Translate this page into:
Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella
⁎Corresponding author. guraishi@yahoo.com (Saleh Al-Quraishy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Coccidiosis has a considerable economic loss in the poultry industry where the parasite Eimeria affect the animal survival. Natural products emerge as an essential and complementary means of controlling avian coccidiosis, in addition to anticoccidial medications. The current study examined the antioxidant activity of Rumex nervosus leaf extract (RE) in chickens infected with Eimeria tenella. RE was able to decrease the induced weight loss due to infection. Also, RE significantly suppressed the oocysts released in chickens’ faeces. Moreover, the oxidative status due to E. tenella infection had been changed after treatment with RE where the level of glutathione, malondialdehyde and nitric oxide was improved indicating the antioxidant activity of the R. nervosus. Our findings suggested that RE could boost the induced oxidative stress in the caecum of infected birds, but studies are needed to determine the mechanism of RE action.
Keywords
Rumex nervosus
Coccidiosis
Chickens
Oxidative status
Caecum
1 Introduction
Coccidiosis is a dangerous parasitic disease of the intracellular genus Eimeria protozoan parasite (Mehlhorn, 2014). This parasite causes injuries to the intestinal tissue leading to a reduction in feed intake and nutrient absorption, and also makes the host more susceptible to secondary bacterial infections (Morris et al. 2007). Every year, coccidiosis causes a global loss in the poultry industry of over US$ 2.4 billion (Quiroz-Castañeda and Dantán-González, 2015). Eimeria acervulina, E. tenella, E. maxima, and E. praecox are often found in broiler chickens (Hamidinejat et al., 2010).
Intensive chicken farming is based on effective coccidiosis prophylaxis with anticoccidial in-feed drugs. Because of the development of drug resistance, overuse of coccidiostats becomes less successful, resulting in poor weight gain and high feed intake (Jenkins et al., 2010; Wajiha et al., 2018), and impairing economic performance (Shirzad et al., 2011). The prophylactic use of anticoccidium chemicals as feed additives in European countries has been strictly limited since a short time (Muthamilselvan et al., 2015). Researchers are seeking to find natural product with low coast and reduced side effects against coccidiosis (Abbas et al., 2012; Bozkurt et al., 2013; Quiroz-Castañeda and Dantán-González, 2015). Because of their presumed safe, nutritious and therapeutic value, the use of natural antioxidants found in plant and other biological materials has recently attracted considerable interest (Al-Naqeb, 2015; Alhotan and Abudabos, 2019). Here, we determined the antioxidant activity of Rumex nervosus leaf extracts against coccidiosis induced by E. tenella in chickens. R. nervosus belongs to the Polygonaceae family and contains important anti-microbial phytochemicals and a set of vitamins (Al Yahya et al., 2018).
2 Materials and methods
2.1 Plant collection and extraction
R. nervosus leaves were collected from Taif region, Saudi Arabia. Leaves had been authenticated by a specialist in the herbarium at King Saud University. The leaves had been air-dried and milled into powder by the use of an electric blender then extracted in 70% methanol (Amer et al., 2015). Filtration and evaporation of the filtrate had been achieved at 50 °C in a rotary evaporator (Chikoto and Eloff, 2005). The extract was dissolved for treatment of chickens using dimethyl sulfoxide (DMSO) (Al Yahya et al., 2018).
2.2 Infrared spectroscopy
A small quantity of the sample was mixed with an excess potassium bromide powder (1: 99 wt%) and ground to form a uniform consistency, then finely pulverized and pellet-forming die. The instrument used for the study of Infrared (IR) is Thermo Scientific’s optical spectrometer NICOLET 6700 Fourier-transform infrared spectroscopy (FT-IR). Maximum absorption was reported in the number of waves (cm-1). Spectra were registered at 25 °C from 4000 cm to 1 to 400 cm−1 at a resolution of 4 cm−1.
2.3 Birds and infection
A total of 70 broiler chicks (one-day-old) were purchased from a local hatchery, Saudi Arabia. Upon arrival, they were placed immediately in disinfected wire cages with identical size, and were grown under healthy conditions up to 21 day of age. During the experiment period, water and feed were provided ad libitum for all of the chickens' groups.
Unsporulated Oocysts of E. tenella were obtained from the faeces of naturally infected chickens. These oocysts were sporulated using 2.5% potassium dichromate at 25 °C for 72 h (El-Ashram and Suo, 2017). Sporulated oocysts were identified through the morphological features and morphometry as described by McDougald et al. (1997). Furthermore, the sporulated oocysts were passaged from two to three times in healthy chickens to confirm of the site of lesions. The experimental chickens were orally gavaged with 1 mL distilled water containing 10,000 sporulated oocysts (Lee et al., 2012).
2.4 Experimental design
The birds were allocated randomly into seven groups with approximately homogeneous weights. The groups included an uninfected-untreated control, uninfected-treated with RE (200 mg kg−1 bird weight), infected-untreated control with E. tenella (10,000 oocysts). The groups 4, 5, 6 were infected and treated with RE 50, 100, 200 mg kg−1, respectively. The group 7 was infected and medicated with Amprolium (Veterinary and Agricultural Products Company (VAPCO), Jordan) with rate of 25 mg kg−1 (EMEA, 2001). Administration of RE concentrations and Amprolium began in 21 day of age after two hours of challenge and lasted one time daily for 6 days.
2.4.1 Oocysts counting
In order to test efficacy of RE against E. tenella, the feces samples were collected from the infected chickens' groups on day 6 postinfection. The numbers of E. tenella oocysts per gram of feces were counted using McMaster technique as described by Long et al. (1976).
2.5 Body weight and feed intake
The experimental bird performance indicators included increase in body weight (BWG) and consumption of feed (FI). All birds were weighted individually and the BWG was calculated by subtract the initial body weight (at day 0p.i.) from the final body weight (at day 6p.i.). FI of each replicate was calculated by subtracting the weight of the residual feed from the weight of the feed offered at the start of the experiment at the end of the experiment.
2.6 Oxidative status in cecum
Parts of the chickens' cecum were weighed and homogenized in phosphate buffer immediately (10%, w/v) and centrifuged for 15 min at 5000×g, at 4 °C. The supernatant was used for different biochemical estimates. The levels of glutathione (GSH) and malondialdehyde (MDA) were measured by Ellman (1959) and Satoh (1978), respectively. Nitric oxide levels (NO) were measured using the method of Green et al. (1994).
2.7 Statistical analysis
Statistical comparison between the groups studied was performed using a one way variance analysis (ANOVA). Values were presented as means ± SD. The statistical significance was set at p ≤ 0.05.
3 Results
The analysis of R. nervosus extract using FTIR showed major bands at 3410.33 cm−1, 1609.92 cm−1, 1526.88 cm−1, 1445.41 cm−1, 1364.43 cm−1, 1285.28 cm−1, 1210.93 cm−1, 1107.57 cm−1, 1032.89 cm−1, 765.22 cm−1 (Fig. 1, table 1). N—H stretching was indicated by band at 3410.33 Cm−1 confirming the presence of aliphatic primary amine. The band at 1609.92 implied to C⚌C stretching for the presence of α, β-unsaturated ketone. N—O stretching confirming the presence of nitro compound at 1526.88 cm−1. Aromatic ester, alkyl aryl ether and aliphatic ether were present at 1285.28, 1210.93 and 1107.57 cm−1, respectively. However, other compounds were present (Fig. 1, Table 1).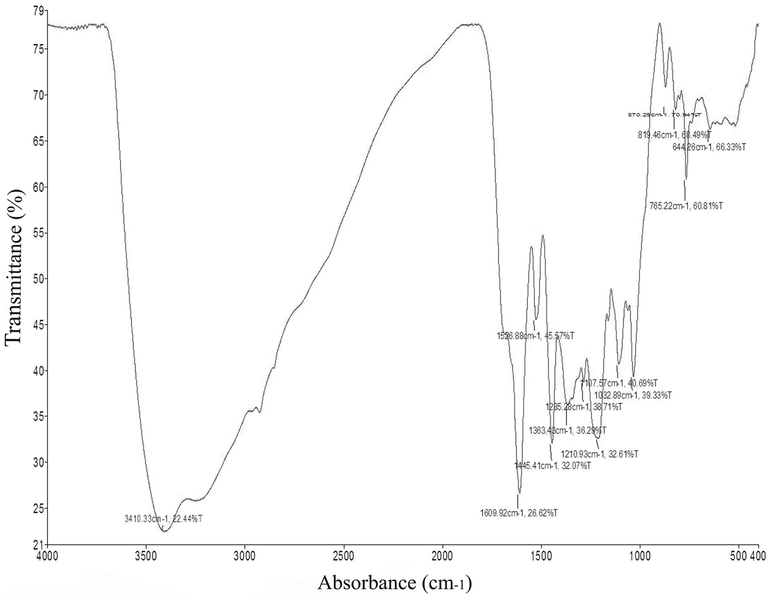
Infrared spectroscopy of Rumex nervosus leaf extracts.
Absorption (cm−1)
Appearance
Transmittance (%)
Group
Compound class
3410.33
medium
22.44
N—H stretching
aliphatic primary amine
1609.92
Strong
26.62
C⚌C stretching
α,β-unsaturated ketone
1526.88
Strong
45.57
N—O stretching
nitro compound
1445.41
medium
32.07
C—H bending
alkane
1364.43
36.29
1285.28
strong
38.71
C—O stretching
aromatic ester
1210.93
Strong
32.61
C—O stretching
alkyl aryl ether
1107.57
Strong
40.69
C—O stretching
aliphatic ether
1032.89
strong
39.33
S⚌O stretching
sulfoxide
765.22
strong
60.81
C—H bending
monosubstituted
The non-infected chickens have significant increase in body weight when compared to the infected bird (Fig. 2). RE-challenged birds showed a significant increase in body weight compared to infected birds, especially at the 200 mg/kg RE dose showing more weight gain than the reference drug, Amprolium (Fig. 2).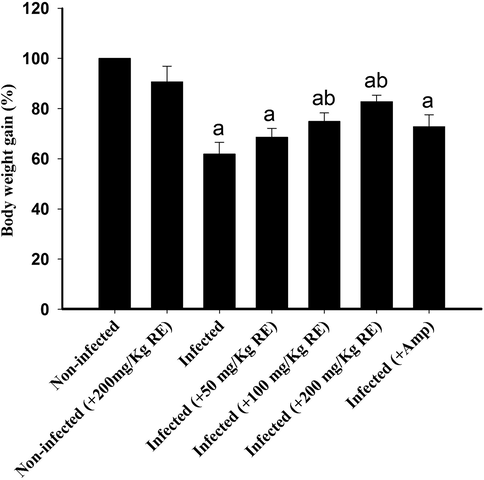
Change in weight of chicken during E. tenella infection and after treatment with RE. Significance at p < 0.01 against control group (a) and against infected group (b).
On day 6 post E. tenella infection, the feed intake was 438.40 ± 15.27 g/bird in the infected group (Fig. 3). This decrease was ameliorated by the treatment of animals with RE that reached 576.00 ± 18.48 g/bird (Fig. 3). Birds treated with Amprolium also significantly appeared with increased feed intake (Fig. 3).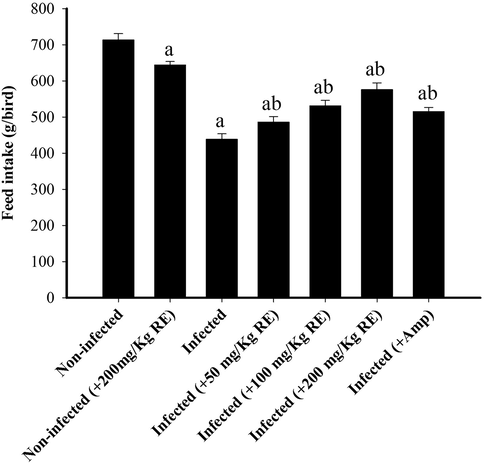
Suppression of E. tenella oocysts in infected and infected-treated birds. Significance at p < 0.01 against control group (a) and against infected group (b).
Examination of chicken faeces at day 6 post-infection revealed high number of oocysts output reaching 32.88 ± 2.46 × 106 (Fig. 4). This number of oocysts significantly decreased (p ≤ 0.01) after the treatment of the infected chickens with 200 mg/kg. RE treated group appeared with better oocyst output inhibition than Amprolium treated group (Fig. 4).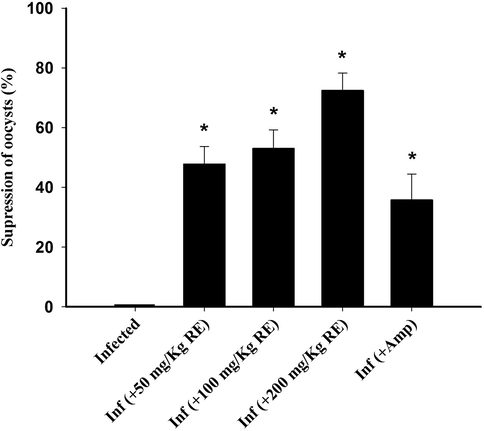
Change in chicken’s feed intake during E. tenella infection and after treatment with RE. *Significance at p < 0.01 against infected group.
Eimeriosis induced by E. tenella disrupted the oxidative status in chicken’s cecum. This was evidenced through the decrease in GSH level in the caecum of infected chickens and the significant increase of MDA and NO (Fig. 5). After treatment of chickens with RE, the oxidative status in chicken's cecum was changed where the level of GSH was 5.26 ± 0.68 mmol/g. However, the level of MDA and NO were reduced to 17.75 ± 2.54 U/g and 4.25 ± 0.55 µmol/L, respectively (Fig. 5). Comparing the results of RE group to that of the Amprolium treated group, it is clear that RE could act as an antioxidant agent with similar effects to Amprolium.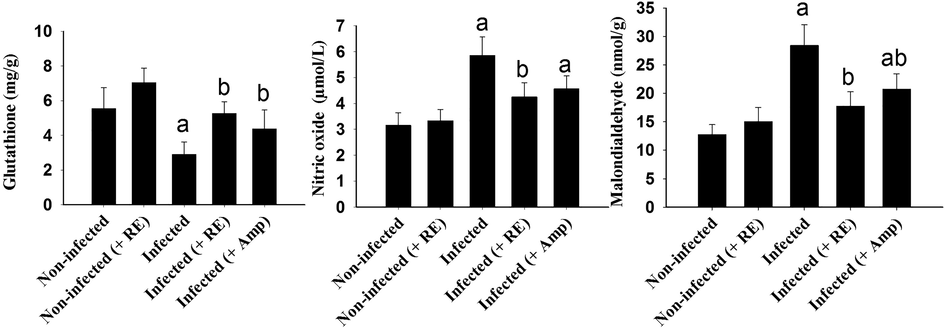
Induced changes in the level of glutathione (GSH), Nitric oxide (NO) and malondialdehyde (MDA) in chicken caecum infected with E. tenella and treated with RE. Significance at p < 0.01 against control group (a) and against infected group (b).
4 Discussion
The development of effective agents against coccidiosis in animals is urgently needed. Today, the poultry industry is under growing pressure to reduce the use of anticoccidium drugs and the search for natural source active compounding that enhances and the immune function of animals. This study aimed to evaluate RE anticoccidial and antioxidant effect against E. tenella infection.
Animal infected with Eimeria show a significant weight loss and feed intake (Mehlhorn, 2014), due to the poor nutrient absorption and decreased immune response resulting in intestinal tissue damage (Chapman, 2014). Our finding demonstrated this weight loss following infection with E. tenella, as well as a decline in fee intake in the infected birds. This increase in weight and feed consumption could be due to the presence of certain essential compounds in the plant extract used. Ott et al. (2018) reported that β.glucans improved the decrease in weight and feed intake induced by E. tenella.
The caecum is the site of E. tenella infection where the parasite invades the caecum epithelium causing severe injuries (Mehlhorn, 2014). Different developmental stages were developed in caecum epithelium and finally oocysts were developed and go out with faeces.
In many diseases, including parasitic diseases, oxidative stress arises when the equilibrium between the process of producing free radicals and their removal process is disrupted, which is normally balanced under natural conditions, when the production of free radicals by antioxidant compounds is greater than the process of removal (Georgieva et al., 2006). Consequently, the oxidative stress occurs, the intestinal epithelial barrier and tight junctions are destroyed throughout lipid peroxidation (increased malondialdehyde) and antioxidants insult, as a result, reduced feed intake, a poor absorption of nutrients and the body weight gain decreased of the infected birds (Naidoo et al., 2008). During coccidiosis nitric oxide and other reactive oxygen species (ROS) are produced as an immunological response to Eimeria sporozoites that invade the intestinal epithelium causing inflammation. In spite, the nitric oxide and other ROSs are considered toxic cellular products for Eimerian sporozoites, but also have negative side effects on the host if not protected by an antioxidant system (Halliwell and Gutteridge, 1989).
Using antioxidants from natural sources could be beneficial for the oxidative status of the cell in the caecum inducing oxidant/antioxidant balance and lead to improvement in the oxidative damage induced by E. tenella infection (Wang et al., 2018). In our study, RE could significantly reduce the severity of the infection in caecum by ameliorating the level of lipid peroxidation by neutralizing reactive oxygen species (ROS). Like diclazoril, a common anticoccidial drug, RE was able to limit the degree of lipid peroxidation (Eraslan et al., 2004). The parasite induces a change in the caecum oxidative status and cause inflammatory cellular effects (Pourali et al., 2014). Similar results have been obtained in rodents infected with Eimeria papillata due to the increased MDA and NO levels. This may be caused by serious inflammatory response due to oxidative damage caused by eimeriosis (Al-Quraishy et al. 2019).
Our results collectively show that RE is effective agent that improved the change in weight and feed intake as well as it reduced the number of E. tenella oocysts expelled in faeces. In addition, RE may control the oxidative status of the infected birds in the caecum but studies are needed to know the mechanism of RE action on both the host and the parasite.
Acknowledgement
This study was supported by Research Supporting Project (RSP-2019/23), Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Botanicals: an alternative approach for the control of avian coccidiosis. World’s Poult. Sci. J.. 2012;68(2):203-215.
- [Google Scholar]
- Phytochemicals and antimicrobial activities of Rumex nervosus natural populations grown in Sarawat Mountains, Kingdom of Saudi Arabia. Arab. J. Sci. Eng.. 2018;43:3465-3476.
- [Google Scholar]
- Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ. Sci. Pollut. Res. Int.. 2019;26(14):14194-14199.
- [Google Scholar]
- Antioxidant and antibacterial activities of some yemeni medicinal plants. Int. J. Herb. Med.. 2015;3(3):06-11.
- [Google Scholar]
- Salvadora persica protects mouse intestine from eimeriosis. Rev. Bras. Parasitol. Vet.. 2019;28(4):605-612.
- [Google Scholar]
- Antioxidant and anti-inflammatory activities of pomegranate (Punica granatum) on Eimeria papillata-induced infection in mice. BioMed. Biomed. Res. Int.. 2015;2015:219670
- [Google Scholar]
- An update on approaches to controlling coccidia in poultry using botanical extracts. Br. Poult. Sci.. 2013;54(6):713-727.
- [Google Scholar]
- Milestones in avian coccidiosis research: a review. Poult. Sci.. 2014;93(3):501-511.
- [Google Scholar]
- Chikoto, H., Eloff, J.N., 2005. Antioxidant. Patent. NR. 2005/09681.
- Electrical cream separator coupled with vacuum filtration for the purification of eimerian oocysts and trichostrongylid eggs. Sci. Rep.. 2017;7:43346.
- [Google Scholar]
- EMEA, 2001. Committee for Veterinary Medicinal Products (Amprolium). The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Inspections. /MRL/767/00-FINAL.
- Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J.. 2006;172(3):488-492.
- [Google Scholar]
- Nitric oxide: cytokine-regulation of nitric oxide in host resistance to intracellular pathogens. Immunol. Lett.. 1994;43(1–2):87-94.
- [Google Scholar]
- Lipid peroxidation: a radical chain reaction. In: Free Radical’s Biology and Medicine (second ed.). New York: Oxford University Press; 1989. p. :188-218.
- [Google Scholar]
- Characterization of Eimeria species in commercial broilers by PCR based on ITS1 regions of rDNA. Iranian J. Parasitol.. 2010;5:48-54.
- [Google Scholar]
- Comparison of Eimeria species distribution and salinomycin resistance in commercial broiler operations utilizing different coccidiosis control strategies. Avian Dis.. 2010;54:1002-1006.
- [Google Scholar]
- Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab. Anim. Res.. 2012;28(3):193-197.
- [Google Scholar]
- A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet. Lat.. 1976;6(3):201-217.
- [Google Scholar]
- A survey of coccidia on 43 poultry farms in Argentina. Avian Dis.. 1997;41(4):923-929.
- [Google Scholar]
- Encyclopedic Reference of Parasitology (4th ed.). Berlin: Springer; 2014.
- Investigating a persistent coccidiosis problem on a commercial broiler–breeder farm utilizing PCR-coupled capillary electrophoresis. Parasitol. Res.. 2007;101(3):583-589.
- [Google Scholar]
- Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. Evid. Based Complement. Alternat. Med.. 2015;2016:2657981.
- [Google Scholar]
- The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol.. 2008;153(3–4):214-219.
- [Google Scholar]
- The impact of β-glucans on performance and response of broiler chickens during a coccidiosis challenge. Poult. Sci.. 2018;97(8):2713-2721.
- [Google Scholar]
- Antioxidant and anticoccidial effects of garlic powder and sulfur amino acids on Eimeria-infected and uninfected broiler chickens. Iranian J. Vet. Res.. 2014;15(3):227-232.
- [Google Scholar]
- Control of avian coccidiosis: future and present natural alternatives. Biomed. Res. Int.. 2015;2015:430610
- [Google Scholar]
- Serum Lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica. Chimica. Acta.. 1978;90:37-43.
- [Google Scholar]
- Prevalence and risk factors for subclinical coccidiosis in broiler chicken farms in Mazandaran province. Iran. Trop. Anim. Health. Prod.. 2011;43:1601-1604.
- [Google Scholar]
- Comparative analysis of egg adapted vaccines and salinomycin against coccidiosis in chicks. Microb. Pathog.. 2018;123:454-460.
- [Google Scholar]
- Anticoccidial effects of areca nut (Areca catechu L.) extract on broiler chicks experimentally infected with Eimeria tenella. Exp. Parasitol.. 2018;184:16-21.
- [Google Scholar]







