Translate this page into:
Salinity-induced modulations in sexual and asexual reproduction in the freshwater planarian Dugesia bursagrossa (nomen nudum species): Insights from microtubular cytoskeleton and oxidative stress marker analyses
⁎Corresponding author at: Department of Zoology, College of Science, King Saud University, P.O. Box 2455, 11451 Riyadh, Saudi Arabia. hharrath@ksu.edu.sa (Abdel Halim Harrath)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Global warming-induced environmental changes have resulted in salinity fluctuations that markedly impact aquatic organisms. Consequently, the salinization of freshwater has become a major concern. Among these organisms, planarians are highly sensitive to environmental changes, especially Dugesia bursagrossa, the first freshwater planarian species identified in Saudi Arabia, which reproduces both sexually and asexually through fission. This study examined the reproductive response of sexual and asexual D. bursagrossa specimens to salinity changes. Asexual reproduction rates were found to increase with elevated salinity, whereas sexual reproduction was sensitive to high salinity, evidenced by the absence of cocoons in treated groups. This phenomenon was further explored by investigating the microtubular cytoskeleton’s organization in the sexual form’s ovary using immunolabeled α-tubulin and confocal microscopy. Results revealed a significant decline in the tubulin cytoskeleton with rising salinity levels, supporting the notion that increased salinity restricts sexual reproduction. Given that heightened salinity induces stress, the activities of malondialdehyde (MDA) and various antioxidant indicators, including glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT), were evaluated. Elevated salinity led to a notable increase in MDA levels as well as reduced GSH, SOD, and CAT levels in both sexual and asexual specimens, with sexual organisms exhibiting more pronounced effects. In conclusion, salinity directly impacts the homeostasis and reproduction of D. bursagrossa, with sexual specimens facing greater challenges than their asexual counterparts.
Keywords
Global warming
Salinization
Freshwater planarian
Sexual
Asexual
Ovary
Oxidative stress
1 Introduction
Salinity in freshwater ecosystems is an escalating issue that has intensified due to the impacts of global climate change (Schallenberg et al., 2003). Salinity poses major environmental implications, subjecting aquatic organisms to stressful conditions and disrupting their ability to maintain internal stability, i.e., homeostasis (Flöder et al., 2010; Mutshinda et al., 2013). Salinity levels are primarily influenced by human-induced climate change, with global warming altering precipitation patterns and the hydrological cycle, resulting in reduced rainfall and runoff, increased evaporation, and rising sea levels. Consequently, these changes contribute to heightened salinity levels in water ecosystems (Field and Barros, 2014).
Numerous studies have highlighted the importance of increased salinity as a major stressor for species in freshwater environments, particularly affecting the water concentration of soluble salts, notably sodium chloride (NaCl), which is crucial for the acclimatization of many aquatic animals to their environment (Flöder et al., 2010; Mutshinda et al., 2013). Freshwater organisms, particularly euryhaline species, demonstrate the capacity to adapt to or evade saline stress, enabling survival and reproduction in these dynamic environments (Rokneddine and Chentoufi, 2004). However, stenohaline species, lacking adaptability to high salinity conditions, face detrimental effects due to salinity fluctuations, including increased mortality rates, higher metabolic costs, and reduced growth, posing a serious risk of extinction (Flöder et al., 2010; Beatty et al., 2011; Mutshinda et al., 2013). Salinity variations impact physiological processes, influencing behavior, oxidative metabolism, reproductive function, and even genomic composition in animals (Montory et al., 2014; Saravanan et al., 2018). Disturbances in salinity within ecosystems can disrupt egg fertilization, embryonic development, and larval growth in both freshwater and marine species (Bœuf and Payan, 2001; Wang et al., 2016). Assessing these effects involves well-established parameters, such as the lipid peroxidation indicator malondialdehyde (MDA) and various antioxidant parameters, including superoxide dismutase (SOD), glutathione (GSH) peroxidase, and catalase (CAT), among others, considered highly suitable indicators of cellular and tissue health status (Orun et al., 2005; Gulhan and Selamoglu, 2016).
Understanding the mechanisms through which salinity affects reproductive processes is crucial for developing effective conservation strategies and mitigating negative impacts on reproduction in freshwater ecosystems. Freshwater planarians serve as a suitable model for ecological monitoring owing to their sensitivity to environmental changes, including salinity fluctuations, limited documented responses to salinity’s reproductive effects, and unique ability to reproduce both sexually and asexually. Given that salinity fluctuations and the associated environmental changes can cause oxidative stress in aquatic organisms (Lee et al., 2017), we hypothesized that increased salinity exposure would induce oxidative metabolism changes in the ovary of the freshwater planarian Dugesia bursagrossa nomen nudum. Thus, we investigated the effects of increased salinity exposure on a) sexual and asexual reproduction rates, b) ovary cytoskeleton structure, and c) the oxidative damage levels.
2 Methodology
2.1 Study design
Dugesia planarian specimens were sourced from the Ain Al-Dhiba Al-Soudah spring in the Abha region, located in southern Saudi Arabia (Harrath et al., 2023). Adult planarians (2.0 ± 0.1 cm long) of reproductive age were used in this study. The experimental design included three replicates for each of three groups of sexually reproducing specimens (control, 0.375 g/L salinity, and 0.750 g/L salinity groups) and three replicates for each of the same three groups of asexually reproducing specimens, with each replicate containing 11 organisms. Planarians were housed for 45 days in 200-mL glass flasks, each containing 100 mL of experimental solution, which was replaced weekly after ad libitum feeding with chicken liver. The organisms were maintained in the dark at 22 °C ± 1 °C and monitored daily. Reproduction rates were evaluated by daily counting of asexual planarian specimens as well as sexual planarian specimens and cocoons.
2.2 Immunofluorescence staining and confocal microscopy
The tissue section slides were placed on a hotplate set to 60 °C. They were then deparaffinized using xylene, rehydrated, and rinsed twice with distilled water, followed by three rinses with 1 × phosphate-buffered saline (PBS). After drying, the slides were placed in a suitable container. To permeabilize the tissue sections, a solution containing 0.1 % Triton X-100 and 0.1 % sodium citrate was applied, followed by treatment with blocking buffer (1 % bovine serum albumin in PBS) at room temperature. The slides were then placed in a humid box and incubated overnight at 4 °C with the primary antibody solution (anti-tubulin; 1:500 dilution; DGpeptides Co., Ltd., China). The next day, the slides were washed four times with 1 × PBS and treated with the secondary antibody FITC (1:2000 dilution; ab6717, Abcam, Cambridge, UK) for 45 min at room temperature in the dark. After washing with PBS and TE buffer, Hoechst solution (1:15,000 dilution; Hoechst 33,342, Life Technologies, USA) was added. Finally, the sections were immersed in a 50 % glycerol/TE solution, and the edges were sealed with nail polish. To quantify the signal, the sections were observed and imaged using a Zeiss spinning disk confocal microscope. The signal intensity of protein expression was analyzed using the Zen 3.1 service (ZEN lite) and quantified using GraphPad Prism 9.3.1 (GraphPad Software).
2.3 Measurement of lipid peroxidation and GSH concentrations
Lipid oxidation was assessed by quantifying the levels of lipid peroxidation products, mainly MDA (thiobarbituric acid reactive substances). The measurement of lipid peroxidation levels was conducted spectrophotometrically, following the established protocol by Ruiz-Larrea et al. (1994). In summary, the samples were exposed to thiobarbituric acid under acidic conditions, and the absorbance of the resulting pink chromogen was recorded at 532 nm. To determine the total GSH (glutathione) levels, the rapid colorimetric method described by Beutler et al. (1963) was employed. This method relies on the color change to yellow when 5,5′-dithiobis-2-nitrobenzoic acid is added to sulfhydryl compounds, enabling the measurement of GSH concentration at 405 nm.
2.4 SOD and CAT assays
SOD activity was determined using the method established by (McCord and Fridovich, 1969), involving inhibition of the cytochrome c reduction rate via superoxide radicals and SOD measurement at 550 nm. CAT activity was measured following the method of (Maehly and Chance, 1954), determining the hydrogen peroxide dissociation rate/min due to CAT, measured at 240 nm.
2.5 Statistical analysis
The data were analyzed using GraphPad Prism version 9. Statistical comparisons were conducted using one-way analysis of variance (ANOVA), followed by Tukey's multiple comparison test. All data are expressed as mean ± standard deviation values, and statistical significance was considered at a p-value of less than 0.05.
3 Results
3.1 Effect of salinity on planarian reproduction
Planarian specimen numbers remained consistent across sexual groups (control, 0.375 g/L salinity, and 0.750 g/L salinity groups). A significant dose-dependent increase in asexual specimens was observed with increasing salinity (Fig. 1), suggesting that elevated salinity stimulated asexual reproduction through fission. Conversely, sexual reproduction was inhibited, evident from the absence of cocoons in treated groups compared with the control.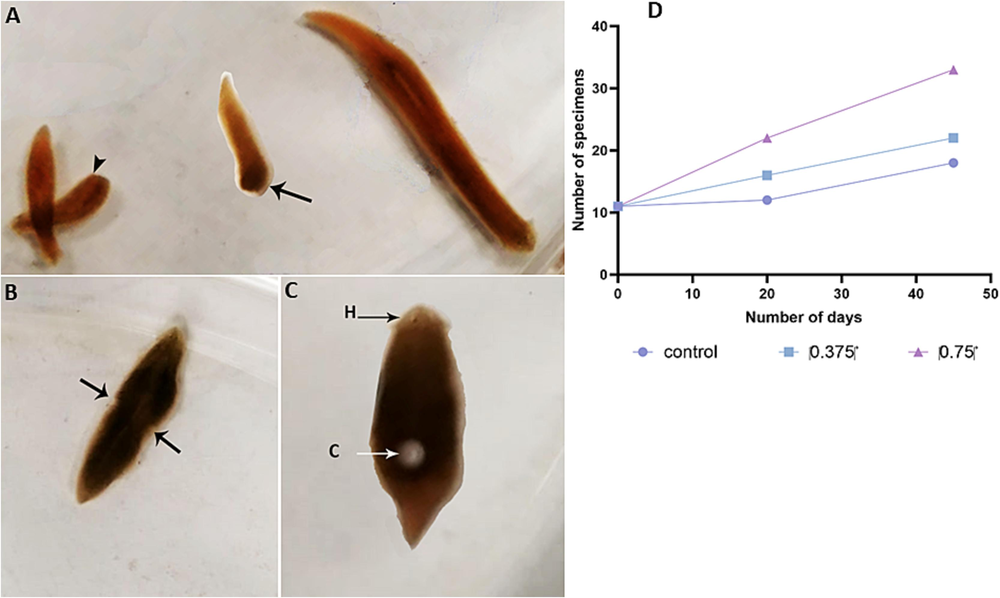
A. Asexual forms of D. bursagrossa reproducing asexually by scissiparity (arrowhead: anterior fragment; arrow: posterior fragment). B. Asexual specimens during fission, leading to a natural and spontaneous isolation of a fragment from the parental organism. C. Sexual form of D. bursagrossa producing a cocoon. D. Reproduction rate of the asexual form of D. bursagrossa, showing an increase in the number of specimens with elevated salinity. H: head; C: cocoon.
3.2 Effect of salinity on the ovarian microtubular cytoskeleton
Immunofluorescence staining (Fig. 2) revealed significantly higher Alexa Fluor 488 fluorescence intensity in the control group’s ovaries (Fig. 2A–C) compared with the treated groups’ ovaries (Fig. 2F). Furthermore, quantification analysis confirmed significantly higher (p < 0.0001) tubulin protein expression levels in the control group’s ovaries (Fig. 1) compared with the treated groups’ ovaries (Fig. I).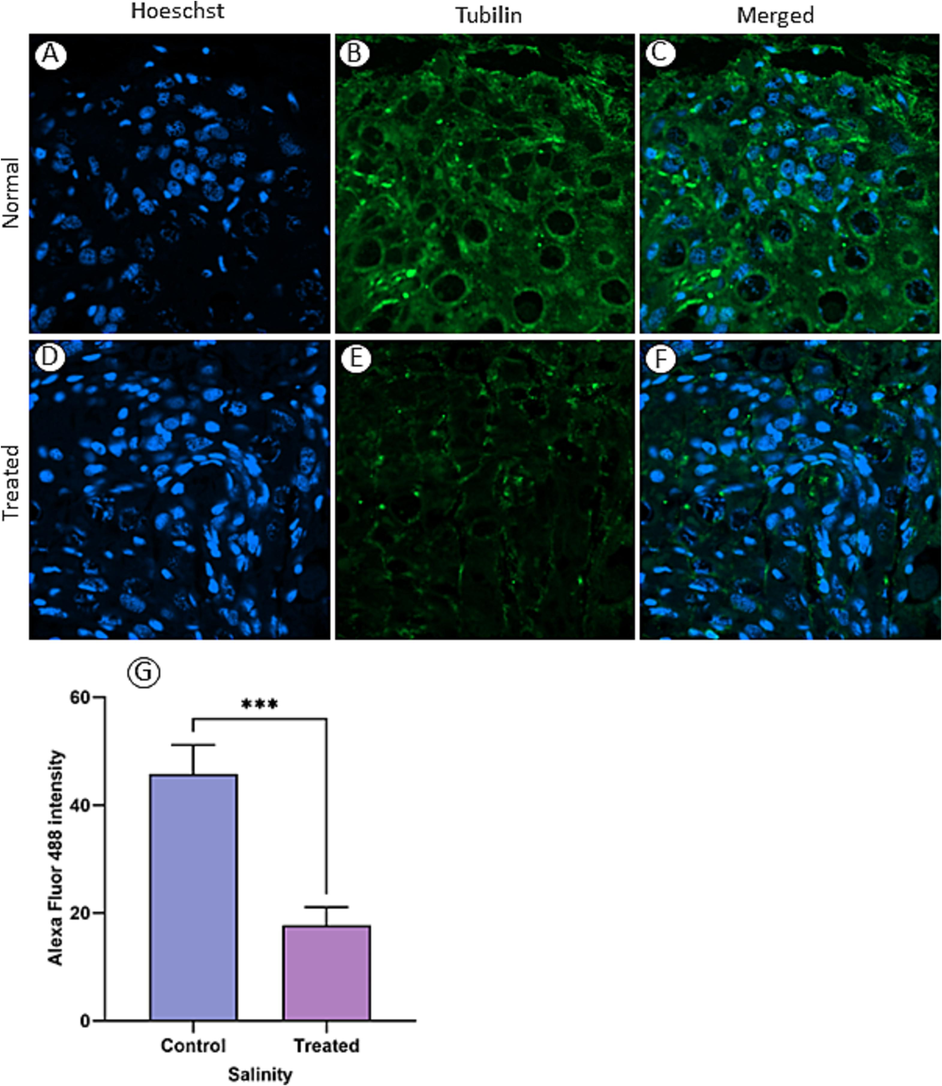
Tubulin distribution in ovarian tissue was assessed through immunofluorescence staining of ovaries from sexual specimens in normal (A–C) and one of the treated (D–F) groups (0.375 g/L). The relative fluorescence intensity of tubulin was notably higher in normal ovarian sections compared with those from treated groups (G). (***) indicates P < 0.0001.
3.3 Evaluation of lipid peroxidation
Fig. 1 illustrates MDA levels, a lipid peroxidation marker, in sexual and asexual experimental animals. Both treated groups of sexual specimens (0.375 and 0.750 g/L salinity) showed a significant dose-dependent increase in MDA levels (p < 0.05 and p < 0.01, respectively) compared with the control group (Fig. 3A). Notably, the lower salinity concentration had no effect on MDA levels in asexual specimens, whereas the higher salinity concentration significantly increased lipid peroxidation (p < 0.01) compared with the control (Fig. 3B).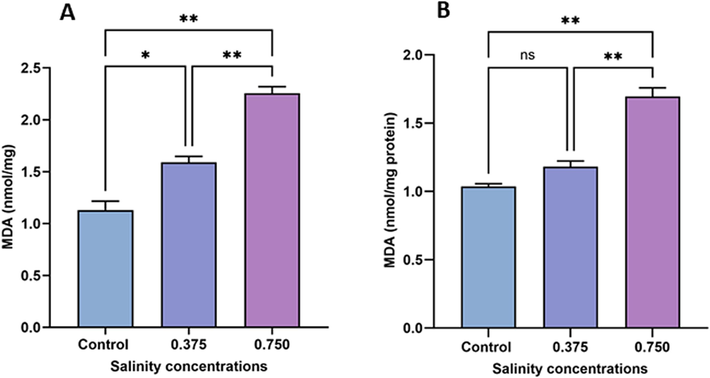
Variations in MDA content in sexual (A) and asexual (B) salinity-treated groups. Values are expressed as the mean ± standard deviation of three planarians from each group. (*) indicates p < 0.05, and (**) indicates p < 0.01. ns: not significant.
3.4 Effect of salinity on GSH levels
As shown in Fig. 4A, a significant decrease in GSH levels in sexual specimens was observed in the low-salinity group (0.375 g/L) compared with the control group (p < 0.01). However, asexual specimens remained unaffected by the low-salinity treatment. Additionally, both sexual and asexual specimens exposed to the high salinity treatment (0.750 g/L) exhibited decreased GSH levels (19.48 % and 41.51 %, respectively) compared with the control group. Moreover, 0.750 g/L salinity caused greater damage to GSH content in sexual specimens (p < 0.001) than in asexual specimens (p < 0.05).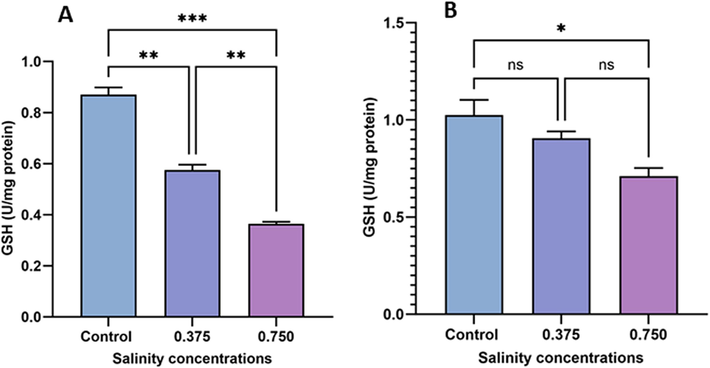
Evaluation of GSH content in sexual (A) and asexual (B) salinity-treated groups. Values are expressed as the mean ± standard deviation of eight rats from each group. (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001. ns: not significant.
3.5 Effect on CAT activity
As illustrated in Fig. 5, exposure to both salinity concentrations (0.375 and 0.750 g/L) resulted in a significant decrease in CAT activity in sexual specimens compared with the control group. Similarly, CAT activity in asexual specimens decreased under 0.375 and 0.750 g/L salinity treatments, respectively, compared with the control (Fig. 5B). In asexual specimens, no significant difference was observed in CAT activity between the high (0.750 g/L) and low (0.375 g/L) salinity groups. However, a significant difference was observed in sexual specimens between these salinity treatments (p < 0.01). Notably, the detrimental effects of 0.375 and 0.74 g/L salinity treatments on CAT activity were more pronounced in sexual specimens (p < 0.001 and p < 0.0001, respectively) than in asexual specimens (p < 0.05 and p < 0.01, respectively).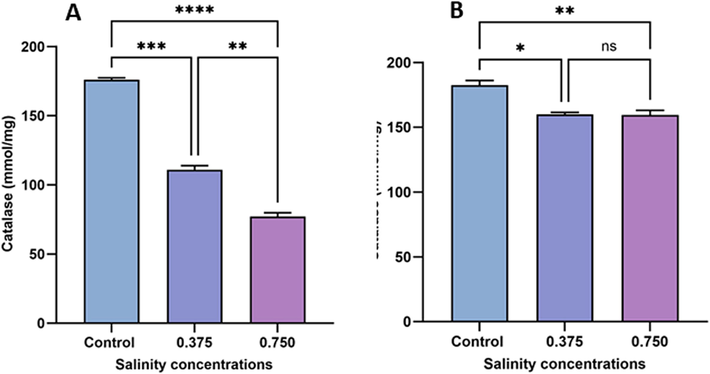
Changes in CAT activity in sexual (A) and asexual (B) salinity-treated groups. Values are expressed as the mean ± standard deviation of eight rats from each group. (*) indicates p < 0.05, (**) indicates p < 0.01, (***) indicates p < 0.001, and (****) indicates p < 0.0001. ns: not significant.
3.6 Evaluation of SOD activity
Fig. 6 depicts SOD activity among treatment groups. Both salinity concentrations (0.375 g/L and 0.750 g/L) dose-dependently inhibited SOD activity in sexual (19.48 % and 41.51 %, respectively; Fig. 5A) and asexual (19.48 % and 41.51 %, respectively; Fig. 5B) specimens compared with the control group.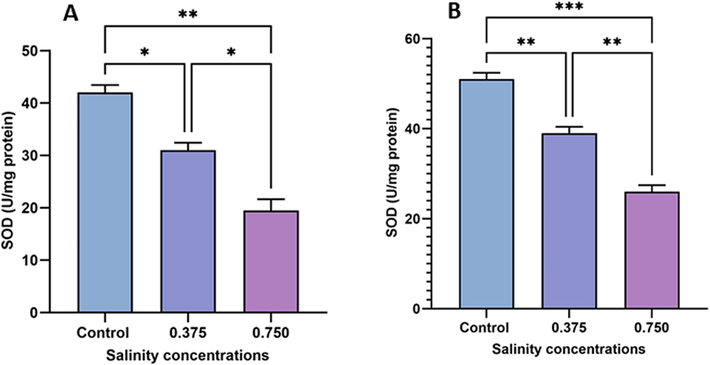
Changes in SOD activity in sexual (A) and asexual (B) salinity-treated groups. Values are expressed as the mean ± standard deviation of eight rats from each group. (*) indicates p < 0.05, (**) indicates p < 0.01, and (***) indicates p < 0.001. ns: not significant.
4 Discussion
Numerous studies have examined the impact of increased salinity on freshwater ecosystems (Hall and Burns, 2003; Montory et al., 2014), particularly its effects on highly vulnerable organisms, such as freshwater planarians with permeable teguments making them susceptible to dissolved chemicals (Buttarelli et al., 2000). This study focused on determining how elevated salinity levels influence the reproductive capabilities of the freshwater planarian D. bursagrossa. The findings suggest that increased salinity may induce a shift from sexual to asexual reproduction, with the asexual form of D. bursagrossa becoming significantly more abundant with increasing salinity. This aligns with previous studies reporting the reproductive responses of sexual and asexual species to various abiotic factors. For example, (Rivera and Perich, 1994) found that Dugesia tigrina showed tolerance to increased salinity that promoted asexual reproduction. In the field, the influence of water flow on the sexual development of this planarian species has been noted, with the sexual form commonly found in running water, whereas the asexual strain tends to thrive in ponds and still water (Hyman, 1943), environments where higher salinity levels may be prevalent. These findings align with those reported in another study, which revealed a significant reduction in blastema regeneration size in D. tigrina due to NaCl exposure (Dornelas et al., 2020). This reduction could affect asexual reproduction through fission, although NaCl exposure did not significantly alter the fecundity rate (Dornelas et al., 2020).
We explored the microtubular cytoskeleton’s organization in the ovary of the sexual form of D. bursagrossa using immunolabeled α-tubulin and confocal microscopy. Results indicated a dose-dependent decrease in the tubulin cytoskeleton with rising salinity, highlighting the potential impact of increased salinity on reproductive events in D. bursagrossa, i.e., affecting sexual reproduction and promoting asexual reproduction. Cytoskeletal elements, such as microfilaments and microtubules, are integral in various cellular processes, including cell cycle progression, cell differentiation, oocyte maturation, fertilization, and early embryo development (Lai et al., 1988; Wilson et al., 1997; Silvestre and Tosti, 2010; Maurizii and Taddei, 2012). In Ciona intestinalis, oocyte growth undergoes characteristic changes in the distribution of microtubules and cortical mRNAs (Prodon et al., 2006). Similar relocalizations have been observed the Xenopus previtellogenic oocyte, where microtubular reorganization and mRNA translocation to the cortex are well-documented (Gard, 1993; Kloc and Etkin, 2005). Microtubules in vertebrates are essential for the formation of the Balbiani body, a transient organelle complex in oogonia and the first lineage-specific morphological feature of female germ cells (Lei and Spradling, 2016). Consequently, factors such as toxins that block or destabilize cytoskeleton proteins can disrupt interactions, leading to functional impairment (Silvestre and Tosti, 2010). Considering the cytoskeleton’s vital role in ovarian cell development and differentiation, the significant decrease observed in the ovary of sexual planarians exposed to salinity compared with normal conditions suggests regression in ovarian structure, potentially affecting sexual reproduction.
In line with our findings, extreme salinity levels adversely affected both sexual and asexual reproduction in other invertebrates. For instance, the rotifer Brachionus plicatilis exhibited a more pronounced reduction in reproduction in sexual females compared with asexual females under high salinity conditions (Snell, 1986). (Lubzens et al., 1985) found that asexual reproduction rates displayed exponential growth, leading to an increase in population size. In specific environmental extremes, including high salinity, exclusive asexual reproduction was observed due to physiological constraints limiting sexual reproduction. This phenomenon aligns with observations of changes in macroinvertebrate communities and diversity occurring even at relatively low-salinity levels (Horrigan et al., 2005).
In most animals, GSH, SOD, and CAT activities are crucial indicators of antioxidant capacity, defending against oxidative cell damage by eliminating reactive oxygen species generated under stressful conditions (Bouayed and Bohn, 2010). SOD plays a role in detoxifying radicals, whereas CAT detoxifies peroxides. GSH, present in aerobic cells, is vital for scavenging reactive oxygen species and typically present at high concentrations in vertebrate and invertebrate tissues (Cnubben et al., 2001). Our findings revealed a significant reduction in SOD and CAT activity with increasing salinity levels in both sexual and asexual forms of D. bursagrossa. Regarding GSH, a significant decrease occurred in sexual forms exposed to both 0.375 and 0.750 g/L salinity, whereas in asexual forms, this reduction was only observed at the higher concentration. The compromised antioxidant capacity, evident in the significant increase in MDA levels, failed to protect the freshwater planarian from reactive oxygen species effects induced by salinity stress. This increase occurred in sexual specimens during exposure to both 0.375 and 0.750 g/L salinity, whereas in asexual specimens, it was observed solely at the higher concentration. Consequently, the damage inflicted was more severe in sexual specimens than in their more tolerant asexual counterparts. Similar negative effects of salinity on oxidative stress and reproduction have been reported in other invertebrate species, such as the copepod Acartia sp., where salinity-induced changes affected reproduction and oxidative status, potentially leading to long-term consequences for copepod communities (von Weissenberg et al., 2022).
5 Consent to participate
Not applicable.
6 Consent to publish
Not applicable.
CRediT authorship contribution statement
Abdel Halim Harrath: Conceptualization, Methodology, Investigation, Software, Writing – original draft preparation. Waleed Aldahmash: Investigation, Writing – original draft preparation. Lamjed Mansour: Investigation, Validation. Khalid Elfeki: Software, Validation. Saleh Alwasel: Supervision, Writing – review & editing.
Acknowledgments
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (14-Env2212-02).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Health economics of dengue: a systematic literature review and expert panel's assessment. Am. J. Trop. Med. Hygiene. 2011;84:473.
- [Google Scholar]
- Improved method for the determination of blood glutathione. J. Lab. Clin. Med.. 1963;61:882-888.
- [Google Scholar]
- How should salinity influence fish growth? Comp. Biochem. Physiol. C: Toxicol. Pharmacol.. 2001;130:411-423.
- [Google Scholar]
- Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev.. 2010;3:228-237.
- [Google Scholar]
- Acetylcholine/dopamine interaction in planaria. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol.. 2000;125:225-231.
- [Google Scholar]
- The interplay of glutathione-related processes in antioxidant defense. Environ. Toxicol. Pharmacol.. 2001;10:141-152.
- [Google Scholar]
- Lethal and sublethal effects of the saline stressor sodium chloride on Chironomus xanthus and Girardia tigrina. Environ. Sci. Pollut. Res.. 2020;27:34223-34233.
- [Google Scholar]
- Climate change 2014–Impacts, adaptation and vulnerability: Regional aspects. Cambridge University Press; 2014.
- Dominance and compensatory growth in phytoplankton communities under salinity stress. J. Exp. Mar. Biol. Ecol.. 2010;395:223-231.
- [Google Scholar]
- Ectopic spindle assembly during maturation of Xenopus oocytes: evidence for functional polarization of the oocyte cortex. Dev. Biol.. 1993;159:298-310.
- [Google Scholar]
- Comparison of the effects of propolis and pollen extracts in the same concentrations on some biochemical and hematological parameters in rainbow trout (Oncorhynchus mykiss) J. Surv. Fisheries Sci. 2016:1-8.
- [Google Scholar]
- Responses of crustacean zooplankton to seasonal and tidal salinity changes in the coastal Lake Waihola, New Zealand. N. Z. J. Mar. Freshw. Res.. 2003;37:31-43.
- [Google Scholar]
- Using autophagy, apoptosis, cytoskeleton, and epigenetics markers to investigate the origin of infertility in ex-fissiparous freshwater planarian individuals (nomen nudum species) with hyperplasic ovaries. J. Invertebr. Pathol.. 2023;199:107935
- [Google Scholar]
- Response of stream macroinvertebrates to changes in salinity and the development of a salinity index. Mar. Freshw. Res.. 2005;56:825-833.
- [Google Scholar]
- The alpha-tubulin gene family expressed during cell differentiation in Naegleria gruberi. J. Cell Biol.. 1988;106:2035-2046.
- [Google Scholar]
- Interrelationship of salinity shift with oxidative stress and lipid metabolism in the monogonont rotifer Brachionus koreanus. Comparative Biochem. Physiol. Part a: Mol. Integrative Physiol.. 2017;214:79-84.
- [Google Scholar]
- Mouse oocytes differentiate through organelle enrichment from sister cyst germ cells. Science. 2016;352:95-99.
- [Google Scholar]
- Salinity dependence of sexual and asexual reproduction in the rotifer Brachionus plicatilis. Mar. Biol.. 1985;85:123-126.
- [Google Scholar]
- Microtubule organization and nucleation in the differentiating ovarian follicle of the lizard Podarcis sicula. J. Morphol.. 2012;273:1089-1095.
- [Google Scholar]
- Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein) J. Biol. Chem.. 1969;244:6049-6055.
- [Google Scholar]
- Effects of low salinity on adult behavior and larval performance in the intertidal gastropod Crepipatella peruviana (Calyptraeidae) PLoS One. 2014;9:e103820.
- [Google Scholar]
- Which environmental factors control phytoplankton populations? A Bayesian variable selection approach. Ecol. Model.. 2013;269:1-8.
- [Google Scholar]
- Effects of various sodium selenite concentrations on some biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss) Fresen. Environ. Bull.. 2005;14
- [Google Scholar]
- Establishment of animal–vegetal polarity during maturation in ascidian oocytes. Dev. Biol.. 2006;290:297-311.
- [Google Scholar]
- Effects of water quality on survival and reproduction of four species of planaria (Turbellaria: Tricladida) Invertebrate Reprod. Develop.. 1994;25:1-7.
- [Google Scholar]
- Study of salinity and temperature tolerance limits regarding four crustacean species in a temporary salt water swamp (Lake Zima, Morocco) Anim. Biol.. 2004;54:237-253.
- [Google Scholar]
- Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383-388.
- [Google Scholar]
- Influence of environmental salinity and cortisol pretreatment on gill Na+/K+− ATPase activity and survival and growth rates in Cyprinus carpio. Aquacult. Rep.. 2018;11:1-7.
- [Google Scholar]
- Consequences of climate-induced salinity increases on zooplankton abundance and diversity in coastal lakes. Mar. Ecol. Prog. Ser.. 2003;251:181-189.
- [Google Scholar]
- Impact of marine drugs on cytoskeleton-mediated reproductive events. Mar. Drugs. 2010;8:881-915.
- [Google Scholar]
- Effect of temperature, salinity and food level on sexual and asexual reproduction in Brachionus plicatilis (Rotifera) Mar. Biol.. 1986;92:157-162.
- [Google Scholar]
- Combined effect of salinity and temperature on copepod reproduction and oxidative stress in brackish-water environment. Front. Mar. Sci.. 2022;9:952863
- [Google Scholar]
- Combined effects of cadmium and salinity on juvenile Takifugu obscurus: cadmium moderates salinity tolerance; salinity decreases the toxicity of cadmium. Sci. Rep.. 2016;6:30968.
- [Google Scholar]
- Differential expression of two γ-tubulin isoforms during gametogenesis and development inDrosophila. Dev. Biol.. 1997;184:207-221.
- [Google Scholar]







