidant result (Fig. 2b). It was indicated that the T. ornata extract result has excellent anti-oxidant activity and DPPH scavenging activity. The result was driven by fluctuated marine environment, and increased anti-oxidant activity was obtained by influencing of environmental parameters in physiochemical parts of T. ornata (Yang et al., 2020).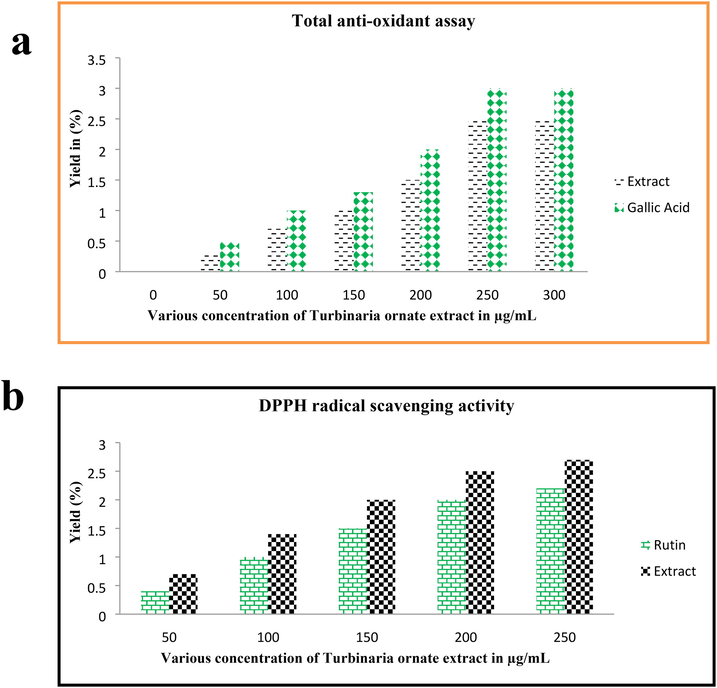
Total anti-oxidant (a) and DPPH radical scavenging assay (b) result of Turbinaria ornata extract by respective invitro studies.
3.3 Inactivation of bacteria
Bacterial inactivation in the presence of T. ornata extract was successfully identified by agar well diffusion experiment. Based on the zone of inhibition measurement, 21 mm zone of inhibition in the 350 µg/mL of the well was calculated by measuring scale. Additionally, the initial inhibition zone was detected at the concentration of 100 µg/mL with 6 mm zone. Instead, the methanol well was shown with no zone of inhibition, and third generation cephalosporin antibiotic ceftazidime disc was revealed that the P. aeruginosa was multi drug resistant strain due to the absence of zone (Fig. 3a, b). Final result of this study was suggested that the T. ornata extract has excellent anti-bacterial activity against tested multi drug resistant P. aeruginosa strain. Similar study result was discussed by Arumugam et al. (2019), and marine seaweed has more anti-bacterial activity against multi drug resistant bacteria.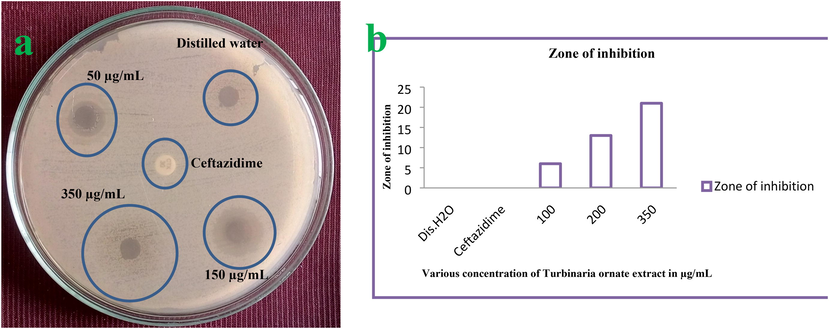
Bacterial inactivation based on the zone of inhibition by agar well diffusion method (a) and differentiation of zone of inhibition based on the various concentration (b).
3.4 Minimum inhibition concentration study
Antibacterial activity result was clearly proved by minimum inhibition concentration assay and it shown more inhibition at increased concentration of 300 µg/mL. In this experiment, the inhibition was started at 50 µg/mL. The result was stated that the T. ornata extract has antibacterial alteration at increasing concentration. The half inhibition of MIC was detected at 150 µg/mL concentration. All these evidences were detected visibly by turbidity of wells after interpretation of control well. The turbidity was initially started at the concentration of below 50 µg/mL and more turbidity was shown in 175 µg/mL concentration. When the O.D values of treated wells result was suggested that the result was exhibited with concentration-dependent turbidity. When converted to percentages based on the equation, the highest inhibition growth of 94% was observed at 275 µg/mL and 300 µg/mL concentrations (Fig. 4). Hence, the 300 µg/mL was fixed as a minimum inhibition concentration for this study. Our result suggested that the different marine environment natures such as nutrients, stress, carbon and nitrogen contents may influenced the increased activity (Oktaviani et al., 2019). Sometimes, rich polysaccharide levels of T. ornata extract influenced the anti-oxidant and biological properties against bacteria (Chakraborty et al., 2013). The statement was agreed by Arumugam et al. (2019), Southeastern coast of India sea has the different variety of seaweeds and these seaweeds posse more polysaccharide derivatives.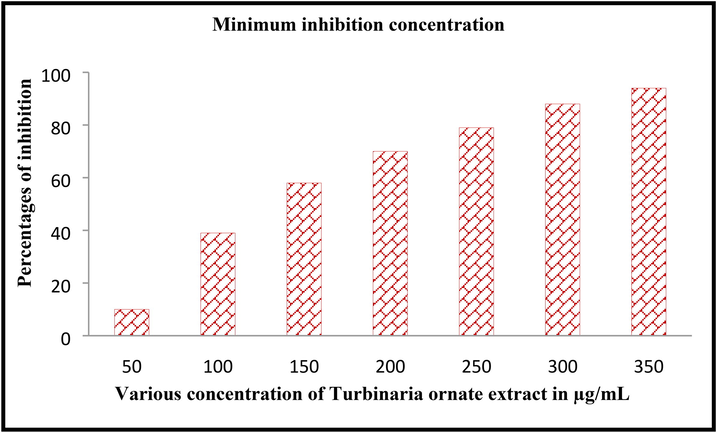
Minimum inhibition concentration of Turbinaria ornata extract against P. aeruginosa by microbroth dilution method.
3.5 Time dependent inhibition assay
Bacterial inactivation at time to time in the liquid culture of T. ornata extract treated result was shown in Fig. 5. After interpretation, the excellent result was shown in 24 h compared with other time interval. Compare with other time, the bacterial inactivation was started at 6 intervals; even 12 h is the suitable time for basic inhibition study. After conversion of percentages, the 96% of inhibition percentages were observed at treated concentration of 300 µg/mL. At 300 µg/mL, 59% inhibition rate and 65% inhibition rate was observed for 6 h and 12 h respectively. Whereas, the untreated control result was shown with no any turbidity and 0% was indicated. The result was suggested that the inhibition range was shown with time and concentration dependent inhibition. Whereas, the 50% of inhibition was observed at 200 µg/mL, 150 µg/mL and 100 µg/mL concentration at 6, 12 and 24 h time interval (Fig. 5). The result was indicated that the Turbinaria ornata was inhibited the P. aeruginosa at time dependent inhibition. Among the time interval, 12 and 24 h time intervals were very effective compared with other time interval. The time based inhibition study was used to detect the effective inhibition of tested compounds (Maruthupandy et al., 2020). It was more advantages to inhibit bacteria at virulence, gene level and enzyme alteration. Finally, our study was suggested that the T. ornata extract was inhibited the P. aeruginosa at very minimum concentration at 24 incubation.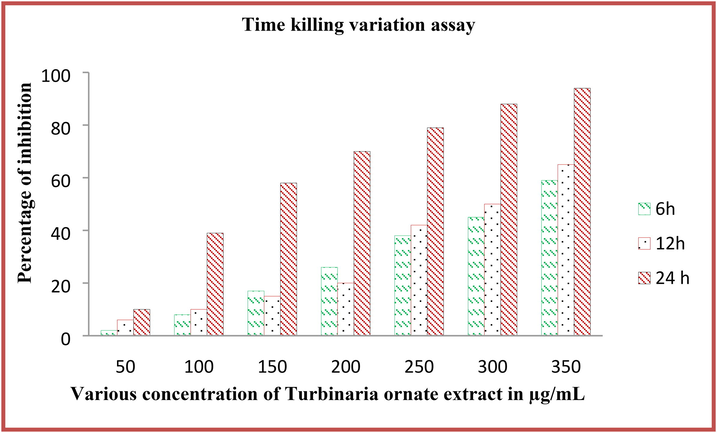
Time variation killing assay of Turbinaria ornata extract against P. aeruginosa by microtitre plate assay.
3.6 Intracellular alteration of bacteria
At the 300 µg/mL, the extract of T. ornata extract was shown with more death cells compared with control cells. The result was shown more necrotic nature with cytoplasmic membrane damage was shown by leakages of intracellular materials. The cells were condensed by T. ornata extract extract and the membranes were lost their organelles in inside. After addition of AO/EB stains, the red dye was entered into the inside of the damaged cells of bacteria and AO is bind with normal cell morphology and it exhibited their respective color emittance in control and treated images (Fig. 6a, b). AO/EB dye combination was used to detect the live and death cells differentiation after treatment with test compound (Ramachandran et al., 2020). Initially, the AO dye enters on bacterial surface and permeates in inside of the bacteria through damaged cells of negative charge. Positive charges of T. ornata extract were entering on the negative surface of the bacteria through electrostatic interactions. The porin channels and ions are transferred from AT-rich region of bacteria to outer cellular membrane and damage in inside of the bacterial cells (Yang et al., 2020). Our result was suggested that the extract of T. ornata extract has increased intracellular damages.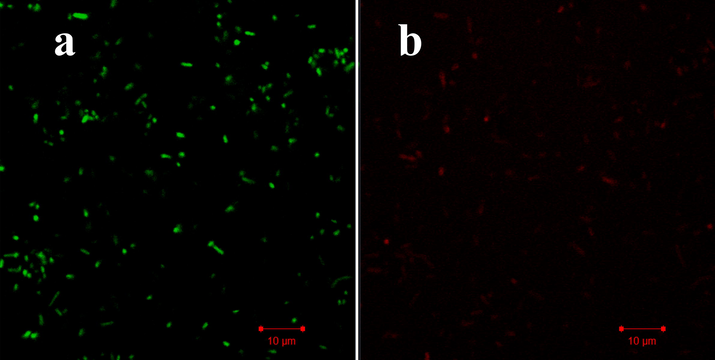
Intracellular modification of Turbinaria ornata extract treated P. aeruginosa by confocal laser scanning electron microscope.
3.7 Size and shape changes of P. aeruginosa
Size and shapes of the P. aeruginosa was changed by influence of MIC of T. ornata extract after 12 h incubation was effectively shown in Fig. 7. Test result image was shown with cytoplasmic damages due to the some leakage materials and transferred seaweed chemical compounds (Ramachandran et al., 2020). Compared with control, more damages with belbing were indicated in treated cells. Also, the size was shrinkled and shape was decreased due to the effect of T. ornata extract. Its looking like, the entire arrangement of polysaccharide materials like proteins, nucleic acid were shown in outside of the cells (Shafay et al., 2016). Complete rod shape morphology was looking with distracted morphology in treated cells (Fig. 7b). In addition, the rough morphology with extract treated outer cellular shape of the cells was shown on control cells (Fig. 7a). Whereas, the smooth, surface attractive colonies were observed in control cells. Therefore, the SEM result was boosted the all other invitro experiments and it suggested that the T. ornata extract as a promising anti-bacterial agent against multi drug resistant P. aeruginosa.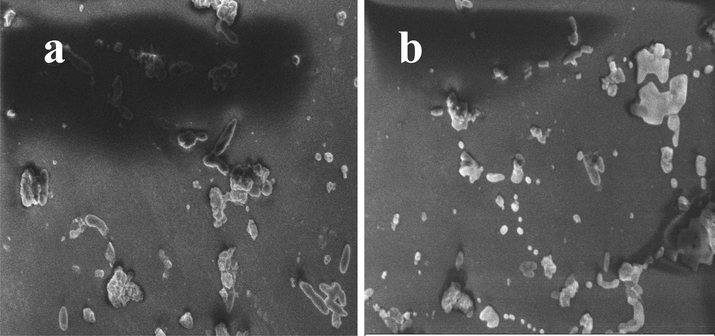
Altered size and shape of the Turbinaria ornata extract treated P. aeruginosa by scanning electron microscope.
4 Conclusion
Phenolic, flavonoids, anti-oxidant and bioactive compounds of T. ornate was identified by GC–MS. The total anti-oxidant and DPPH scavenging activity result was suggested that the T. ornata extract was excellent anti-oxidant source. In addition, anti-bacterial activity and minimum inhibition activity results were proved that the T. ornata have more bactericidal properties against P. aeruginosa at very lowest concentration. Finally, increased intracellular death and morphological shape modification in the presence of T. ornata extract confirmed that the T. ornata has important anti-bacterial agent against P. aeruginosa. Hence, the current study result was concluded that the marine T. ornata is a promising anti-bacterial agent through intracellular and extracellular damages.
Acknowledgment
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURG-1438-074.
References
- Structural deformation in pathogenic bacteria cells caused by marine fungal metabolites: An in vitro investigation. Microb. Pathog.. 2020;146:104248
- [Google Scholar]
- Antibacterial substances from marine algae isolated from Jeddah coast of Red sea Saudi Arabia. Saudi J. Biological Sci.. 2014;21:57-64.
- [Google Scholar]
- Anti-candidal and anti-virulence efficiency of selected seaweeds against azole resistance Candida albicans. Biocatal. Agricult. Biotech.. 2019;20:101195
- [Google Scholar]
- Cytotoxicity and Antiangiogenic Activity of Turbinaria ornata Agardh and Padina australis Hauck Ethanolic Extracts. Analyt. Cellular Pathol.. 2018;5:1-8.
- [Google Scholar]
- Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar, Asian Pac. J Trop. Biomed.. 2013;3:8-16.
- [Google Scholar]
- Fucoidan from Turbinaria conoides: A multifaceted ‘deliverable’ tocombat pancreatic cancer progression. I. J. Biolog. Macromol.. 2015;74:447-457.
- [Google Scholar]
- Antitumor potential of carrageenans from marine red algae. Carbohyd. Poly.. 2020;246:116568
- [Google Scholar]
- Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides. 2017;95:94-105.
- [Google Scholar]
- M. Maruthupandy, G. Rajivgandhi, S. Kadaikunnan, T. Veeramani, N.S. Alharbi, W.J. Li, J.M. Khaled, T. Muneeswaran, A.S. Alobaidi. Anti-biofilm investigation of graphene/chitosan nanocomposites against biofilm producing P. aeruginosa and K. pnumoniae, Carbohydrate Polymer, 230 (2020).
- Biologically synthesized zinc oxide nanoparticles as nanoantibiotics against ESBLs producing gram negative bacteria. Microb. Pathog.. 2018;121:224-231.
- [Google Scholar]
- Antibacterial Activity From Seaweeds Turbinaria ornata and Chaetomorpha antennina Against Fouling Bacteria. IOP Conf. Ser.: Earth Environ. Sci.. 2019;255:012045
- [Google Scholar]
- In Vitro Antibacterial and Time-Kill Evaluation of the Erythrina caffra Thunb. Extract against Bacteria Associated with Diarrhoea. Scientific World J.. 2012;738314:1-8.
- [Google Scholar]
- Photocatalytic reduction and antibacterial activity of biosynthesized silver nanoparticles against multi drug resistant Staphylococcus saprophyticus (MN310601) Mater. Sci. Eng.: C. 2020;114:111024
- [Google Scholar]
- Extraction and partial purification of secondary metabolites from endophytic actinomycetes of marine green algae Caulerpa racemosa against multi drug resistant uropathogens. Biocatal. Agricult. Biotechnol.. 2019;17:750-757.
- [Google Scholar]
- Anti-carbapenamase activity of Camellia japonica essential oil against isolated carbapenem resistant klebsiella pneumoniae (MN396685) Saudi J. Biolog. Sci.. 2020;27:2269-2279.
- [Google Scholar]
- Chemical screening and antibacterial activity of essential oil and volatile fraction of Dictyopteris polypodioides. Microchem. J.. 2020;152:104415
- [Google Scholar]
- Screening of selected marine algae from the coastal Tamil Nadu, South India for antibacterial activity. Asian Pacif. J. Trop. Biomed.. 2012;2:S140-S146.
- [Google Scholar]
- Marine Algae-PLA composites as de novo alternative to porcine derived collagen membranes. Mat. Today Chem.. 2020;17:100276
- [Google Scholar]
- Antimicrobial activity of some seaweeds species from Red sea, against multidrug resistant bacteria, The. Egypt. J. Aquat. Res.. 2016;42:65-74.
- [Google Scholar]
- Antioxidant properties of seaweed polyphenol from Turbinaria ornata (Turner) J. Agardh, Asian Pacif J. Trop. Biomed.. 1848;2012:S90-S98.
- [Google Scholar]
- Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chinese J. Nat. Med.. 2012;10:421-428.
- [Google Scholar]
- Pilot-scale production of antibacterial substances by the marine diatom Phaeodactylum tricornutum Bohlin. Algal Res.. 2018;32:113-120.
- [Google Scholar]
- Preparative HPLC fraction of Hibiscus rosa-sinensis essential oil against biofilm forming Klebsiella pneumonia. Saudi J. Biological Sci. 2020
- [CrossRef] [Google Scholar]
- Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biolog. Macromol.. 2020;151:344-354.
- [Google Scholar]







