Role of vitamin D3 on apoptosis and inflammatory-associated gene in colorectal cancer: An in vitro approach
⁎Corresponding authors. arun47biotech@gmail.com (Arun Meyyazhagan), avamiet@yahoo.com (Vijaya Anand Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The association between vitamin D and cancer has been studied with different focuses. As far as the beneficial effects concern, studies have shown the effects of vitamin D in inflammatory response and oxidative stress in relation with cancer regression. Indirect anti-cancer effects of vitamin D also due to its anti-inflammatory properties. Nuclear factor kappa B (NF-κB) is considered as a major mediator of inflammation and key transcription factor linking inflammation of cancer development. On the other hand, caspase protein also has an important role in apoptosis. Further, apoptosis and the BCL-2 family now inextricably linked to human cancer. Since the vitamin D3 has proven to exert numerous tumor suppressive effects, the relationship of vitamin D3 on the regulation of apoptosis and NF-κB is yet to be studied in detail. Hence, the present study aims at evaluating the efficacy of vitamin D3 on NF-κB and Caspase 3 in the human colorectal carcinoma cell line (HCT116). Treatment of HCT cell line with different concentration of vitamin D3 shown a dose-dependent effect on the expression of Caspase 3 not on NF-κB. This finding suggests that vitamin D does not have any effect on NF-κB, but have a positive effect on Caspase3 on HCT116, which might play a vital role in preventing the colon cancer.
Keywords
Vitamin D
Caspase 3
NF-κB
Colorectal cancer
Apoptosis
Inflammation
1 Introduction
Vitamin D was discovered in 1919 during rickets experiments by Edward Mellanby (Mehta and Mehta, 2002; Mellanby, 1919). There are two main forms of vitamin D exist in nature: Photo-chemically synthesized vitamin D2 (ergocalciferol) in plants, and vitamin D3 (cholecalciferol) synthesized in the skin of animals and humans (Vuolo et al., 2012; Norman, 1998). According to recent research, vitamin D may play an important role in preventing and treating many diseases like type 1 and type 2 diabetes (Hyppönen et al., 2001; Pittas et al., 2006), hypertension (Krause et al., 1998), glucose intolerance (Chiu et al., 2004), multiple sclerosis (Munger et al., 2006) and other medical conditions like rheumatoid arthritis (Merlino et al., 2004; Schleithoff et al., 2006).
Cancer is a disease induced by genetic changes that result in uncontrolled development of cells and the formation of tumors. When considering the relationship between cancer and beneficial effect of vitamin D3, different opinions exist in recent years. Many, although not all, studies that associate vitamin D levels with cancer risk measure serum levels of 25(OH)D to determine if the subjects are deficient. For example, a greater proportion of melanoma patients had deficient or insufficient levels of 25(OH)D than healthy controls (Cattaruzza et al., 2019). Similarly, vitamin D levels were lower in patients with thyroid cancer compared to matched controls (Heidari et al., 2017). Women with breast cancer that were deficient in 25(OH)D had larger tumors, more advanced stage and reduced survival than those that were not deficient (Ismail et al., 2018).
Not all studies have shown beneficial effects of vitamin D and/or D3 supplementation in cancer treatment. Experimental evidence of vitamin D has also shown that it may reduce the risk of cancer by regulating anti-proliferative effect, apoptosis, stimulation of differentiation, anti-inflammatory effect, inhibition of invasion and metastasis, and the inhibition of angiogenesis (Liu et al., 2018). Emerging epidemiological data suggest that vitamin D may have a protective effect against colon cancer, but there is no strong evidence for prostate, breast and other cancers (Davis, 2008; Davis et al., 2007). Previous study shown not only the beneficial effect of vitamin D on cancer treatment but has also shown no effect or even increased cancer risk as well. The reasons for such variability is because of multiple attribute in study design and approches. Therefore, adequate research is needed to determine whether the inadequacy of vitamin D can lead to increased risk of cancer or not (Davis et al., 2007). Hence, the present study aims at investigating the efficacy of vitamin D3 as a preventive and/or therapeutic agent for colon cancer through targeting specific gene of interest.
2 Materials and methodology
2.1 Cell culture
Human colorectal carcinoma cell line, HCT116, was obtained from National Centre for Cell Science (NCCS) India which was cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% antibiotics (Penicillin and Streptomycin) in an incubator with the presence of 5% CO2 at 37 °C. The media were changed at regular intervals and hen the cells reached the 80% confluence, the cells were seeded onto 6-well plate at a density of 1X105 for further treatment and analysis.
2.2 MTT assay
MTT Assay was performed to find out the viability and IC50 value at different concentrations of vitamin D3 (Merck) such as 2.5 µM, 5 µM, 7.5 µM, 10 µM, 15 µM, 20 µM, 40 µM. Briefly, cells were seeded on 96-well plate and treated with indicated concentrations of vitamin D3 at 80% confluence level for 24 h. At the end of the experiment, MTT was added and read at 590 nm for the absorbance.
2.3 RT-PCR and gene expression
To depict the beneficial effect of vitamin D3 as prevention and/or therapeutic agent for colon cancer, the HCT119 cells were cultured on 6-well plate and treated with different concentration of vitamin D3 viz 5 µM, 15 µM and 20 µM (dosage was fixed based on the MTT assay) for 24 h. At the end of the experiment, cells were tripzinzed and total RNA was isolated by the Trizol method as previously described (Rio et al., 2010). Approximately, 2 μg of RNA was used for cDNA synthesis using a PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa Bio Inc., Japan) by reverse transcriptase PCR (Cyberlab, USA). For the expression of Caspase 3 in the treated cell line, the primes 5′-AGTAGATGGTTTGAGCCTGAGC-3′ (Forward) and 5′-TTTCTTACTTGGCGATGGCG-3′ (Reverse) was used. Similarly, for NF-κB, 5′-CCCACGAGCTTGTAGGAAAGG-3′ (Forward) and 5′-CTGGATGCGCTGACTGATAG-3′ (Reverse) primers was used. The house keeping gene, β actin, was used as internal control and the primers used for actin was 5′-AAGAGAGGCATCCTCACCCT-3′ (Forward) and 5′-TACATGGCTGGGGTGTTGAA-3′ (Reverse). The intensity of the band was calculated using image pro software and the results obtained from three different experiments were used for the expression analysis.
3 Results
In this present investigation, we evaluated the toxicity of vitamin D3 through percentage of cell viability upon treatment with different concentrations as shown in Fig. 1. The result shown that the viability of cells treated with vitamin D3 decreases with increasing concentrations. The higher the concentration (40 µM) shows the maximum of 20% cell viability approximately. Hence, vitamin D3 has a significant effect on cell survival in a dose-dependent manner (Fig. 1). Further, the IC50 value of vitamin D3 observed at a concentration of 15 μM in HCT116 cell line.
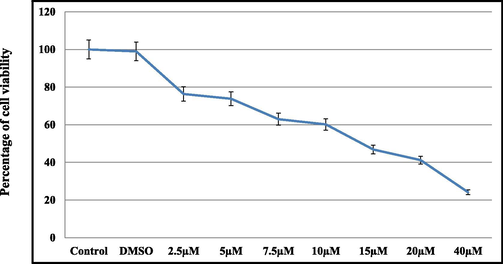
- MTT assay for the percentage of cell viability in HCT119 cell lines which treated with different indicated concentrations of vitamin D3. values for the percentage indicates mean ± SD of three different experiments.
3.1 Expression of Caspase 3 in HCT116
To know the effect of vitamin D3 on expression of Caspase 3, HCT116 cells were treated vitamin D3 as shown in Fig. 2. In this, the cells were treated with three different concentrations of vitamin D3 as mentioned along with DMSO as a vehicle control. The cells treated with vitamin D3 has significant upregulation of Caspase 3 with respect to the vitamin D3 concentration. On the other hand, a dose-dependent increase in the expression of Caspase 3 observed in the present investigation (Fig. 2A and B).
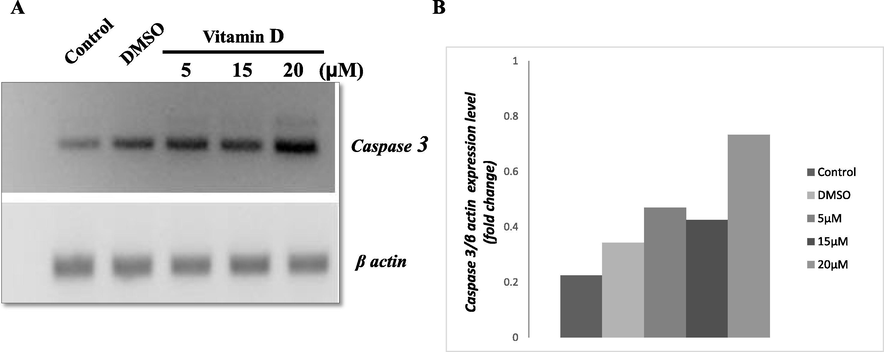
- (A) RT-PCR for Caspase 3 gene expression in HCT119 treated with different concentrations of vitamin D3. (B) Represents the densitometry analysis for the level of gene expression among vitamin D3 treated and control groups. The values shown are obtained from there different experiments. β actin served as internal control.
3.2 Expression of NF-κB in HCT116 treated with vitamin D3
We further intended to investigate if vitamin D3 has a beneficial effect on the inflammatory response in colon cancer. Hence, the HCT116 cells treated with the different concentrations of vitamin D3 as shown in Fig. 3. The expression of NF-κB was observed among the treated cells. However, the cells treated with vitamin D3 shows no significant changes in the expression of NF-κB when compared with each other (Fig. 3A and B).
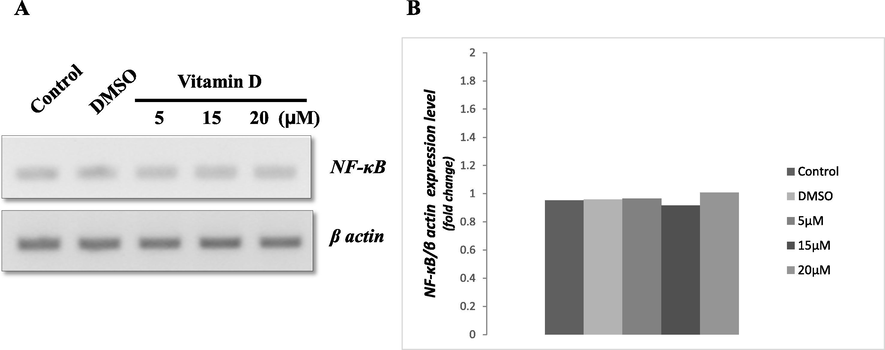
- (A) RT-PCR for NF-κB gene expression in HCT119 treated with different concentrations of vitamin D3. (B) Represents the densitometry analysis for the level of gene expression among vitamin D3 treated and control groups. The values shown are obtained from there different experiments. β actin served as internal control.
4 Discussion
In this present study, a dose-dependent effect of vitamin D3 on the expression of Caspase 3 and NF-κB was performed to make out beneficial role of vitamin D3 in cancer prevention on HCT116. MTT assay has depicted the viability of cells and also the IC50 value of vitamin D3. Cells treated with different concentrations of vitamin D3 such as 2.5 µM, 5 µM, 7.5 µM, 10 µM, 15 µM, 20 µM and 40 µM shows the significant changes in cell viability with increased concentrations of vitamin D3, which suggest that vitamin D3 might play a role in cell homeostasis. Further, the IC50 value of vitamin D3 was found to be at a concentration of 15 μM. Few studies shown similar type of experiments in which the cells treated with vitamin D have lesser significant results. Shruthi et al., (2017) finds the cellular uptake of vitamin D by the HCT116 treated with different concentration of vitamin D3. Similarly, Diesing et al. (2006) used supraphysiological concentration and physiological concentration to find cellular uptake of vitamin D and confirmed the uptake of vitamin D by cancer cells. In respect with this result and evident, HCT116 cells were treated with the three different concentration to understand the effect on the gene expression such as Caspase 3 and NF-κB. The results suggest that the supplement of vitamin D3 increase apoptotic regulatroy protein Caspase 3 expression, but this result is not enough to prove the effect on NF-κB. Previous studies have demonstrated that the NF-κB has a very important role in the CRC as CRC patients will develop inflammation (Barbáchano et al., 2018). It has been shown that vitamin D3 reduce the expression of NF-κB suggesting that vitamin D3 has an anti-inflammatory property which will gradually reduce the CRC from further developing into inflammation. However, our results are not consistent with this study and do not provide a valid conclusion on this part. Because, NF-κB is inhibited by vitamin D3 through several pathways and it may depend on the types of cancer as well.
The expression of Caspase 3 shows there is an increased expression of the genes as of concentration of vitamin D3 increases. This result further suggests that vitamin D3 regulates on the expression of Caspase 3, an important mediator of apoptosis. Since apoptosis is a major target for control of cancer growth, the increased intex of Caspase 3 in vitamin D3-HCT116 might have a beneficial role on colon carcinoma as a homeostatic regulator. Previous report suggests that the treatment with the active component of vitamin D3 will cause apoptosis through the increase in several pro-apoptotic proteins, decrease in anti-apoptotic proteins and associated proteins (Barbáchano et al., 2018). In support with the previous data, our result suggests that vitamin D3 upregulates Caspase 3 which in turn regulate apoptosis in colon cancer.
The present study also investigated the effect of vitamin D3 on the inflammatory and apoptotic factors on the human colon carcinoma cell HCT116. Vitamin D3 has direct or indirect effect as anti-inflammatory (Lin and Li, 2016) and other cellular processes like anti-proliferative, stimulation of differentiation, inhibition of metastasis and invasion and inhibition of angiogenesis (Liu et al., 2018). Previous studies have focused on the anti-inflammatory and inhibition of apoptosis property. As both the factors are important for reducing the CRC, we focused on NF-κB and Caspase 3. In CRC patients, inflammation is a major factor which in severe cases does not have treatment. The active component of vitamin D known as the calcitriol exerts anti-inflammatory properties and inhibits IL-1β, IL-6, IL-8, TNF-α and possibly other cytokines. Further, indirect anti-cancer effects of vitamin D can also be due to its anti-inflammatory properties. Men with prostate cancer had reduced 25(OH)D and increased inflammatory mediator levels compared to controls (Xie et al., 2017). The results of our study indicate that NF-κB has no significant changes in their expression in vitamin D3 treated HCT119 cell line. This observation may be because of sufficient supplementation of vitamin D3 concentration.
On the whole, we conclude that vitamin D3 does not affect the level of NF-κB in a particular concentration. At the same time, it was found that Caspase 3 is upregulated in the vitamin D3 treated cells in a dose-dependent manner. Thus, from this present study, we conclude that vitamin D3 could promote apoptosis in the HCT116. However, there is need of more supporting experimental evidence for the use of vitamin D3 as a potential therapeutic agent for the treatment of colorectal cancer.
Acknowledgements
The authors thank the management of Bharathiar University for providing infrastructure facilities for this research work. The authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this research through Researchers Supporting Project number (RSP-2020/19).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Barbáchano, A., Larriba, M.J., Ferrer-Mayorga, G., González-Sancho, J.M,, Muñoz, A., 2018. Vitamin D and colon cancer. In Vitamin D, Academic Press-Elsevier. 2(99), 837-862.
- 25-Hydroxyvitamin D serum levels and melanoma risk: a case-control study and evidence synthesis of clinical epidemiological studies. Eur. J. Can. Prev.. 2019;28(3):203-211.
- [Google Scholar]
- Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am. J. Clin. Nut.. 2004;79(5):820-825.
- [Google Scholar]
- Vitamin D and cancer: current dilemmas and future research needs. Am. J. Clin. Nut.. 2008;88(2):565S-5699S.
- [Google Scholar]
- Vitamin D and cancer: Current dilemmas and future needs. Nutr. Rev.. 2007;65(8Pt2):S71-S74.
- [Google Scholar]
- Vitamin D-metabolism in the human breast cancer cell line MCF-7. Antican. Res.. 2006;26:2755-2760.
- [Google Scholar]
- Vitamin D deficiency associated with differentiated thyroid carcinoma: a case-control study. As. Pac. J. Can. Preven.. 2017;18(12):3419-3422.
- [Google Scholar]
- Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. The Lancet. 2001;358(9292):1500-1503.
- [Google Scholar]
- Prognostic significance of serum vitamin D levels in Egyptian females with breast cancer. As. Pac. J. Can. Preven.. 2018;19(2):571-576.
- [Google Scholar]
- Ultraviolet B and blood pressure. Lancet (British edition). 1998;352(9129):709-710.
- [Google Scholar]
- The role of vitamin D and its analogs in inflammatory disease. Curr. Topics Med. Chem.. 2016;16(11):1242-1261.
- [Google Scholar]
- The anti-inflammatory effects of vitamin D in tumorigenesis. Int. J. Mol. Sci.. 2018;19(9):2736.
- [Google Scholar]
- Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women's Health Study. Arthritis Rheumatism. 2004;50(1):72-77.
- [Google Scholar]
- Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- [Google Scholar]
- Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. Am. J. Clin. Nut.. 1998;67(6):1108-1110.
- [Google Scholar]
- Vitamin D and calcium intake in relation to type 2 diabetes in women. Diab. Care.. 2006;29(3):650-656.
- [Google Scholar]
- Purificaiton of RNA using TRIzol (TRI reagent) Cold Spring Harbour Protocol. 2010;2010(6):2010.
- [Google Scholar]
- Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nut.. 2006;83(4):754-759.
- [Google Scholar]
- Analysis of the cytotoxic effects of vitamin D3 on colorectal, breast and cervical carcinoma cell lines. Biochem. Anal Biochem.. 2017;6(2)
- [Google Scholar]
- Low vitamin D status is associated with inflammation in patients with prostate cancer. Oncotarget. 2017;8(13):22076-22085.
- [Google Scholar]







