Translate this page into:
Rumex hastatus derived silver nanoparticles development and their potential applications as hepatic-protection agent along with antimicrobial activity
⁎Corresponding authors. doctor_shahgee@yahoo.com (Muhammad Awais), mikhan@math.qau.edu.pk (M. Ijaz Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Current study aimed to develop eco-friendly and cost-effective approaches for silver nanoparticle (AgNPs) synthesis using leaf extract of Rumex hastatus and characterization using various analytical techniques. Besides, the crystallinity of Ag+, reduction/stability, functional groups and structural morphology were determined through X-ray diffraction (XRD), Fourier transforms infrared spectroscopy (FT-IR spectroscopy) and scanning electron microscopy (SEM) respectively. The biosynthesized RH-AgNPs were investigated for hepatoprotective and cytotoxic effect, antioxidative activities and antimicrobial potential. Results revealed that RH-AgNPs were effective against hepatotoxin (carbon tetrachloride) at all tested concentrations by exhibiting very valuable results; showed high antioxidant potential and cytotoxic effect against HepG2 cancer cell lines. RH-AgNPs showed very promising antibacterial results as well. Overall, these results highlighted the potential of RH-AgNPs to be used for the treatment of various diseases.
Keywords
Herbal plants
Rumex hastatus
Silver nanoparticles
Hepatoprotective activity
Cytotoxic activity
Antioxidant activity
Antibacterial activity
1 Introduction
Nanoparticles are atomic or molecular mass with dimensions ranging from 1 to 100 nm, which can substantially amend their physical and chemical properties associated with the bulk material (Chen et al., 2013). Nanoparticles (NPs) can be synthesized using chemical, physical, and green synthesis. Among them, green synthesis is considered as more preferable due to its cost-effective and environment-friendly nature (Pugazhendhi et al., 2018). Plants have been used to determine the oxidizing and reducing properties of green material responsible for metal ion reduction to synthesize specific nanoparticles. (Annu et al., 2018). Numerous studies have been recorded to use Ag-NPs as an antibacterial agent (Siddiqui et al., 2018). Moreover, AgNPs enhance antioxidant activities as well as anti-cancerous activities against various cell lines, e.g., MCF (human breast adenocarcinoma), A549 (Human lung carcinoma cells) and HepG2 (human liver cancer cells) (Zangeneh, 2020).
The primary toxicological problems accompanying multiple diseases have focused liver for their effects. The liver has played a pivotal role in the detoxification of hepatotoxic compounds that severely affects liver cells and are the reason for hepatic injury and oxidative damage. Therapeutic effects of plant medicines against synthetic drug toxicity have got chief importance recently, and natural treatments have been extensively used against liver diseases (Mahmoodzadeh et al., 2017).
Medicinal properties of plants played a significant role in the existence of primordial societies for their reliability on local plants for curing different ailments. Increased resistance against antibiotics persuaded scientific community to evaluate novel plants for the development of herbal drugs with more efficacies and a lesser amount of side effects (Zangeneh et al., 2019). Therefore, in this study, the Rumex hastatus plant was used that belongs to the family Polygonaceae. It is a herbal plant; and is widely distributed in Pakistan, Afghanistan, and China. This plant possesses several biologically active compounds, especially antioxidants, having many therapeutic properties like wound healing, jaundice, arthritis, diarrhea dysentery, antitumor and cytotoxic activities. Despite the documented potential of the plant for curing tumors and cancers, the detailed studies reporting its therapeutic capabilities of are still scarce. The plant has been used as antioxidants to treat liver injuries (Sahreen et al., 2015). However, there is no such study reporting the hepatoprotective effect of the leaves of this plant against toxicity in Mice. So, taking it as an initiative, this work is intended for demonstrating the hepatoprotective activity of RH AgNO3 nanoparticles against CCl4 induced hepatotoxicity in male Albino Mice.
2 Materials and methods
2.1 Identification and Sample preparation
This medicinal plant, Rumex hastatus, was taken from Kotli Sattian, located in Rawalpindi District and was identified with the services of plant taxonomists Prof. Dr. Mushtaq Ahmad and Dr. M. Zafar, Department of Plant Sciences, Quaid I Azam University, Islamabad. A voucher specimen (RHH-84) was submitted at Herbarium of Pakistan (ISL) for future records. Sample powder 100 g was extracted by with 100 mL of each solvent (Methanol and Water) separately (Gul et al., 2017).
For the synthesis of silver oxide nanoparticles, a solution of 20 mM (40 mL) AgNPs was added in a 200 mL beaker with aqueous plant extract (40 mL) and subjected to heating by ultrasonic bath for 2 h with continuous stirring. Then centrifuged at 5000 rpm for 20 min. The greyish-colored pellet was then subjected to hot air drying for 6 h in the oven (65 °C) and calcinated in Muffle-furnace (500 °C) for 2 h.
The characterization of nanoparticles was done using XRD (X-ray diffraction, GNR Analytical instruments Groups) FTIR and SEM (Scanning electron microscope, TESCAN, MIRA3) with EDX detector (Iram et al., 2020).
2.2 Cytotoxicity testing
Human Liver cancer cell lines were used to measure the efficacy of RH-AgNO3, RHM and RHH in culture medium by using SRB assay as protocol used by Gul et al. (2020).
2.3 Antioxidant activity
The free radical scavenging capacity of AgNO3, RHM and RHH was investigated using the DPPH and Hydroxyl radical scavenging assay to measure antioxidant activity. (Braca et al., 2001). Hydroxyl radical assay was carried out by the deoxyribose method (Nagai et al., 2005). ABTS: The ABTS (2,2-azinobis[3-ethylbenzothiazoline-6-sulfonate]) free radical scavenging activity of RHM, RHH and AgNO3 NPs was evaluated by method used by Zhu et al. (2011). The IC50 value is the actual concentration at which free radicals were quenched by 50%, and its value is attained by linear regression analysis.
2.4 Antimicrobial activity
The antimicrobial testing of AgNO3 and plant samples were estimated by agar well diffusion method with the inoculums comprising 108 bacterial cells/mL. Samples were subjected to analysis for minimum inhibitory concentration by broth dilution method (Rahman et al., 2018).
2.5 Animal management
Forty-five male Swiss Albino mice weighing 30–35 g were used in the current study and were brought from the National Institute of Health Sciences (NIH), Islamabad, Pakistan. Experimental animals were kept at the temperature of 23 ± 2 °C and humidity of 50–60% with 12-hour light and 12-hour dark cycle and allowed to freely access the pellet diet and water ad libitum.
2.6 Experimental strategy
Animals were divided into nine groups, and each category comprises five mice.
Group-I: pellet diet and water with no CCl4 extract or RH-AgNPstreatment.
Group-II; Control: CCL4 with 1:1 olive oil, 0.7 mL/Kg body weight [B.W].
Group-III: CCl4 + 100 mg/Kg B.W methanol extract.
Group IV: CCl4 + 200 mg/Kg B.W doses of methanol extracts
Group V: CCl4 + 100 mg/kg B.W. of aqueous extract.
Group VI: CCl4 + 200 mg/kg B.W) aqueous extracts
Group VII: CCl4 + 100 mg/Kg B.W of RH- AgNPs.
Group VIII: CCl4 + 200 mg/kg B.W. of RH- AgNPs.
Group IX: CC l4 + 10 mg/Kg B.W of Silymarin.
Blood was obtained by cardiac puncture, and after that, serum was used for further analysis of liver function tests. Then, the liver was separated and sustained in a 10% solution of formalin for histopathological examination.
2.7 Biochemical parameters
Liver tests were estimated using markers (ALT, ALP and AST) and bilirubin by kit method (AMS diagnostic kit, Italy). Catalase (CAT), Superoxide dismutase (SOD) and Glutathione peroxidase (GPx) were measure by Aebi (1984), Misra and Fridovich (1972) and Paglia and Valentine (1967). Glutathione reductase (GSSH) was measured as the quantity of NADPH (Nicotinamide adenine dinucleotide phosphate hydrogen) being consumed GSSH has been expressed as moles of NADPH oxidized/min/mg at a temperature of 30 °C.
2.8 Histopathological examination
The processed organ was preserved in 10% formalin solution, and 5 µm thickness tissue in paraffin slices was made and stained with H & E (Hematoxylin and Eosin) to observe histopathological changes under the microscope at 40x.
2.9 Statistical analysis
All the readings are expressed as mean values for replicates (3) ± SD (standard deviation). Results were analysed by using one-way ANOVA at the significant difference of p < 0.05. The 50% inhibition concentration (IC50) was calculated in analysis by linear regression 3.
3 Results
3.1 Characterization of RH-AgNO3
The change observed in the color of the reaction mixture during the process indicates the synthesis of nanoparticles. X-ray diffraction (XRD) provides spectra to comprehend the crystalline structure of NPs. The diffraction configuration of RH-AgNPs is represented in (Fig. 1) and displayed four fine peaks at 2θ values of 38.15, 44.25, 64.4 and 77.5 corresponding to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) plane. The average size of RH-AgNPs crystallite was 32.18 nm obtained from all projecting peaks (20.34–54.67 nm).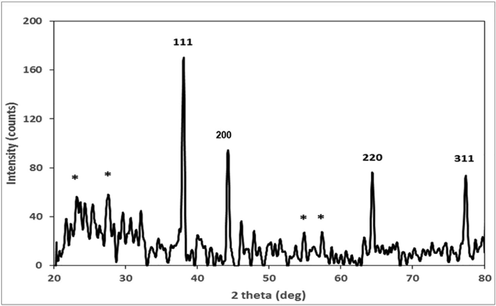
XRD analysis of RH synthesized AgNPs.
FT-IR analysis of RH-AgNPs showed several bands that specify functional groups of biological molecules being adsorbed on NPs (Fig. 2). FT-IR spectra illustrated peaks between 500 and 4000 cm−1 and absorption peaks were observed corresponds to OH, N—H, SH, C⚌C, C⚌O, N—H bending, COOH and C—O—C. SEM analysis confirmed the structure and morphology of RH-AgNPs (Fig. 3a and b). RH-NPs were agglomerated, relatively spherical shaped, and image magnification ranged from 1 µm to 500 nm with a size of 77 nm. EDX images confirmed RH-AgNPs existence with the depiction of strong peaks. EDX analysis revealed the presence of silver (58.66%) and also some other elements, i.e., O, C, Cl, Fe, K, P and Ca (Fig. 3c and d).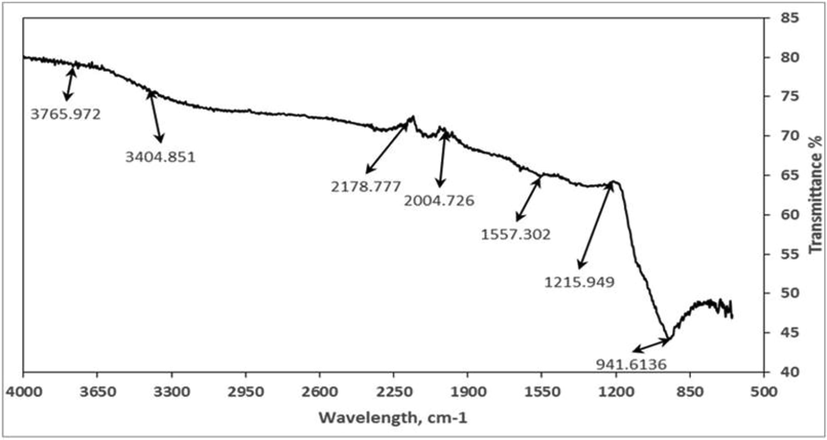
FTIR analysis of RH synthesized AgNPs.
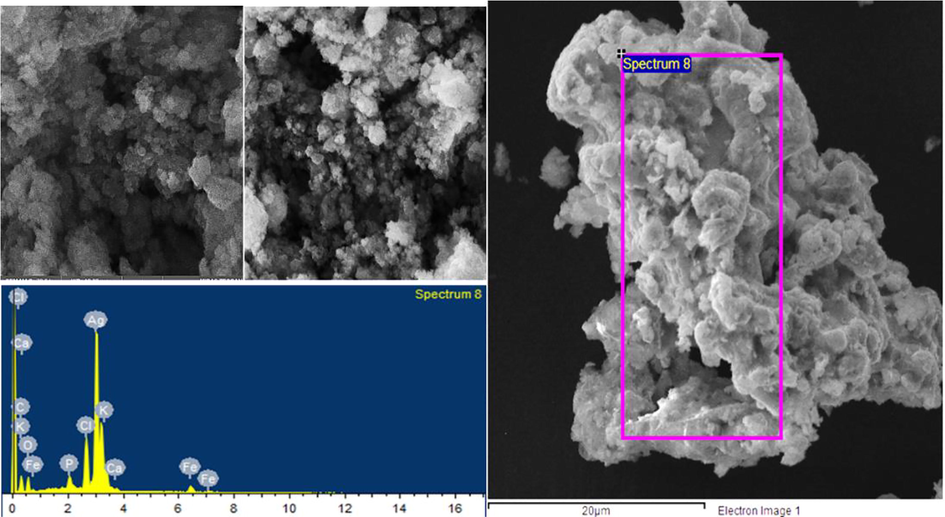
SEM (A/B) and EDX (C/D) analysis of RH-AgNPs.
3.2 Antioxidant, antimicrobial and cytotoxic assay
Further investigation of RH extracts and AgNPs was done for antioxidant potential performed with numerous radical scavenging assays, i.e., the DPPH, ABTS and hydroxyl radical scavenging assays. RH-AgNPs showed the highest IC50 in DPPH assay was 9 µg/mL and the RH methanol extract exhibited slightly more potential than aqueous extract, as given in Fig. 4.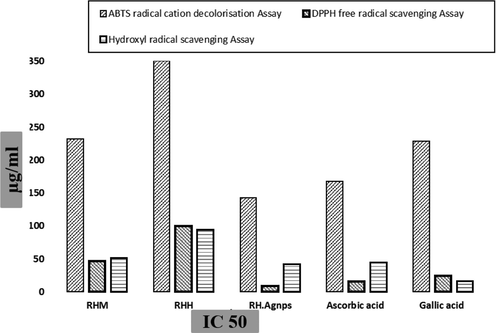
Antioxidant assay (IC 50) of RH extracts and RH-NPs with standard drugs.
RH-AgNPs showed significant inhibition zones as given in (Table 1) (Fig. 6). Subsequently, RH-AgNPs MIC value against pathogenic bacteria ranged from 0.2 to 3 mg/mL given in Table 2. Considerable variation indicates HepG2 viable alive tumor cells (black cells) when treated with different extracts whereas treated with RH-AgNPs resulted in less viable tumor cells and the high toxicity (87.89%) (Fig. 5). Zones of Inhibition (avg ± SD) mm of various bacterial strains in response to different concentrations of RH-AgNPs and RHM and RHH extract along with positive control (n = 3).
Strains
AgNPs 20 µg/mL
AgNPs 40 µg/mL
AgNPs 60 µg/mL
AgNPs 80 µg/mL
AgNPs 100 µg/Ml
RHM extract 200 µg/mL
RHH extract 200 µg/mL
Positive control20 µg/mL
Staphylococcus aureus
8 ± 1.5
8 ± 0.7
10 ± 0.2
11 ± 0.5
19 ± 0.2
Nil
Nil
25 ± 0.8
Escherichia coli
8 ± 0.2
8 ± 0.2
10 ± 0.6
11 ± 0.1
12 ± 0.6
Nil
5 ± 0.3
22 ± 0.7
Salmonella gallinarum
7 ± 0.5
10 ± 0.4
13 ± 0.2
13 ± 0.9
15 ± 0.8
Nil
Nil
11 ± 0.4
Klebsiella pneumonia
8 ± 0.4
10 ± 0.5
10 ± 0.3
11 ± 0.3
15 ± 0.1
Nil
Nil
19 ± 0.8
Enterobacter aerogenes
9 ± 0.7
12 ± 0.2
12 ± 0.5
13 ± 0.1
16 ± 1.2
Nil
Nil
17 ± 0.6
Pseudomonas aeruginosa
6 ± 0.3
9 ± 0.1
10 ± 0.9
14 ± 0.5
17 ± 0.2
7 ± 0.6
Nil
24 ± 0.5
Micrococcus lotus
6 ± 0.5
7 ± 0.3
9 ± 0.8
9 ± 0.2
16 ± 0.7
Nil
Nil
21 ± 0.3
Bacillus brevis
9 ± 0.5
11 ± 0.2
12 ± 0.8
15 ± 0.1
18 ± 0.3
Nil
4 ± 0.5
19 ± 1.7
Strains
MIC (mg/mL)
Staphylococcus aureus
0.2
Escherichia coli
0.4
Salmonella gallinarum
1
Klebsiella pneumonia
0.6
Enterobacter aerogenes
2
Pseudomonas aeruginosa
0.8
Micrococcus lotus
3
Bacillus brevis
0.9
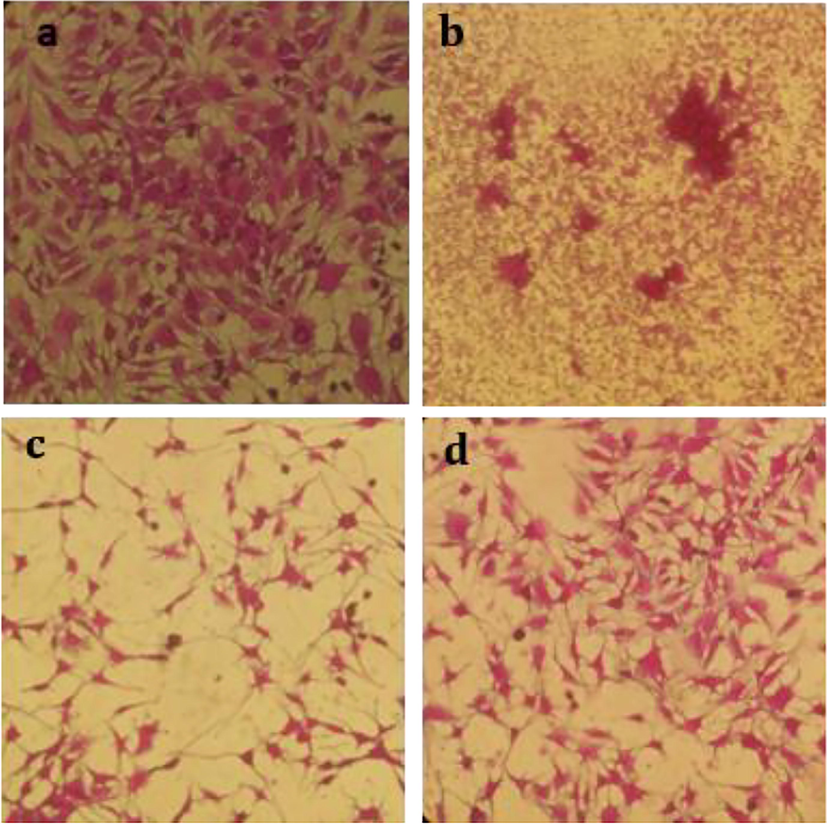
Cytotoxicity analysis (a) Control (b) RH-AgNPs (c) RHM (d) RHH.
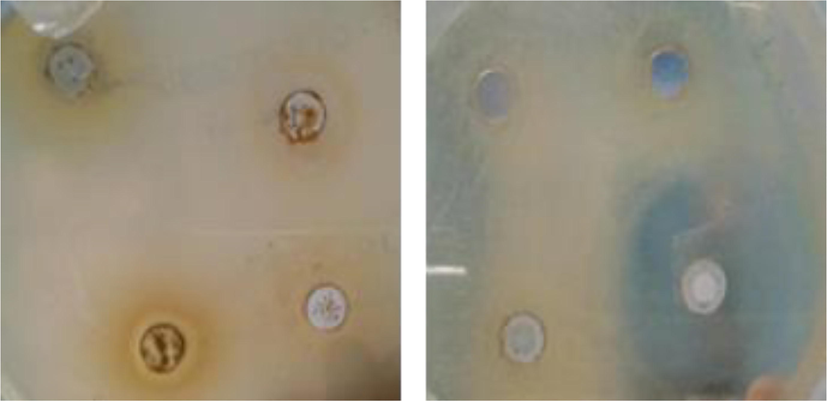
(a) RH Methanol extracts showed zone of inhibition against Pseudomonas aeruginosa (b) RH-AgNPs displayed inhibition zones against Staphylococcus aureus.
3.3 Hepatoprotective effect
CCL4 induced abnormal changes in the weight. The weight of animals has improved mainly with RH-AgNPs, Silymarin drug, and RHM200 (Fig. 7A). Liver toxicity in animals was detected by the rise of biochemical markers ALT (145 ± 9), AST (127.8 ± 8.6), and ALP (321 ± 15) in group II after compared with group I ALT. Level of serum markers in group VII and VIII were reduced to normal. According to the results revealed in Fig. 6D. action of antioxidant enzymes CAT (7.96 ± 2.3 mmol/min/mg protein), SOD (10.1 ± 1.8 U SOD/mg protein), GPX (31 ± 0.2 µmol/min/mg protein) and GSSH (41.07 ± 0.43 nmol/min/mg protein) in group VII were higher as compared to group III to VI but lower than the effect of group VIII. Hepatic toxicity highly damages the normal function of antioxidant enzymes, however, biosynthesized NPs are found to be defensive against reactive oxygen species (Fig. 7D). The histopathological section indicated normal liver architecture among group I and showed abnormal hepatic cells in animals (group II) with a fragmented central vein. Fig. 8 showed attenuated cellular organization.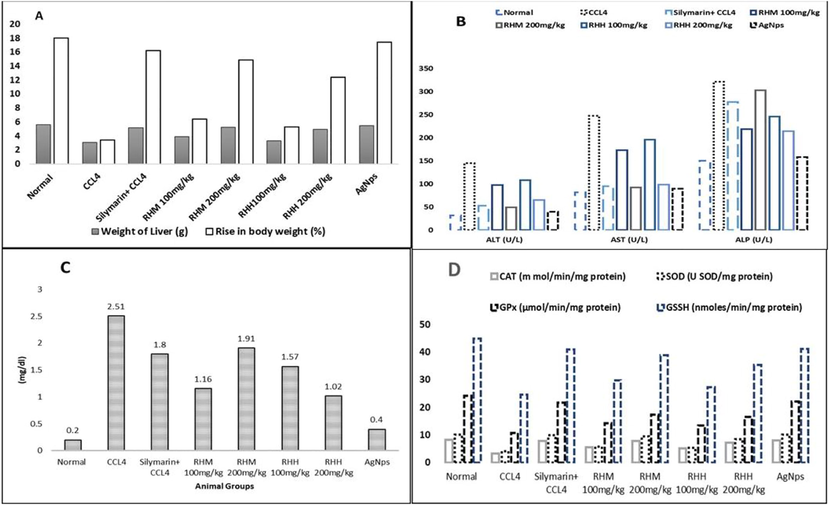
Hepato-protective assay (A) Effect of doses on the weight of different groups, (B) Serummarkers analysis, (C) Bilirubin estimation and (D) ROS (Reactive oxygen species) assays.
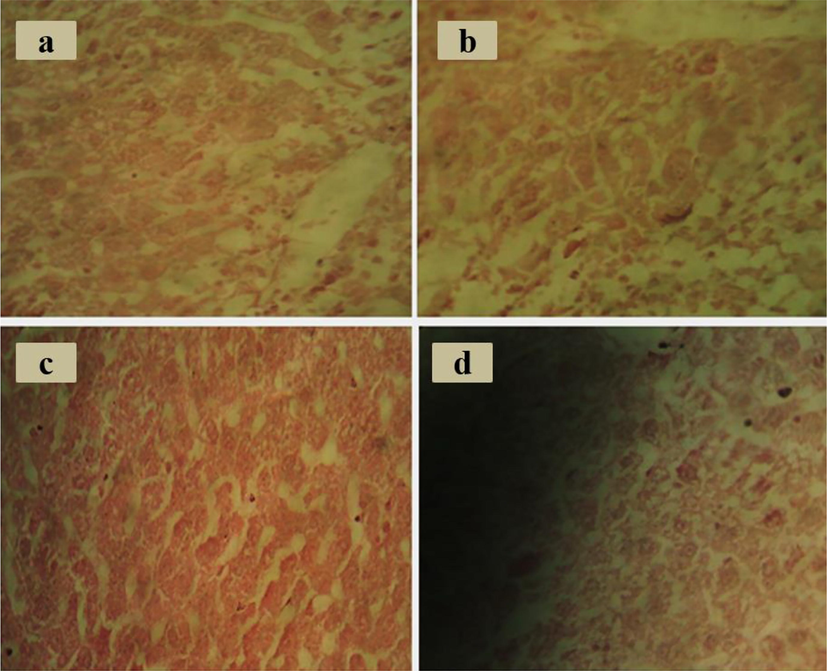
Histopathological studies (A) CCl4 group (B) RHM (C) RH-AgNPs (D) Silymarin.
4 Discussion
The green synthesis of nanoparticles has grabbed consideration due to its suitable approaches, i.e., cost-effectiveness, environment-friendly, and application in the biomedical field (Rautela et al., 2019). The plant extract-based synthesis of silver NPs is widely used for liver protection, cancer treatment and antimicrobial purposes (Zhang et al., 2019). R. hastatus leaves been known due to their medicinal value against different ailments (Ali and Qaiser, 2009) and effectively synthesized into RH-AgNPs and further undergoes characterization for topographies (Morphological and structural) with FT-IR, XRD, SEM, and EDX. Broad-bands in FTIR spectra indicate the biomolecules absorption on AgNPs and these molecules are responsible for reducing silver ions to AgNPs (Zomorodian et al., 2016).
Stretching vibrations corresponds to the functional group of alcohol, esters, ethers and carboxylic and are stated to have a pivotal capping and stabilization role in reducing Ag+to Ag0 for the synthesis of NPs (Pirtarighat et al., 2019). RH synthesized AgNPs showed high-intensity XRD peaks at 38.15°, 44.25°, 64.4° and 77.45° correspond respectively to (158.69), (91.62), (76.13) and (71.6) Bragg reflection and these peaks showed exact positions and characteristics as given for metallic face center cubic (fcc) structure of Ag (Mousavi et al., 2018: Jain and Mehata, 2017). The SEM-EDX analysis investigates the morphology of nanoparticles and images presented approximately spherical shape AgNPs (µm) with agglomeration caused by solvent vanishing. EDX results illustrated clear silver peaks, displayed element configuration, i.e., a greater percentage of Ag (58.66 %) and few other elements. Other detected peaks in EDX analysis could be biomolecules originated elements that acted as capping agents due to bounding with the peripheral surface of AgNPs (Parveen et al., 2016).
The antimicrobial activity of R. hastatus extracts and RH-AgNPs has been analyzed against pathogenic bacteria. This study’s observed antibacterial activity can be attributed to the combined effect (AgNPs and Ag2O or AgO) (Mohammadlou et al., 2017; Siddiqi et al., 2018; Das et al., 2019).
The cytotoxicity of RH-AgNPs and RH extracts was validated against the HepG2cancer cell line. This cytotoxic effect may be triggered by cellular damage, instigation of immunological reactions and attraction of treated tumor cells electrostatically with RH-AgNPs (Zangeneh, 2019).
The liver is found to be a primary site for the accumulation of silver nanoparticles. This is evident from a recent study that AgNPs have been used as a coating material to dress burns. This is attributed to oxidative stress or increased ROS (reactive oxygen species) effect (Faedmaleki et al., 2014). RH-NPs and extracts showed good inhibition against free radicals. The occurrence of functional groups, i.e., a phenolic group on the AgNPs surface, manifests the reducing property and antioxidant potential of AgNPs (Molyneux, 2004: Govindappa et al., 2018).
CCl4 intensified serum transaminases, declined antioxidant enzymes and, caused lipid peroxidation and results in damage of functional integrity in hepatic mitochondria, similar to earlier studies (Akhtar et al., 2013: Lim et al., 2016). In our research, adversarial changes were observed in serum enzymes (ALT, AST and ALP/SOD, CAT, GSH and GPx) contribute to hepatic injury. RH-AgNPs treatment (Group VII) exhibited decreased serum transaminases, closely related to normal levels that specify rejuvenation of hepatocytes and curative effect on hepatic parenchyma (Chatterjee et al., 2012; Domitrović and Potočnjak, 2016). The biochemical and histopathological studies signify the effectiveness of AgNPs in protection against toxicity of CCL4. Lipid peroxidation and cellular damage of liver results in halting antioxidant system (He et al., 2019). Therefore, the antioxidant effect of RH-AgNPs might show an active role for the restoration of parameters, i.e., biochemical/histopathological to near standard in the treated animals (Singh et al., 2016; Keshari and Bhat, 2019), and this makes silver nanoparticles a valued aspirant for curative purposes, especially for hepatoprotective effect.
5 Conclusions
Results revealed that RH-AgNPs were effective against hepatotoxin (carbon tetrachloride) even at low concentration and showed great antioxidant potential and higher cytotoxic effect against HepG2 cancer cell line. The promising antibacterial activities showed by RH-AgNPSencourages their potential prospects as antimicrobial agents. Overall, results highlighted the effective applications of RH-AgNPs for the treatment of various diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel approaches to preventing ischemia-reperfusion injury during liver transplantation. In: Transplantation Proceedings. Elsevier; 2013. p. :2083-2092. No. 6
- [Google Scholar]
- The ethnobotany of Chitral valley, Pakistan with particular reference to medicinal plants. Pak. J. Bot.. 2009;41(4):2009-2041.
- [Google Scholar]
- Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punicagranatum mediated silver nanoparticles. Toxicology. 2018;7(5):923-930.
- [Google Scholar]
- Antioxidant principles from bauhinia tarapotensis. Journal of natural products. 2001;64(7):892-895.
- [Google Scholar]
- In vivo hepatoprotective activity of ethanolic extract of Russulaalbonigra against carbon tetrachloride-induced hepatotoxicity in mice. Res. J. Pharm. Technol.. 2012;5(8):1034-1038.
- [Google Scholar]
- In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater.. 2013;25(23):3144-3176.
- [Google Scholar]
- Green synthesis and characterization of silver nanoparticles using belladonna mother tincture and its efficacy as a potential antibacterial and anti-inflammatory agent. Mater. Chem. Phys.. 2019;228:310-317.
- [Google Scholar]
- A comprehensive overview of hepatoprotective natural compounds: mechanism of action and clinical perspectives. Arch. Toxicol.. 2016;90(1):39-79.
- [Google Scholar]
- Toxicity effect of silver nanoparticles on mice liver primary cell culture and HepG2 cell line. Iran. J. Pharm. Res.: IJPR. 2014;13(1):235.
- [Google Scholar]
- Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllumtomentosum leaves extract. Results Phys.. 2018;9:400-408.
- [Google Scholar]
- The in vitro and in vivo biological activities of the leaf of cape myrtle, Myrsineafricana L. Phytotherapy Res.. 2017;31(9):1305-1309.
- [Google Scholar]
- Chemical profiling, in vitro and in vivo bioactivities of leaf extracts of Vitexneugundo. Pak. J. Pharm. Sci.. 2020;33(4)
- [Google Scholar]
- Functional teas from the stems of PenthorumchinensePursh.: phenolic constituents, antioxidant and hepatoprotective activity. Plant Foods Hum. Nutr.. 2019;74(1):83-90.
- [Google Scholar]
- Nanostructured lead sulphide depositions by AACVD technique using bis (Isobutyldithiophosphinato) lead (II) complex as single source precursor and its impedance study. Nanomaterials. 2020;10(8):1438.
- [Google Scholar]
- Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep.. 2017;7(1):1-13.
- [Google Scholar]
- Evaluation of Hepatoprotective potential of Rhododendron arboreum Sm. Stem Bark as AbhavaPratinidhiDravya (Substitute) of Rohitaka (Tecomellaundulata (Sm.) Seem.) against paracetamol induced hepatotoxicity in experimental rats. Pharmacogn. J.. 2019;11(5)
- [Google Scholar]
- Amomumcardamomum L. ethyl acetate fraction protects against carbon tetrachloride-induced liver injury via an antioxidant mechanism in rats. BMC Complementary Altern. Med.. 2016;16(1):1-10.
- [Google Scholar]
- Hepatoprotective effect of methanolicTanacetumparthenium extract on CCl4-induced liver damage in rats. Toxicology. 2017;4:455-462.
- [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem.. 1972;247(10):3170-3175.
- [Google Scholar]
- Hydrothermal green synthesis of silver nanoparticles using Pelargonium/Geranium leaf extract and evaluation of their antifungal activity. Green Process. Synth.. 2017;6(1):31-42.
- [Google Scholar]
- The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity.Songklanakarin. J. Sci. Technol.. 2004;26(2):211-219.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS) Artif. Cells, Nanomed., Biotechnol.. 2018;46(sup1):499-510.
- [Google Scholar]
- Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem.. 2005;91(3):389-394.
- [Google Scholar]
- Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med.. 1967;70(1):158-169.
- [Google Scholar]
- Microwave-assisted green synthesis of silver nanoparticles from Fraxinus excelsior leaf extract and its antioxidant assay. Appl. Nanosci.. 2016;6(2):267-276.
- [Google Scholar]
- Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem.. 2019;9(1):1-9.
- [Google Scholar]
- Synthesis of highly stable silver nanoparticles through a novel green method using Mirabillisjalapa for antibacterial, nonlinear optical applications. Opt. Mater.. 2018;79:457-463.
- [Google Scholar]
- Structural, optical, magnetic and antibacterial properties of Nd doped NiO nanoparticles prepared by co-precipitation method. J. Alloys Compd.. 2018;742:421-429.
- [Google Scholar]
- Green synthesis of silver nanoparticles from Tectonagrandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol.. 2019;10(1):1-10.
- [Google Scholar]
- Evaluation of phytochemical content, antimicrobial, cytotoxic and antitumor activities of extract from Rumex hastatus D. Don roots. BMC Complementary Altern. Med.. 2015;15(1):1-6.
- [Google Scholar]
- Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett.. 2018;13(1):1-13.
- [Google Scholar]
- Green synthesis of silver nanoparticles and study of their antimicrobial properties. J. Polym. Environ.. 2018;26(2):423-433.
- [Google Scholar]
- Drug-induced liver toxicity and prevention by herbal antioxidants: an overview. Front. Physiol.. 2016;6:363.
- [Google Scholar]
- Green synthesis and chemical characterization of silver nanoparticles from aqueous extract of Falcaria vulgaris leaves and assessment of their cytotoxicity and antioxidant, antibacterial, antifungal and cutaneous wound healing properties. Appl. Organomet. Chem.. 2019;33(9):e4963
- [Google Scholar]
- Green synthesis and formulation a modern chemotherapeutic drug of Spinaciaoleracea L. leaf aqueous extract conjugated silver nanoparticles; Chemical characterization and analysis of their cytotoxicity, antioxidant, and anti-acute myeloid leukemia properties in comparison to doxorubicin in a leukemic mouse model. Appl. Organomet. Chem.. 2020;34(1)
- [Google Scholar]
- Green synthesis and chemical characterization of silver nanoparticles obtained using Allium saralicum aqueous extract and survey of in vitro antioxidant, cytotoxic, antibacterial and antifungal properties. Appl. Organomet. Chem.. 2019;33(7)
- [CrossRef] [Google Scholar]
- Albumin enhances PTX delivery ability of dextran NPs and therapeutic efficacy of PTX for colorectal cancer. J. Mater. Chem. B. 2019;7(22):3537-3545.
- [Google Scholar]
- Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem.. 2011;126(3):1122-1126.
- [Google Scholar]
- Biosynthesis and characterization of silver nanoparticles by Aspergillus species. BioMed Res. Int. 2016
- [Google Scholar]







