Translate this page into:
Rose Bengal induced formation of thrombosis in rats and an investigation of cardiovascular worries by haematological changes
⁎Corresponding author at: Department of Interventional Therapy, General Hospital of Northern Theater Command, Shenyang 110016, China. hongjiangman@sina.com (Hong Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University
Abstract
Background
The present investigation planned to study the cardiovascular worries in Rose Bengal (RB) induced thrombotic rats by analysing biochemical profile of plasma, platelet membrane and cardiac tissue.
Methods
Male Wistar rats were categorized into two groups. The group I was Control (C) rats and received glucose, whereas group II was Rose Bengal (50 mg/kg) induced thrombotic rats. The animals sacrificed and biological samples collected were analysed for various biochemical parameters.
Results
In rats RB induced heart damage/thrombosis showed significantly increased levels of lipid peroxidation (LPO), nitrate and nitrite. In RB administered group the antioxidant activities were lowered significantly i.e., reduced glutathione, glutathione S-transferase, glutathione peroxidase, superoxide dismutase and catalase when compared with control rats. Plasma enzymes SGOT, SGPT were decreased and Na+-K+ ATPase was significantly increased in group II in comparison with group I. There is no significant change in plasma hexokinase enzyme in RB administered rats when compared with control rats. The non-enzymatic and enzymatic antioxidants levels were decreased significantly in experimental groups II to compare with control group I rats. A significant increase in platelet membrane fluidity observed in group II rats when compared with group I rats in DPH and pyrene employed method. A significant raise in LPO of platelet membrane was observed in the experimental rats. Plasma nitrite and nitrate levels were increased in group II when compared to group I. Furthermore, the haematological parameters were also increased in group II when compared to group I rats.
Conclusion
This investigation pointed out that the nitric oxide scavenging levels increased and might be protected the oxidative stress and free radicals generated by RB induced cardiac thrombosis in rats.
Keywords
Rose Bengal
Thrombosis
Platelet membrane fluidity
Antioxidants
Nitric oxide
1 Introduction
Annually more than 19 million mortalities resulting from cardiovascular diseases (CVD) and significant part of these mortalities are contributing by coronary heart disease. The disease affected individuals appears healthy but at once surrender to CVD without earlier symptoms (Naghavi et al., 2013). The events such as atherosclerotic lesions interaction, formation of thrombus and participation of platelets are the key processes involved in the development and progression of CVD (Fuentes et al., 2013). The rupture of plaque and adhesion of platelets secret the contents from granules initiate the formation of thrombus. The last stage of the process is activation which leads to obstruct blood flow and results ischemic damage that proceeds to organ failure (Ruggeri, 1997). In arteries the interruption of the vascular endothelium is the major event of pathogenesis of thrombosis. Vascular endothelium and its homeostasis are considered as crucial for prevention of thrombosis. It regulates platelet adhesion and suppresses activation by releasing the molecule like nitric oxide.
Animal usage is the most preferred cost-effective model to investigate the thrombosis pathophysiology. Different types of methods and techniques were applied for the induction of thrombosis in animal models. Among them, foremost method is ferric chloride compound application that initiates damage of endothelium which causes adventitia to intima injury and further proceeds to loss of endothelium integrity. Second method is stasis which leads to the interrupted blood flow causing rupture of blood vessels that further proceeds to hypercoagulable condition (Aghourian et al., 2012). Other methods also applied such as ultra sound (Hechler and Gachet, 2011) and laser treatment. Laser irradiation induces photosensitization performing thrombi formation by executing endothelial damage (Fukuoka et al., 2012). The interaction of Rose Bengal (RB) compound with laser initiates the generation of reactive oxygen species that plays a crucial role in tissue necrosis which promotes pathological events and formation of thrombosis in rat models (Inamo et al., 1996). Among all these methods the laser administered injury seems to be a model of thrombosis mostly like the injury caused by inflammation.
RB is a dye compound denoted as 4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluorescein (Redmond and Kochevar, 2019). In most of biological and non-biological tasks the RB is employed as photosensitizer. With the illumination of green light, RB gets activated and produces (ROS) reactive oxygen species (Talley Watts et al., 2015). The ROS generated by RB triggers activation of tissue factor which is the initiator of coagulation cascade. The initiation of coagulation process enhances ischemic lesion which is pathologically similar to clinical stroke. Alternatively, the process of ROS generation leads to the destruction of endothelial cells and activation of clotting to execute thrombosis. Therefore, RB is strongly recommended in the induction of photothrombotic lesion in murine animal models (Piao et al., 2009). The current study aimed to investigate the induction of thrombosis by treated with RB in male Wistar rats. In this study, we targeted to analyse the effect and underlying mechanisms of RB induced thrombosis. Our findings elucidated that the increased nitric oxide levels executed the protection against the oxidative stress and free radicals in cardiac thrombosis rats along with the support of various other biochemical parameters.
2 Methods
2.1 Animals
Sixty-days-old male Wistar rats weighing approximately 120 to 140 g were housed in the animal house. The rats were procured from the National Institute of Nutrition (NIN), Hyderabad, Telangana, India. They maintained in cages in an air-conditioned room (25 ± 1 °C) with sunlight between 7:00 am and 7:00 pm. These animals were fed ad libitum with pelleted feed and tap water. The food and water intake of each rat was recorded daily and their body weight was recorded daily. This study was approved by the Ethics Committee (No/12/1/2023-AWD).
2.2 Experimental protocol
Rats were divided into 2 groups. Group I control 12 rats (C), experimental group II thrombus –induced 12 rats. The effective final dose of RB was fixed to 50 mg/kg with preliminary dose dependent study by injection of RB at different doses (10, 20, 50 and 75 mg/kg). RB was intramuscularly administered after applying a light on tail with 3 min warm-up. Animals were sacrificed by cervical puncture and heart tissues were immediately removed, washed with chilled isotonic saline, and used for these studies. This study used the blood samples taken from rats which were fasted overnight. Blood was drawn from rats and plasma was separated for further study.
2.3 Biochemical analysis
Plasma protein carbonyls (Reznick and Packer, 1994), TBARS for LPO (Sreejayan and Rao, 1997), glucose levels (Trinder, (1969), superoxide dismutase (SOD) (Mishra and Fridovich 1972), catalase (CAT) (Reznick and Packer, 1994), glutathione peroxidase (GPx) (Rotruck et al., 1973), and reduced glutathione (GSH) content (Ellman 1959), blood pressure, viscosity (mm/Hg), heart rate (beats/min), haematocrit (%) (Henry, 1984), platelet count 104/ml blood (Aster and Jandl, 1964), platelet membrane (Wrzyszcz et al., 2016), phospholipids (Fiske and Subbarow, 1925), cholesterol (Zlatkis et al., 1953), C/P, membrane lipid peroxidation (Ohkawa et al., 1979) membrane fluidity in the membrane (Shinitzky and Barenholz, 1978), serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT) (Reitman and Frankel, 1957), Na+-K+ATPase (Ismail and Edelman, 1985) were estimated.
2.3.1 Determination of plasma nitric oxide
The nitroprusside production was predicted by the Greiss reaction followed by modified method of Narendra et al., (2007). Blood was centrifuged and packed cell volume (PCV) was prepared. PCV was added with phosphate-buffered saline (5 mM) and mixed with sodium nitroprusside in various concentrations and incubated at 25 °C for 150 min. Greiss reagent was prepared in O-phosphoric acid (5%), sulfonamide (1%) and naphthaleneethylenediamine (0.01%) composition and was reacted with the above formula. The absorption of the chromophore formed was checked during the diazotization of the nitrite and sulfonamide followed by naphthylenediamine mixture in a UV–Vis spectrophotometer at 546 nm.
2.4 Statistical analysis
The statistical study performed by Duncan’s Multiple Range (DMR) test with consideration of p value as p < 0.05. Results were expressed as means ± SD.
3 Results
The results clearly revealed a significant (p < 0.05) increase in plasma LPO levels, platelet count, haematocrit, peroxy nitrites, protein carbonyls, systolic and diastolic blood pressure in thrombosis rats compared to controls (Table 1). The results in Table 2 represented a significant increase (p < 0.05) in platelet membrane cholesterol (C) and a significant decrease (p < 0.05) in the content of phospholipid (P) in thrombosis induced group II rats when comparing to the normal control group I rats. Table 2 also exhibited significantly (p < 0.05) increased platelet membrane LPO in thrombosis exerted group. Furthermore, data in Table 2 indicates the platelet membrane fluidity with significant increased levels in thrombosis group rats in comparison with control group rats. Fig. 1a-1d revealed various antioxidants such as GST, GPx, CAT, SOD and GSH showed significant (p < 0.05) decrease levels in the heart tissues of RB induced thrombosis rats when compared to control rats. Fig. 2a-b exhibited the significant (p < 0.05) increased levels of plasma parameters namely plasma glucose levels, nitrate, and nitrites in control and thrombosis rats. Data in Fig. 3a–c enumerated the significantly decreased in SGOT, SGPT levels in RB administered rats compared to control rats. The erythrocytes Na+-K+ATPase activities were increased significantly (p < 0.05) in thrombosis rats compared with control rats and there was no significant (p < 0.05) change in erythrocyte glycolated enzymes. All the tables and figure values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 12. All the tables and figure values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 12.
Parameter
Groups
Controls rats
Rose Bengal treated formation of thrombosis rats
Plasma lipid peroxidation (p mole of MDA formed/mg protein)
1.94 ± 0.01a
2.13 ± 0.20b
Platelet count (%)
3.8 ± 0.5a
4.2 ± 0.42b
HCT (%)
56.8 ± 2.2a
42.1 ± 0.64b
Peroxynirites
2 ± 0.11a
3.2 ± 1.10b
Protein carbonyls
4 ± 0.22a
6.2 ± 1.33b
Systolic blood pressure (SBP)
112.54 ± 10.22a
116.3 ± 6.32b
Diastolic blood pressure (DBP)
72.14 ± 9.34a
82.20 ± 6.12b
Parameter
Groups
Controls rats
Rose Bengal treated formation of thrombosis rats
Cholesterol (ng/1 105 platelets)
0.182 ± 0.009a
0.204 ± 0.009b
Phospholipid (ng/1 105 platelets)
0.198 ± 0.009a
0.168 ± 0.006b
Platelets Cholesterol/Phospholipid ratio
0.91a
1.21b
Lipid Peroxidation (MDA formed (µmoles × 105 platelets)
0.60 ± 0.04a
1.16 ± 0.05b
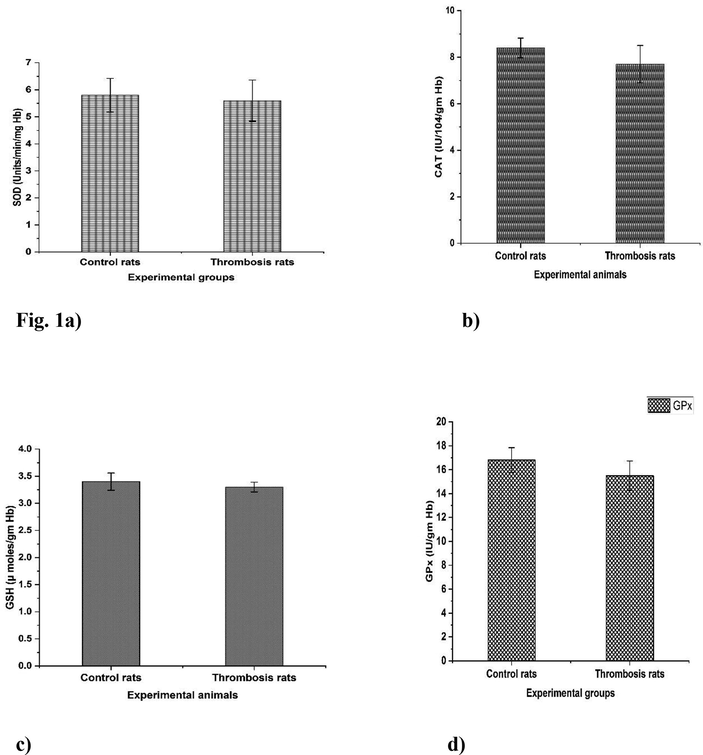
a–d. Rose Bengal treated formation of thrombosis rats with effect of antioxidant enzymes. All the tables and figure values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 12.
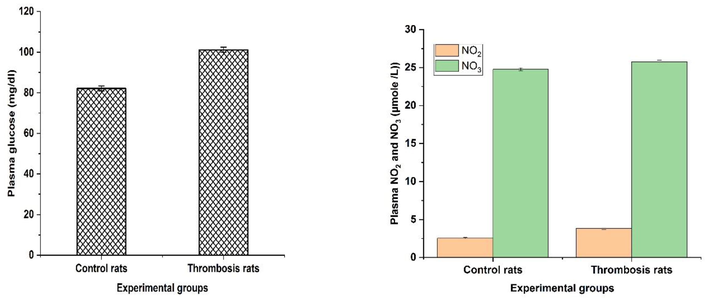
a–b. Rose Bengal treated formation of thrombosis rats with effect of plasma glucose and NO2 and NO3 levels. All the tables and figure values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 12.
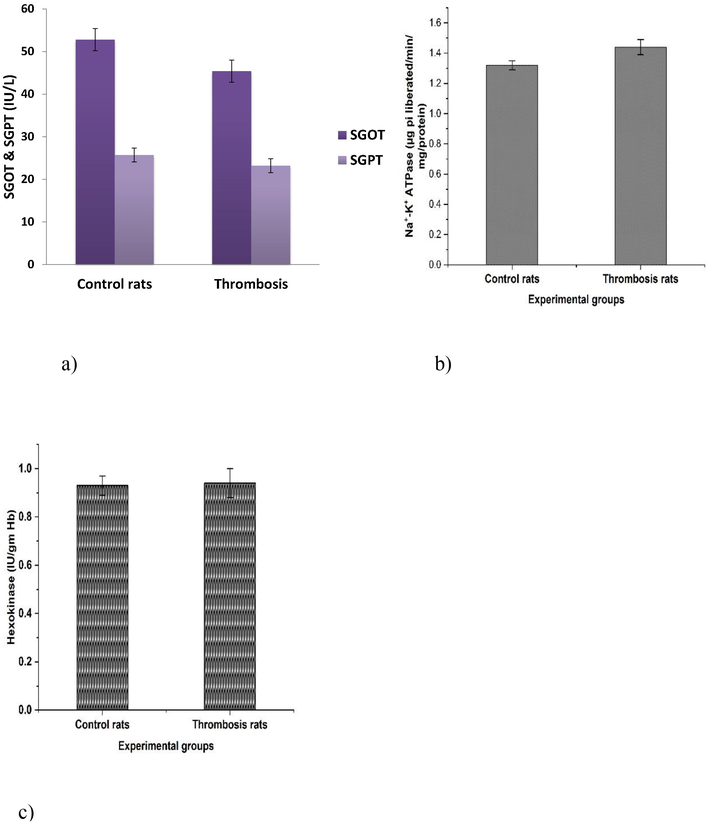
a–c. Effect of Rose Bengal treated formation of thrombosis rats on the activities of GOT, GPT, Na+-K+-ATPases, Hexokinase of rats. All the tables and figure values are expressed as Mean ± SEM, in each column followed by the same letter are not significantly different (P ≤ 0.05) from each other according to Duncan’s Multiple Range (DMR) test, n = 12.
4 Discussion
Isoenzyme that catalyzes the binding of GSH to various electrophilic compounds; GST is a multigene family of isoenzymes and thus plays a decisive role in protecting cells from ROS (Wilce and Parker, 1994). Due to decreased GST activity, the detoxification of the toxic aldehyde 4-hydroxynonenal is impaired and LPO is produced. Thus, it may cause a decrease in enzyme activity or cardiotoxicity that may be derived from the GST isozyme-specific drug and its metabolites (Hayes and Pulford, 1995). Side-by-side detoxification reactions and their GSH-based redox reactions preserve cell association and function (Alin et al., 1985). Therefore, GSH plays an important role in the coordination of the antioxidant defence mechanism, as it is the main non-protein thiol. Glutathione is a tripeptide (Gueeri, 1995) found in almost all cells and plays an important role in metabolism, cell formation and transport in addition to the oxidation of reactive oxygen species and free radical intermediates in group II, which is like group I compared to controls (Das et al., 1997). This type of reactive oxygen requires the antioxidant enzyme glutathione reductase (Das, 1997) to reduce GSSG to GSH, and the use of NADPH, shunt HMP, glutathione peptide peroxidases catalyze and reduce organic hydroxide. hydrogen peroxide (Oberley, 1998) SOD, GSH catalyzes the reduction of superoxide radicals to hydrogen peroxide (Jacob, 1995) and CAT reduces hydrogen peroxide to water and multifunctional proteins, glutathione S-Glucosaceyl-catalyzes and contributes to GSH conjugation It transforms into several minor substrates that are very important in xenobiotic detoxification (Ashour et al., 1999). Endogenous glutathione and the combination of glutathione peroxidase and catalase is an important antioxidant and cell protective device in drug-exposed hepatocytes (Deisseroth and Dounce, 1970). GR is concerned with the storage of concentrated GSH at the cellular level. This study aimed to evaluation of lipid composition changes in male albino Wistar rats treated with RB, showing an increase in heart function and blood aspartate aminotransferase and alanine aminotransferase levels, followed by a decrease in heart rate and a significant increase in blood glucose. Therefore, our findings show that it causes changes in lipid composition and lipid toxicity.
5 Conclusion
RB administration notably altered the systolic and diastolic blood pressures, antioxidants, membrane bound sodium–potassium ATPase, plasma membrane fluidity, LPO and NO in plasma and heart tissues of rats. Thus, our study clearly revealed that RB induced thrombosis in rats by oxidative stress and generating free radicals, and the increased NO levels might be involved in the protection.
Funding
The authors appreciate Researchers Supporting Program for funding this research through Researchers Supporting Project number (RSP2023R371), King Saud University, Riyadh, Saudi Arabia.
Contributions of authors
All authors were involved in the scientific assessment, analytical analysis described in the research. All authors read carefully and agreed the concluding version.
Acknowledgement
The authors acknowledge their sincere thanks to Researchers Supporting Program for providing fund to conduct this work through Researchers Supporting Project number (RSP2023R371), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vivo monitoring of venous thrombosis in mice. J. Thromb. Haemost.. 2012;10:447-452.
- [Google Scholar]
- 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett.. 1985;179:267-270.
- [Google Scholar]
- Antioxidant status and insulin dependent diabetes mellitus. J. Clin. Biochem.. 1999;29:99-107.
- [Google Scholar]
- Glutathione status of some homeothermic vertebtate species and its relation with diabetogenesis. Indian J. Exp. Biol.. 1997;35:66-662.
- [Google Scholar]
- Hydroxy radical is the major causative factor in stress induced gastric ulceration. Free Radic. Biol. Med.. 1997;23:8-18.
- [Google Scholar]
- Catalase: physical and chemical properties. Mechanism of catalysis and physiological role. Physiol. Rev.. 1970;50(3):319-375.
- [Google Scholar]
- The colorimetric determination of inorganic phosphorus. J. Biol. Chem.. 1925;66:375-404.
- [Google Scholar]
- Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2013;24:255-262.
- [Google Scholar]
- Laser-induced thrombus formation in mouse brain microvasculature: effect of clopidogrel. J. Thromb. Thrombolysis. 2012;34:193-198.
- [Google Scholar]
- Influence on prolonged ethanol intake on the level and turnover of metformin and aldehyde dehydrogenase and glutathione. Adv. Exp. Med. Biol.. 1995;23:12-14.
- [Google Scholar]
- The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol.. 1995;30:445-600.
- [Google Scholar]
- Comparison of two murine models of thrombosis induced by atherosclerotic plaque injury. Thromb. Haemost.. 2011;105(Suppl 1):S3-S12.
- [Google Scholar]
- In: Clinical diagnosis and management by laboratory methods (seventeenth ed.). Philadelphia: Wb saunders Company; 1984. p. :580-589.
- Importance of photo activation of rose bengal for platelet activation in experimental models of photochemically induced thrombosis. Thromb. Res.. 1996;83:229-235.
- [Google Scholar]
- The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem.. 1972;247:3170-3175.
- [Google Scholar]
- From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies Part I. Circulation. 2013;108:1664-1672.
- [Google Scholar]
- Prallethrin induced biochemical changes in erythrocyte membrane and red cell osmotic haemolysis in human volunteers. Chemospheres. 2007;67:1065-1071.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- A mouse model of photochemically induced spinal cord injury. J. Korean Neurosurg. 2009;46:479-483.
- [Google Scholar]
- Review: medical applications of Rose Bengal- and Riboflavin-photosensitized protein crosslinking. Photochem. Photobiol.. 2019;95:1097-1115.
- [Google Scholar]
- A coloricmetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol.. 1957;28:8-15.
- [Google Scholar]
- Oxidative damage to proteins: spectroscopic method for carbonyl assay. Methods Enzymol.. 1994;233:357-363.
- [Google Scholar]
- Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588-590.
- [Google Scholar]
- Mechanisms initiating platelet thrombus formation. Thromb. Haemost.. 1997;78:611-616.
- [Google Scholar]
- Fluidity parameters of lipid regions determined by fluorescence polarization. Biochem Biophys Acta. 1978;515(4):367.
- [Google Scholar]
- Rose Bengal photothrombosis by confocal optical imaging in vivo: a model of single vessel stroke. J. Vis. Exp. JoVE. 2015;100:e52794
- [Google Scholar]
- Determination of glucose-by-glucose oxide method. Ann. Clin. Biochem.. 1969;6:24-26.
- [Google Scholar]
- An efficient method for isolation of representative and contamination-free population of blood platelets for proteomic studies. Platelets. 2016;28:43.
- [Google Scholar]
- A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med.. 1953;4:486-492.
- [Google Scholar]







