Translate this page into:
Sakuranetin counteracts polyethylene microplastics induced nephrotoxic effects via modulation of Nrf2/Keap1 pathway
⁎Corresponding author. ali6265207@gmail.com (Ali Akbar) ali0703593@gmail.com (Ali Akbar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Polyethylene microplastics (PEMPs) are widely distributed in environment and exerts deleterious effects on animal as well as human health. Sakuranetin (SKN) is a natural flavonoid that manifests profound therapeutic potential. Albino rats (n = 24) were partitioned into 4 groups i.e., Control, PEMPs 1.5 mg/kg, PEMPs 1.5 mg/kg + SKN 10 mg/kg and SKN 10 mg/kg administered group. After 30 days of treatment, our results revealed that PEMPs exposure reduced nuclear factor erythroid 2–related factor 2 (Nrf-2) and antioxidant genes while enhancing the expression of kelch-like ECH-associated protein 1(Keap-1). Besides, PEMPs intoxication reduced the level of renal biomarkers i.e., creatinine clearance and increased the level of creatinine, urea, neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1). Additionally, it lessened the activities of glutathione S-transferase (GST), superoxide dismutase (SOD), glutathione reductase (GSR), catalase (CAT), glutathione peroxidase (GPx) and heme oxygenase-1 (HO-1) whereas the levels of malondialdehyde (MDA) and reactive oxygen species (ROS) were increased. Conversely, it increased the levels of nuclear factor-kappa B (NF-kB), tumor necrosis factor-alpha (TNF-a), interleukin-1beta (IL-1b) and IL-6 as well as escalated the activity of cyclooxygenase-2 (COX-2). Furthermore, the expression of bcl-2-associated X protein (Bax) and caspase-3 were elevated, while the expression of B-cell lymphoma 2 (Bcl-2) was lowered. However, SKN treatment significantly (P < 0.05) restored aforementioned renal impairments. Therefore, it is proposed that SKN may be applied as a nephroprotective agent against the PEMPs-prompted renal toxicity.
Keywords
Sakuranetin
Polyethylene microplastics
Toxicity
Kidney damage
Oxidative stress
1 Introduction
The increasing environmental pollution caused by plastics has garnered substantial global attention (Hwang et al., 2020). Recent reports have revealed that plastic pollution has become ubiquitous in terrestrial as well as marine ecosystems (Borrelle et al., 2020). Microplastics (MPs) originate from the deterioration of plastic materials due to biological factors as well as the incineration of plastic materials (Wright and Kelly, 2017; Andrady, 2011). Humans are exposed to MPs through several ways, such as inhalation and oral administration, owing to their occurrence in air, water and food (Zhang et al. 2020). Once MPs invade the human body, they travel via the circulatory network to various body parts, affecting normal physiological functions by provoking cellular stress (Vethaak and Legler, 2021).
The most commonly recognized microplastics in the terrestrial and aquatic environments are polyethylene microplastics (PEMPs) (de Souza Machado et al., 2018). Recent studies have reported that PEMPs can induce various adverse effects including cytotoxicity, developmental toxicity and hematological disturbances in the body (Ge et al., 2021). It is evident that, PEMPs treatment leads to neurotoxicity and disrupts the normal physiological mechanism of germ cell differentiation (Mak et al. 2019). In addition to this, PEMPs treatment has been reported to elicit oxidative stress (OS), which can have potential detrimental influence on intracellular metabolism and disrupt the redox equilibrium (Silva et al., 2021). Recent investigations have reported that PEMPs may increases OS and lipid peroxidation (Ijaz et al., 2024).
Plants are recognized as extensive reservoirs of therapeutic compounds. Flavonoids are phenolic compounds that are abundantly found in grains, flowers, vegetables and fruits. Elsayed et al. (2022) reported that flavonoids can reduce the levels of OS via eliciting the activity of antioxidant enzymes. Sakuranetin (SKN) is a derivative of naringenin that is isolated from multiple plants as well as honey derived from. SKN has been extensively documented for its numerous pharmacotherapeutic characteristics i.e., anti-inflammatory, antitumor, antioxidant as well as neuroprotective (Stompor, 2020). Therefore, ongoing research was aimed to estimate the alleviative action of SKN to counteract PEMPs prompted renal impairments.
2 Materials and methods
2.1 Chemicals
PEMPs (CAS No:9002–88-4) and SKN (CAS No:520–29-6) were obtained from Sigma-Aldrich, Germany.
2.2 Animals
Trial was conducted on albino rats (n = 24) in the institutional research center of the University of Agriculture, Faisalabad. Rats were kept in enclosures and maintained under uniform conditions (temperature, 24 ± 1 °C) and treated with standard diet and water. Rats were acclimated for 1 week. Rats were treated in accordance with the approved protocol of the European Union for Animal Care and Experimentation (CEE Council 86/609).
2.3 Layout of experiment
24 albino rats were apportioned among 4 different groups (n = 6) i.e., Control, PEMPs 1.5 mg/kg, PEMPs 1.5 mg/kg + SKN 10 mg/kg and SKN 10 mg/kg administered group. After 30 days of administration. Animals were anesthetized and decapitated. Kidneys were dissected, and washed employing normal saline. One kidney was preserved in 10 % solution of formaldehyde for histological assessment while, second kidney was utilized for the biochemical assessment
2.4 Assessment of renal biomarkers
The estimation of renal biomarkers was carried out with the help of ELISA kits. The analysis was accomplished following the recommended protocol of the manufacturer.
2.5 Biochemical analysis
The CAT activity was measured through the Aebi (1984) technique. The SOD activity was calculated by following the technique documented by Kakkar et al. (1984) approach. For the quantification of GPx Rotruck et al. (1973) technique was employed. Carlberg and Mannervik (1975) along with Jollow et al. (1974) protocol was followed for the measurement of GSR along with GSH. The Younis et al. (2018) protocol was followed for the measurement of GST. The ROS and MDA level was ascertained using Hayashi et al. (2007) and Ohkawa et al. (1979) approaches.
2.6 Assessment of Nrf2/Keap1, Bcl-2, caspase-3, Bax and antioxidative genes
qRT-PCR was employed to estimate the expressions of Nrf2/Keap1, Bcl-2, caspase-3, Bax and antioxidative genes. Total RNA isolation was accomplished using the TRIzol reagent, followed by reverse transcription to produce cDNA. The evaluation of changes in the gene expression was carried out following 2-ΔΔCT method and β-actin served as an internal control, as outlined by Livak and Schmittgen (2001). Table 1 demonstrates the primer sequence of β-actin as well as target genes as previously stated by Ijaz et al. (2022) and Hamza et al. (2023).
Gene
Primers 5′ −> 3′
Accession number
Nrf-2
F: ACCTTGAACACAGATTTCGGTG
R: TGTGTTCAGTGAAATGCCGGANM_031789.1
Keap-1
F: ACCGAACCTTCAGTTACACACT
R: ACCACTTTGTGGGCCATGAANM_057152.1
CAT
F: TGCAGATGTGAAGCGCTTCAA
R: TGGGAGTTGTACTGGTCCAGAANM_012520.2
SOD
F: AGGAGAAACTGACAGCTGTGTCT
R: AAGATAGTAAGCGTGCTCCCACNM_017051.2
GPx
F: TGCTCATTGAGAATGTCGCGTC
R: ACCATTCACCTCGCACTTCTCANM_030826.4
GSR
F: ACCAAGTCCCACATCGAAGTC
R: ATCACTGGTTATCCCCAGGCTNM_053906.2
HO-1
F: AGGCTTTAAGCTGGTGATGGC
R: ACGCTTTACGTAGTGCTGTGTNM_012580.2
Bax
F: GGC CTT TTT GCT ACA GGG TT
R: AGC TCC ATG TTG TTG TCC AGNM_017059.2
Bcl-2
F: ACA ACA TCG CTC TGT GGA T
R: TCA GAG ACA GCC AGG AGA ANM_016993.1
Caspase-3
F: ATC CAT GGA AGC AAG TCG AT
R: CCT TTT GCT GTG ATC TTC CTNM_012922.2
β-actin
F: TACAGCTTCACCACCACAGC
R: GGAACCGCTCATTGCCGATANM_031144
2.7 Inflammatory biomarkers
The analysis of inflammatory biomarkers was executed using ELISA kits. The analysis was accomplished following the recommended protocol of the manufacturer.
2.8 Statistical analysis
Data were shown as Mean ± SE. The normal distribution of the data was checked by Shapiro–Wilk test, whereas the homogeneity of variances was checked and confirmed by using Levene test. Using one-way ANOVA and Tukey’s test, data were statistically examined through Minitab software. Significance level was set at P < 0.05.
3 Results
3.1 Impact of PEMPs and SKN on Nrf2/keap1 pathway
PEMPs exposure substantially (P < 0.05) decreased Nrf2 and antioxidative genes while escalating the Keap1 expression contrary to control. Co-treatment of SKN + PEMPs substantially increased Nrf2 and antioxidant gene’s expression while decreasing the expression of keap1 in comparison to PEMPs-group. No substantial alterations were detected in Nrf2/keap1 expression among the SKN-supplemented rats and the control (Fig. 1).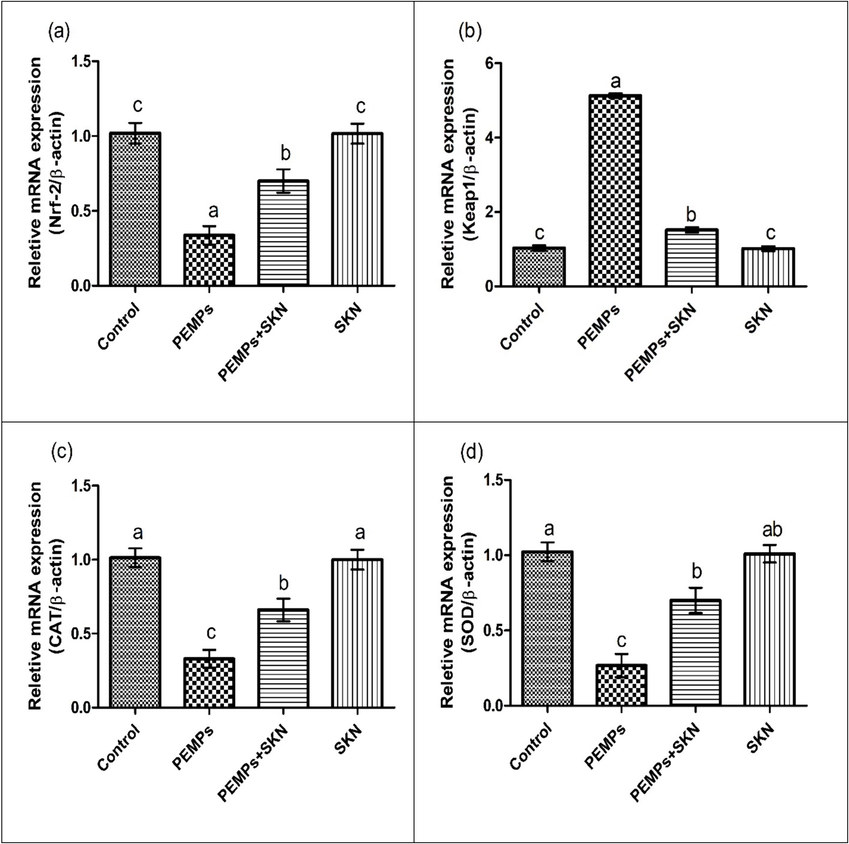
The PEMPs and SKN effect on the expression of (a) Nrf2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1. Data were shown as Mean ± SEM. Dissimilar letters on graph bars denoting substantial distinctions at P < 0.05.
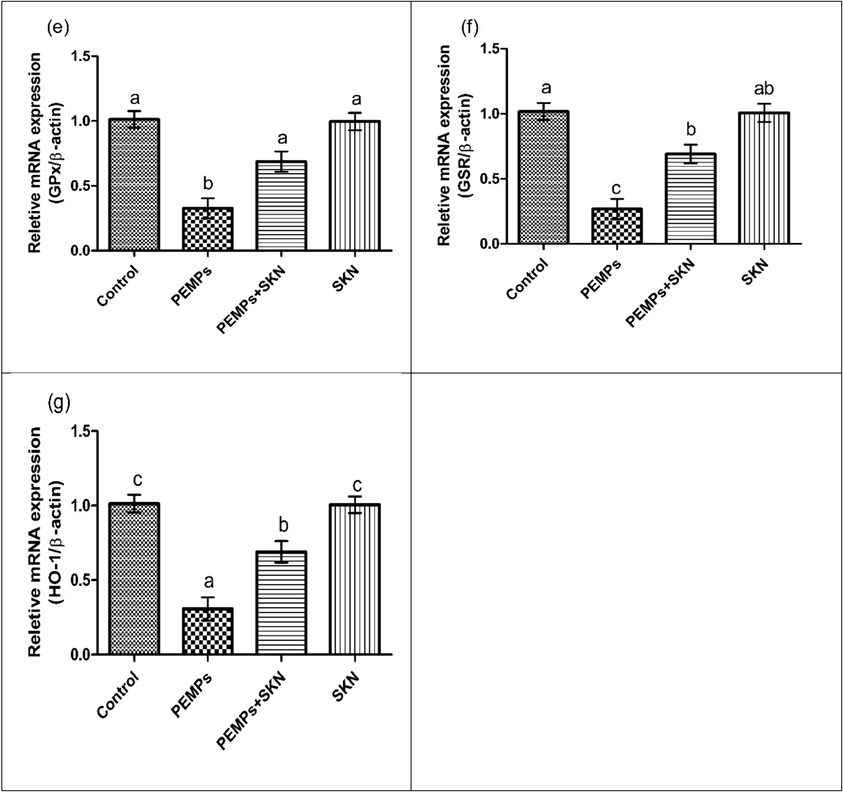
The PEMPs and SKN effect on the expression of (a) Nrf2, (b) Keap1, (c) CAT, (d) SOD, (e) GPx, (f) GSR and (g) HO-1. Data were shown as Mean ± SEM. Dissimilar letters on graph bars denoting substantial distinctions at P < 0.05.
3.2 PEMPs and SKN impact on biochemical indices
PEMPs treatment remarkably lowered GSH, GST, GSR, SOD, HO-1, CAT, GPx and escalated the ROS and MDA level contrary to the control rats. Conversely, the concurrent treatment of PEMPs + SKN recovered the aforesaid dysregulations in contrast to the PEMPs-group. However, only SKN administration showed insignificant differences contrary to the animal in the control as displayed in Table 2. Dissimilar letters accompanying certain values serve to highlight disparity among dissimilar groups.
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
CAT (U/mg protein)
14.21 ± 1.59a
5.99 ± 0.96b
11.28 ± 0.74a
14.75 ± 1.90a
SOD (U/mg protein)
11.48 ± 1.27a
4.56 ± 1.18c
8.18 ± 0.75b
11.77 ± 1.43a
GSR (nM NADPH oxidized/min/mg tissue
8.59 ± 0.88a
3.68 ± 0.47c
6.21 ± 0.62b
8.86 ± 0.96a
GPx (U/mg protein)
25.11 ± 1.43a
12.15 ± 1.84b
21.07 ± 1.52a
25.37 ± 1.92a
GSH (U/mg protein)
17.82 ± 1.28a
8.05 ± 0.44c
14.72 ± 1.17b
18.15 ± 1.50a
GST (U/mg protein)
37.88 ± 2.18a
16.390 ± 0.796c
31.080 ± 1.336b
38.52 ± 2.82a
HO-1 (pmoles bilirubin/ mg protein/h)
287.06 ± 8.35a
64.99 ± 9.48c
20.93 ± 11.38b
299.42 ± 14.54a
MDA (nmol/g)
0.27 ± 0.14c
2.48 ± 0.34a
1.58 ± 0.13b
0.25 ± 0.14c
ROS (nmol/g)
1.28 ± 0.22b
6.86 ± 0.81a
2.05 ± 0.14b
1.25 ± 0.22b
3.3 PEMPs and SKN impact on renal parameters
Analysis of the renal function biomarkers showed that PEMPs treatment instigated renal disturbances as confirmed by a notable amplification in the creatinine, urea, NGAL, KIM-1 and reduction in the creatinine clearance contrary to control. However, combined treatment of SKN and PEMPs restored aforementioned disturbances in comparison to the PEMPs-exposed animals. SKN (only) treatment group displayed mean values of aforesaid markers approximately similar to the animals in the control as displayed in Table 3. Dissimilar letters accompanying certain values serve to highlight disparity among dissimilar groups.
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
Urea (mg/dl)
15.96 ± 2.15a
29.7 ± 22.2a
21.66 ± 2.28a
15.88 ± 2.16a
Creatinine (mg/dl)
1.47 ± 0.20bc
5.40 ± 0.39a
2.19 ± 0.29b
1.39 ± 0.21c
Creatinine Clearance (ml/min)
2.15 ± 0.16a
0.77 ± 0.22c
1.61 ± 0.135b
2.21 ± 0.14a
KIM-1 (mg/dl)
0.48 ± 0.12c
4.48 ± 0.26a
1.45 ± 0.21b
0.43 ± 0.15c
NGAL (ng/day)
0.83 ± 0.15c
6.30 ± 0.25a
1.56 ± 0.20b
0.79 ± 0.17c
3.4 Pemps and SKN impact on inflammatory indices
PEMPs treatment considerably upregulated inflammatory biomarkers level contrary to the control. The co-administration of SKN and PEMPs decreased the levels aforesaid biomarkers in contrast to the PEMPs administrated rats. However, only SKN supplementation displayed normal level of these biomarkers almost equal to the animals in the control as displayed in Table 4. Dissimilar letters accompanying certain values serve to highlight disparity among dissimilar groups.
Parameters
Groups
Control
PEMPs
PEMPs + SKN
SKN
NF-kB (ng/g tissue)
21.47 ± 0.85c
84.40 ± 2.37a
26.26 ± 1.27b
21.39 ± 0.89c
TNFα (ng/g tissue)
9.63 ± 0.54c
57.72 ± 1.54a
17.20 ± 1.63b
9.53 ± 0.61c
IL-1ß (ng/g tissue)
15.10 ± 2.06c
72.92 ± 2.16a
23.77 ± 2.60b
14.94 ± 2.01c
IL-6 (ng/g tissue)
10.38 ± 0.99c
52.48 ± 3.49a
19.81 ± 1.78b
10.12 ± 1.11c
COX-2 (ng/g tissue)
11.99 ± 2.22c
63.54 ± 2.11a
19.64 ± 1.581b
11.37 ± 1.94c
3.5 Pemps and SKN impact on apoptotic biomarkers
PEMPs treatment notably amplified the caspase-3 and Bax while lowering the expression of Bcl-2 in comparison to control. SKN supplementation along with PEMPs restored the expression of these biomarkers as compared to PEMPs group. However, non-significant alterations were examined among SKN and control groups (Fig. 2).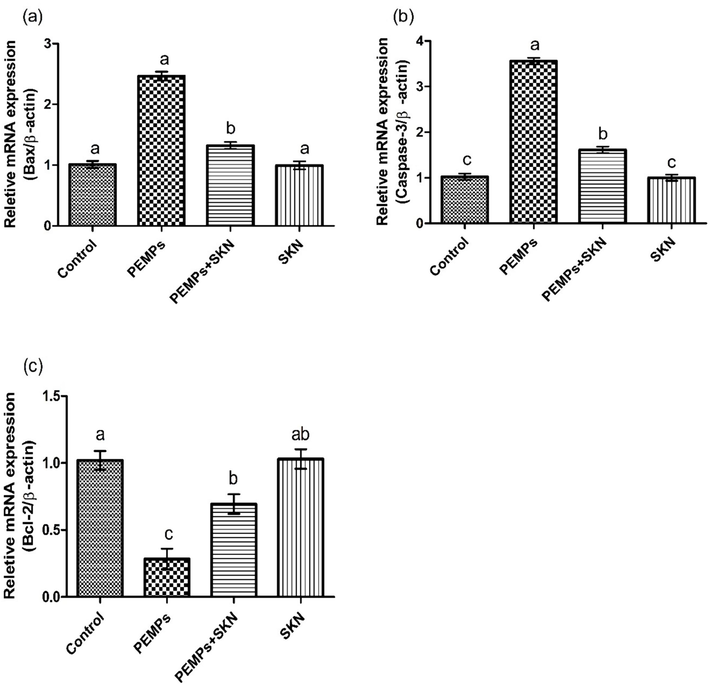
The PEMPs and SKN effect on (a) Bax, (b) caspase-3 and (c) Bcl-2. Dissimilar letters on graph bars denoting substantial distinctions at P < 0.05.
4 Discussion
In our investigation, PEMPs exposure resulted in a downregulation of Nrf2 and an upregulation of Keap1 expression which decreased the expression of antioxidative genes including GSR, SOD, GPx, CAT and HO-1. It has been documented that regulation of antioxidative gene through the Keap1-Nrf2 pathway serves as an inducible defense mechanism to mitigate OS (Yamamoto et al., 2018). Nrf2 coordinates the cellular antioxidative response that can effectively neutralize ROS. Conversely, Keap1 acts as an inhibitor of Nrf2 that facilitates Nrf2 degradation (Bellezza et al., 2018). However, supplementation of SKN substantially recovered the expression of the aforementioned cytoprotectant genes by modulating the Nrf2/Keap1 pathway. Our outcomes are corroborated by the observation of Hamza et al. (2023) who revealed that flavonoids exhibit the potential to regulate Nrf2/Keap1 pathway.
PEMPs intoxication prompted a substantial upsurge in the concentration of creatinine, urea, NGAL, KIM-1 and decreased creatinine clearance. OS is recognized as the major culprit underlying disturbed level of these biomarkers. Changes in the structure of the glomerulus, impaired filtration rate and subsequent dysfunction of nephron are all linked to an increase in the concentration of nitrogenous end product i.e., creatinine and urea (Ijaz et al., 2023). Moreover, increased levels of KIM-1 and NGAL are positively correlated with the vascular damage and dysfunctions of the proximal tubules (Dobrek et al., 2017). However, SKN supplementation significantly restored the disrupted levels of the aforementioned parameters, primarily through its antioxidative and nephroprotective properties.
PEMPs administration lowered the activities GSR, SOD, GST, GPx, CAT HO-1 and escalated the ROS and MDA level. Disturbed equilibrium between pro- and anti-oxidant results in formation of OS. The aforementioned endogenous antioxidants serve as a crucial factor in regulating the levels of ROS and OS, ultimately preventing cellular damage in the body (Sinha et al., 2013). It is reported that increased levels of OS disrupt the normal architecture of plasma membrane (Ishtiaq et al., 2022). Furthermore, excessive generation of ROS reduced the activities of antioxidant enzymes thereby impairing cellular defense system (Ahmad et al., 2023). The occurrence of LP and free radicals formation are correlated to each other and can be diagnosed by the level of its end product i.e., level of MDA (Adejuwon et al., 2015). However, SKN supplementation regulated PEMPs induced imbalance in pro-oxidants and antioxidants by reducing OS and elevating the antioxidant enzymes levels in the kidney. The antioxidative nature of flavonoids is mainly ascribed to their numerous OH groups, which facilitate their ability to alleviate OS (Teixeira et al. 2005).
PEMPS administration raised the inflammatory indices concentration. NF-κB serves as a major element that activates TNF-α, IL-1β along with IL-6 which induce acute inflammation and ROS-associated damage in the body (Khan et al., 2020). Moreover, COX-2 is a notable inflammatory mediator that plays a key part in inducing renal inflammatory response (Agarwal et al., 2009). However, SKN treatment not only suppressed the activation of NF-κB, a major culprit underlying renal inflammation but also regulated the levels of other inflammatory biomarkers. Outcomes of current investigation are in line with the research of Kim and Kang (2016) who reported that SKN constrains the inflammatory response in the macrophages.
PEMPs treatment enhanced Bax and Caspase-3 while lowering the Bcl-2 expression. Apoptosis occurs due to a disruption in the equilibrium between pro- and anti-apoptotic biomarkers. It has been revealed that impairment in the inner and outer mitochondrial membrane movement occurs as a result of disturbed ratio between Bax and Bcl-2 (Gu et al., 2017). Besides, Bax and Bcl-2 play an indispensable role in amplifying the liberation of cytochrome c from mitochondria, thereby triggering the apoptotic response (Caglayan et al., 2019; Kuzu et al., 2019). An upsurge in Bax and a drop in Bcl-2 serves as the mediator for the stimulation of discharge of caspase-3 which ultimately initiates the apoptotic damages (Eldutar et al., 2017). However, SKN treatment restored the expression of these biomarkers due to its anti-apoptotic properties.
5 Conclusion
Present study suggests that SKN possesses the mitigative potential to counteract PEMPs prompted detrimental nephrotoxic effects via modulating the Nrf2/keap1, antioxidative and apoptotic genes expression. Additionally, SKN supplementation restored the disturbed level of renal OS, LP, inflammatory and renal injury biomarkers. These findings provide evidence that SKN demonstrates defensive properties to counteract PEMPs provoked renal impairments. However, clinical trials are recommended in future to evaluate the efficacy and therapeutic potential of SKN against PEMPs-instigated renal impairments.
CRediT authorship contribution statement
Ali Akbar: Writing – original draft, Methodology, Investigation, Data curation, Conceptualization. Fatima Amin: Writing – original draft, Methodology, Investigation, Conceptualization. Moazama Batool: Visualization, Validation, Formal analysis, Data curation. Aisha Khatoon: Visualization, Software, Investigation. Zubair Ahmad: Writing – original draft, Resources, Funding acquisition. Usman Atique: Writing – review & editing, Validation, Software.
Acknowledgment
This work was funded by Researchers Supporting Project number (RSPD2024R1113), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cisplatin-induced testicular dysfunction and its amelioration by Launaea taraxacifolia leaf extract. Andrologia.. 2015;47:553-559.
- [Google Scholar]
- Eicosanoids in inflammation and cancer: the role of COX-2. Expert Rev. Clin. Immunol.. 2009;5:145-165.
- [Google Scholar]
- Ameliorative Effects of Rhamnetin against Polystyrene Microplastics-induced Nephrotoxicity in Rats. Pak. Vet. J.. 2023;43:623-627.
- [CrossRef] [Google Scholar]
- Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta.. 2018;1865:721-733.
- [Google Scholar]
- Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Sci.. 2020;369:1515-1518.
- [Google Scholar]
- Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level oxidative stress apoptosis and inflammation in rats. J. Trace Elem. Med. Biol.. 2019;54:69-78.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol.. 2018;24:1405-1416.
- [Google Scholar]
- Biochemical and histological evaluation of kidney function in rats after a single administration of cyclophosphamide and ifosfamide. J. Nephrol. Kidney Dis.. 2017;1:1002-1008.
- [Google Scholar]
- Restorative effects of Chrysin pretreatment on oxidant–antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats: an experimental and biochemical study. J. Biochem. Mol. Toxicol.. 2017;31:21960-21965.
- [Google Scholar]
- Ameliorating effect of lycopene and N-acetylcysteine against cisplatin-induced cardiac injury in rats. Pak. Vet. J.. 2022;42:107-111.
- [CrossRef] [Google Scholar]
- Review of the toxic effect of microplastics on terrestrial and aquatic plants. Sci. Total Environ.. 2021;791:148333-148335.
- [Google Scholar]
- Inhibition of chemotherapy–induced apoptosis of testicular cells by squid ink polysaccharide. Exp. Ther. Med.. 2017;14:5889-58895.
- [Google Scholar]
- Hepatoprotective effects of astragalin against polystyrene microplastics induced hepatic damage in male albino rats by modulating Nrf-2/Keap-1 pathway. J. Func. Foods.. 2023;108:105771-105775.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. Genet. Toxicol. Environ.. 2007;631:55-61.
- [Google Scholar]
- Chemoprotective effect of vitexin against cisplatin-induced biochemical, spermatological, steroidogenic, hormonal, apoptotic and histopathological damages in the testes of Sprague-Dawley rats. Saudi. Pharm. J.. 2022;30:519-526.
- [Google Scholar]
- Antioxidant, anti-inflammatory and anti-apoptotic effects of amentoflavone on gentamicin-induced kidney damage in rats. J. King Saud Univ. Sci.. 2023;35:102791-102795.
- [Google Scholar]
- Mitigative potential of kaempferide against polyethylene microplastics induced testicular damage by activating Nrf-2/Keap-1 pathway. Ecotoxicol. Environ. Saf.. 2024;269:115746-115750.
- [Google Scholar]
- Therapeutic Effect of Oroxylin A Against Bisphenol A-induced Kidney Damage in Rats: a Histological and Biochemical Study. Pak. Vet. J.. 2022;42:511-516.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol.. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Naringenin prevents doxorubicin-induced toxicity in kidney tissues by regulating the oxidative and inflammatory insult in Wistar rats. Arch. Physiol. Biochem.. 2020;126:300-307.
- [Google Scholar]
- Sakuranetin inhibits inflammatory enzyme, cytokine, and costimulatory molecule expression in macrophages through modulation of JNK, p38, and STAT1. Med: Evid. based Complement. Altern; 2016. p. :9824203.
- Protective effect of morin on doxorubicin-induced hepatorenal toxicity in rats. Chem. Biol. Interact.. 2019;308:89-100.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods.. 2001;25:402-408.
- [Google Scholar]
- Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf.. 2019;182:109442-109445.
- [Google Scholar]
- Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Sci.. 1973;179:588-590.
- [Google Scholar]
- Immune response triggered by the ingestion of polyethylene microplastics in the dipteran larvae Chironomus riparius. J. Hazard. Mater.. 2021;414:125401-125405.
- [Google Scholar]
- Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol.. 2013;87:1157-1180.
- [Google Scholar]
- A review on sources and pharmacological aspects of sakuranetin. Nutr.. 2020;12:513-515.
- [Google Scholar]
- Structure–property studies on the antioxidant activity of flavonoids present in diet. Free Radic. Biol. Med.. 2005;39:1099-1108.
- [Google Scholar]
- Microplastics and Human Health. J. Sci.. 2021;371:672-674.
- Plastic and human health: a micro issue? Environ. Sci. Technol.. 2017;51:6634-6647.
- [Google Scholar]
- The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev.. 2018;98:1169-1203.
- [Google Scholar]
- Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl 4-challanged nephrotoxicity in rats. Pak. J. Pharm. Sci.. 2018;31:1475-1484.
- [Google Scholar]
- Atmospheric microplastics: A review on current status and perspectives. Earth. Sci. Rev.. 2020;203:103118-103120.
- [Google Scholar]







