Translate this page into:
Role of some functional lipids in preventing diseases and promoting health
*Corresponding author. Tel.: +966 14785968x1484, mobile: +966 566091562. sarzoo@ksu.edu.sa (Shaista Arzoo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 27 March 2012
Peer review under responsibility of King Saud University.
Abstract
Fats and lipids are common components of food and may perform essential roles. Their types may be more important with regard to health and disease than their amount. The objective of this review is to summarize the information on the role of functional lipids on human health. New research has linked functional lipids to the prevention and treatment of many diseases. Functional lipids such as omega-3 and omega-6 fatty acids, conjugated linoleic acids, medium chain triglycerides, and phytosterols have many beneficial effects on human health such as in obesity, bone health, and in treating and managing depression, blood pressure, cardiovascular health, etc. The ratio of omega-3 and omega-6 polyunsaturated fatty acids regulates the production of eicosanoids, which are the metabolites of these series of fatty acids. Scientific evidence has shown encouraging improvements in patients and beneficial effects in healthy persons with the use of supplemental and dietary forms of functional lipids.
Keywords
Functional lipids
Omega-3 fatty acid
Omega-6 fatty acid
Medium chain triglycerides
Conjugated linoleic acids
Phytosterols
Obesity
Bone health
1 Introduction
Foods can be regarded as functional if they help in reducing the risk of disease and promote good health (Roberfroid, 2000). They may provide means to reduce the increasing burden on the health care system. Although there is no authoritative definition of functional lipids, one can informally define them as a subset of functional foods, which are considered to be similar in appearance to conventional foods consumed as part of a usual diet, but they have been demonstrated to have physiological benefits and/or reduce the risk of chronic disease beyond basic nutritional functions (Moreau, 2011). The purpose of this review is to summarize the information currently available on selected functional lipids.
2 Components of functional lipids
The following are considered as functional lipids: omega-3 fatty acids (alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)), omega-6 fatty acids (gamma linoleic acid (GLA), and linoleic acid (LA)), conjugated linoleic acid (CLA), medium chain triglyceride oils, and phytosterols.
2.1 Omega-3 and omega-6 fatty acids
Humans can synthesize saturated and monounsaturated fatty acids but cannot synthesize polyunsaturated omega-3 (also referred to as ω-3 or n-3) and omega-6 (also referred to as ω-6 or n-6) fatty acids de novo. This is because humans like other animals, lack the desaturase enzymes required to produce the simplest members of these families (ALA and LA, respectively). Thus ALA and LA are considered “essential fatty acids” (EFAs) that need to be included in the diet. EFAs act as precursors for the synthesis of more highly unsaturated and longer-chain omega-3 and omega-6 fatty acids (Tapiero et al., 2002). Omega-3 and ω-6 fatty acids are essential components of cell-membrane phospholipids and they have several other functional roles (Hardman, 2004).
2.1.1 Omega-3 fatty acids
2.1.1.1 Alpha-linolenic acid (18:3 (ω-3); all-cis-9,12,15 octadecatrienoic acid; ALA)
ALA is mainly of plant origin and is the precursor of two functionally important longer-chain ω-3 fatty acids, eicosapentaenoic acid (EPA; 20:5 (ω-3)) and docosahexaenoic acid (DHA; 22:6 (ω-3)). Conversion efficiency of ALA to EPA and DHA is low (Igarashi et al., 2006; Williams and Burdge, 2006; Kapoor and Patil, 2011) and is estimated to be approximately 4–5% (Brenna, 2002) in men, but greater than this in women (Burdge, 2004). More generally, there are several ways to express the conversion rate and it probably depends on the tissues which are investigated. Furthermore, the turnover of EPA and DHA must be considered. Some studies (Umhau et al., 2009) suggested that the half life of DHA in the human brain may exceed several months and even as long as two years. If these data are confirmed than it means that influence of food must be considered over a long time range.
2.1.1.2 Eicosapentaenoic acid (20:5 (ω-3); all-cis-5,8,11,14,17 eicosapentaenoic acid; EPA) and docosahexaenoic acid (22:6 (ω-3); all-cis-4,7,10,13,16,19 docosahexaenoic acid; DHA)
These are long-chain, polyunsaturated fatty acids naturally found in aquatic foods, especially oily fish and in fish oil supplements. DHA is highly abundant in brain (Sastry, 1985) and retina (Dratz and Holte, 1993) where it plays important structural and functional roles. Consequently, DHA status is important to ensure optimum neural and visual functions (Innis, 2003; Birch et al., 2007; Eilander et al., 2007). EFAs are the precursors of a family of prostaglandins and leukotrienes, which control blood clotting and other arterial functions and inflammation (Simopoulos, 2002; Mori and Beilin, 2004). This may be important in reducing the risk of cardiovascular disease (Angerer and Von Schacky, 2000; Jacobson, 2008). Prostaglandins produced from EPA constitute a minor group compared to eicosanoids produced from arachidonic acid (AA) and may be as much as one hundred fold less potent biologically for inducing pro-inflammatory cellular responses than those derived from AA (Robinson et al., 1988). EPA and DHA also lower blood triglyceride concentrations (Harris, 1997) and are substrates for the synthesis of resolvins, which are believed to play a key role in terminating inflammatory processes (Kohli and Levy, 2009). Thus these ω-3 fatty acids could be important in treating inflammatory diseases (Mori and Beilin, 2004).
2.1.2 Omega-6 fatty acids
2.1.2.1 Gamma linoleic acid (18:3 (ω-6); all-cis-6,9,12 octadecatrienoic acid; GLA)
GLA is produced in the body as an intermediate in the metabolism of LA by the enzyme delta-6 desaturase. This reaction is the rate limiting step in the conversion of LA into its more unsaturated and longer derivatives (Horrobin, 1992; Cheng et al., 2004). GLA is also found in some plant oils. GLA is rapidly elongated to dihomogammalinolenic acid (20:3 (ω-6); DGLA) by the enzyme elongase. Subsequently, DGLA may be incorporated into cell-membrane phospholipids or may be converted into arachidonic acid (20:4 (ω-6); AA) by the enzyme delta-5-desaturase. AA is one of the most important fatty acids associated with membrane phospholipids. AA can be oxidized to a variety of eicosanoid compounds important in cell–cell signalling (Wheelan, 1996).
2.2 Conjugated linoleic acid (main isoform is cis-9, trans-11-octadecadienoic acid; CLA)
CLA refers to a group of polyunsaturated fatty acids (PUFA) that exist as positional and stereo-isomers of linoleic acid with a conjugated double bond. Many forms of CLA are possible (Sehat et al., 1999). The major naturally occurring CLA is cis-9, trans-11 CLA, found in ruminant milks, dairy products and ruminants’ meats and also in CLA supplements. Animal models suggest that some CLA isomers have a role in preventing cancer (Ip et al., 1994). There are suggestions that CLA has a role in treating some human diseases (Nagao and Yanagita, 2005).
2.3 Medium chain triglycerides
Medium chain triglyceride (MCTs) contains fatty acids with 6–10 or 12 carbon atoms in their acyl chain. MCTs are readily digested, not requiring bile salts, and the shorter fatty acids are transported in bloodstream without the need for lipoproteins (Stein, 1999). Hence they are directed to the liver for metabolism. Their oxidation does not require carnitine (Bach and Babayan, 1982). Hence MCTs and their constituent fatty acids are useful as a fat source to those with the abnormalities of fat digestion, absorption, transport and metabolism (Smits et al., 1968; Marten et al., 2006).
2.4 Phytosterols
Phytosterols (plant sterols) are common plant and vegetable constituents. They are normal constituents of the human diet and are considered to be biologically active (Pirronen et al., 2000). They are structurally related to cholesterol, but differ from cholesterol in the structure of the side chain. Examples are campesterol, stigmasterol and beta-sitosterol. Phytosterols inhibit cholesterol absorption and so help to control blood total cholesterol, LDL and HDL levels and so modify the risk of cardiovascular disease (Lichtenstein and Deckelbaum, 2001).
3 Omega-6 to omega-3 ratio of the diet
The healthy ratio of ω-6 to ω-3 fatty acids in the human diet is said to range from 1:1 to 4:1 (Simopoulos et al., 2000; Simopoulos, 2003). Due to the excessive use of vegetable oil, rich in LA, in the human food chain (Blasbalg et al., 2011) and to reduced intake of fish, this ratio is now higher, in most western diets (Kris-Etherton et al., 2002). LA and ALA compete for the same enzymes for conversion to AA and EPA, respectively. Hence the ratio between LA and ALA will partly determine the availability of the biologically active products, AA and EPA. In turn AA and EPA compete for the same enzymes for eicosanoid synthesis and so the ratio between AA and EPA may influence blood clotting, smooth muscle contraction, inflammation and immune responses (Simopoulos, 1991; Simopoulos 2008b) (Fig. 1).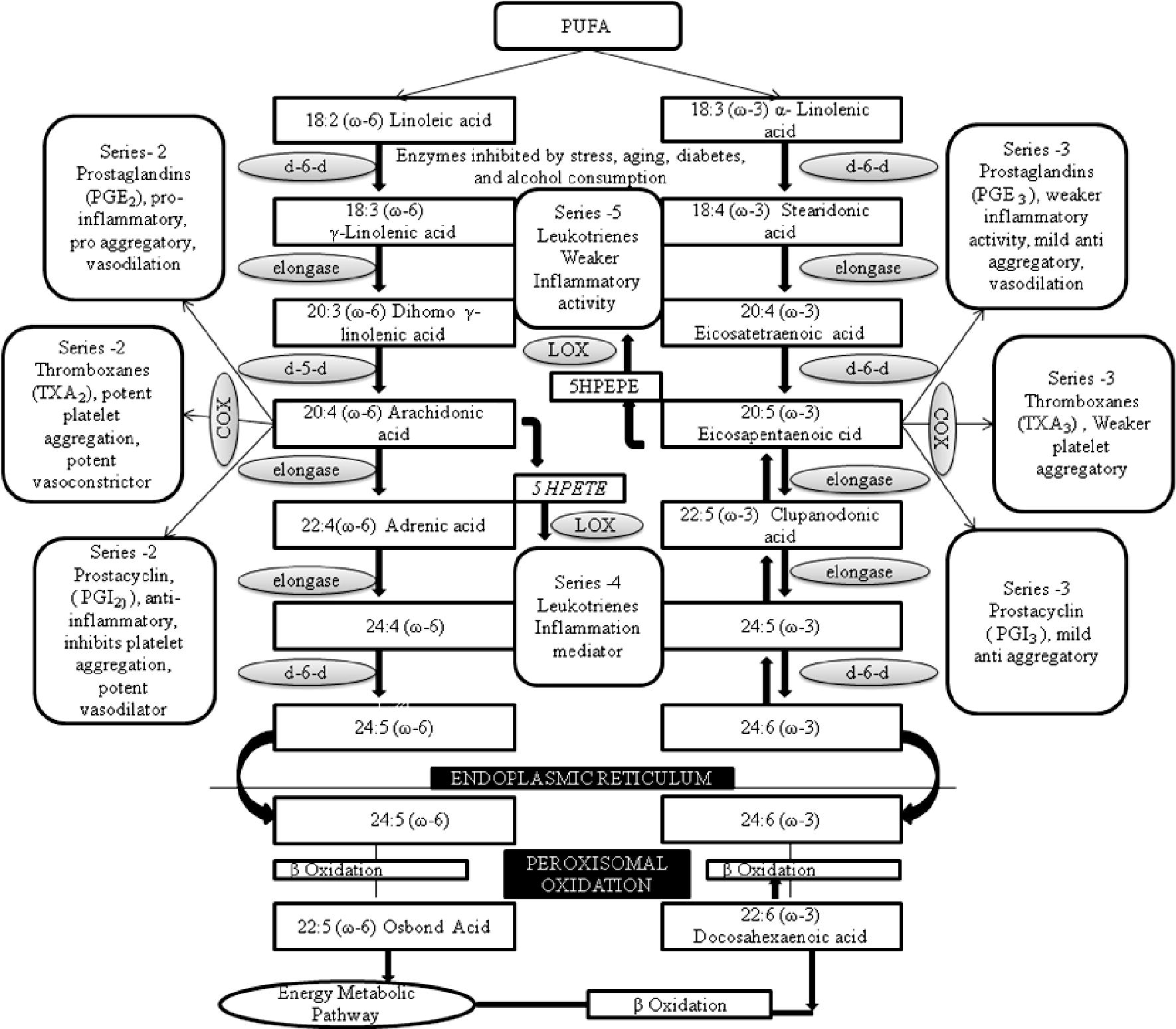
Biosynthesis pathways of omega-3 and omega-6 PUFA and oxidative metabolism of arachidonic and eicosapentaenoic acid by carboxygenase (COX) and 5 Lipoxygenase pathways (LOX), where 5 HPETE denotes 5 hydroperoxyeicosatetraenoic acid, 5HPEPE denotes 5 hydroxyeicosapentaenoic acid, d-6-d denotes delta-6 desaturase and d-5-d denotes delta-5 desaturase. A fatty acid notation represents total number of carbons: number of double bonds, position of the first double bond relative to methyl terminal of the hydrocarbon chain (adapted from San Giovanni and Chew, 2005; Simopoulos, 2010 with modification).
4 Dietary sources of functional lipids and recommendations for their intake
Dietary sources of functional lipids are listed in Table 1. The World Health Organization recommends (for adults), a total ω-3 fatty acid intake of 0.5–2% of energy and a total ω-6 fatty acid intake of 2.5–9% of energy. For adult males and non-pregnant/non-lactating adult females 0.250 g/day of EPA plus DHA is recommended, with insufficient evidence to set a specific minimum intake of either EPA or DHA alone; both should be consumed. For adult pregnant and lactating females, the minimum intake for optimal adult health and foetal and infant development is 0.3 g/d EPA + DHA, of which at least 0.2 g/d should be DHA (FAO, 2008). There are no dietary recommendations for MCTs, CLA or phytosterols. However, a safety factor for free phytosterols has been estimated to be 137 mg/kg/day, equivalent to 9.6 g/person/day for a 70 kg adult (ACNFP, 2002a).
Functional lipids
Dietary sources
Omega-3 fatty acid
Eicosapentaenoic acid and docosahexaenoic acid: Fatty fish such as mackerel, sardine, tuna and micro algae
Alpha linolenic acid: Dark green leafy vegetables, flax seed oil, chia seed oil, egg, meat, lingon berry, sea buckthorn, hemp seed oil, canola oil, walnuts, hazelnuts
Omega-6 fatty acid
Linoleic acids: Vegetable oil, salad dressing, nuts
Gamma linolenic acid: Black currant oil, evening primrose oil, borage oil
Conjugated linoleic acid
Milk, meats (kangaroo meat), grass fed ruminants, egg yolk, fish, fresh ground beef, butter fat, plain yogurt, cultured buttermilk, custard style yogurt, cheddar cheese
Medium chain triglycerides
Animal fat, palm oil, coconut oil, cocoa butter
Phytosterols
Wheat germ, corn oil, canola oil, almonds, brussels, sprouts, flaxseed, peanut butter, cauliflower, olive oil, sesame seeds
5 Roles of functional lipids on health
5.1 Obesity
Obesity and overweight are increasing in epidemic proportions throughout the developed and developing world and are one of the most important growing threats to the health of populations. Obesity management is a modern challenge especially in most of the developed countries and there is a need for effective weight loss solution to avoid adverse consequences. Eating a healthy balanced diet or increasing physical activity appears quite difficult for many individuals to follow. Many studies investigating the effects of functional lipids on weight loss show impressive results. In a three week study in twenty severely obese women, addition of ω-3 PUFA to a very low calorie diet (VLCD) resulted in greater weight and hip circumference losses, and a greater increase in fatty acid oxidation and in ketogenesis compared to control group undergoing the VLCD only (Kunesova et al., 2006). Fish oil improved satiety in overweight and obese volunteers (Parra et al., 2008). In young overweight subjects, 8 weeks of a calorie-restricted diet that included fish or supplemental fish oil resulted in a greater reduction of adipose tissue and of weight than in the calorie-restricted control group (Thorsdottir et al., 2007).
There are some studies which suggest that CLA supplementation reduces body weight, adiposity and plasma leptin concentration (Smedman and Vessby, 2001). However, although such effects are seen commonly in laboratory animals, the findings in humans are less consistent and less significant (Ryder et al., 2001). A double-blind, placebo-controlled randomized trial in 26 men and 28 women who received 1.8 g CLA (mixture of equal amounts of c9, t11 and t10, c12) or placebo per day or 3.6 g CLA (mixture of equal amounts of c9, t11 and t10, c12) or placebo per day, showed that appetite (hunger, satiety and fullness) was favourably and dose-independently suppressed by CLA for 13-weeks consumption but it did not translate into in a lower energy intake at breakfast or an improved body-weight maintenance after weight loss (Kamphuis et al., 2003). Another double-blind design on 22 volunteers indicated that supplementation with 0.7–1.4 g CLA for 4–8 weeks may modulate body fat and serum lipids in humans (Mougios et al., 2001). Larsen et al. (2006) in their one year long double-blind study on one hundred one subjects found that 3.4 g CLA (39% c9, t11 and 41% t10, c12) per day did not prevent weight or fat mass regain in a healthy obese population. In a bicentric, placebo-controlled, double-blind, randomized study, consumption of a drinkable dairy product containing up to 3 g of CLA isomers (c9, t11 or t10, c12) for 18 weeks had no statistically significant effect on body composition in overweight, middle-aged men and women (Malpuech-Brugere et al., 2004). The above data depict the less significant and inconsistent effects of CLA on body weight and composition in humans.
Unlike long-chain triglycerides, MCTs are energetically less dense and provide on average 8.0 kcal/g of metabolized energy as compared with an average of 9.0 kcal provided by long-chain triglycerides (LCTs). The rapid oxidation of MCTs promotes greater ketone body formation which can also be used by the brain as energy source, decreases hunger and increases protein synthesis (Baba et al., 1982; Hoffer et al., 1984; Bach et al., 1996). Increased thermo genesis promoted by MCTs has a lipolytic effect (Krotkiewski, 2001) and so can improve the long-term success of diet therapy of obese patients. In a randomized double-blind study on sixty-six obese women with body mass index (BMI) > 30 kg/m2, 22 were randomly assigned to the control group (VLCD with low fat content) and the remaining 44 were pair-matched VLCD + MCT or VLCD + LCT for a period of four weeks. The MCT group, showed a greater decrease of body fat, body weight and lower intensity of hunger feelings. These changes were observed only for a period of two weeks and gradually declined, indicating subsequent metabolic adaptation (Krotkiewski, 2001). Phytosterols have also shown a positive effect in decreasing obesity in animals (Awika and Rooney, 2004; Ebine et al., 2006; Thornton et al., 2011). However, there is a lack of information about findings in humans.
5.2 Plasma lipid concentrations
The plasma lipid profile is a very important factor that has to be taken into account while considering dietary fat and quality. Studies suggest the beneficial effects of long-chain ω-3 PUFA in preventing sudden death following myocardial infarction (Lee and Lip, 2003; Leaf et al., 2003) and that they may even be superior to statins in secondary prevention (Bhatnagar and Durrington, 2003). Omega-3 fatty acids co-administered with statins have synergistic and additive effects on plasma lipids (Vrablik et al., 2009). In general PUFA are considered as hypocholesterolemic and hypotriglyceridemic compared to saturated fatty acids (Mensink et al., 2003), but different PUFAs have different effects. For example LA is cholesterol and LDL cholesterol lowering whereas marine ω-3 PUFAs are not. There are studies which show a cardio protective effect (such as reduction in hypertryglyceridemia) of ω-3 (60% ω-3 PUFA content, EPA/DHA = 3/2) fatty acids in the patients on haemodialysis (Taziki et al., 2007) and with chronic renal failure (Svensson et al., 2004). In these studies the consumption of 2 g of PUFA (ω-3) per day for 12 weeks (Taziki et al., 2007) or 2.4 g for 8 weeks (Svensson et al., 2004), respectively, resulted in a significant increase in high-density lipoprotein cholesterol levels and a significant decrease in serum triglyceride levels without any changes in total cholesterol or low-density lipoprotein cholesterol levels.
Some studies suggest that MCTs in the diet decrease cholesterol and triglyceride concentrations (Marten et al., 2006), but other studies have indicated that diets containing MCT oil can increase blood cholesterol levels (Cater et al., 1997). The safety of medium chain triacylglycerol in dietary oil has been debated, and related effects on cholesterol metabolism remain unclear (Kitts, 2005). In most of the human studies with CLA, no major effect on blood lipid concentrations was seen (Terpstra, 2004). Phytosterols even at very low dose of 0.45 g/day (as free sterol) were found to lower the blood total cholesterol and remnant-like lipoprotein, cholesterol concentrations which may be helpful in reducing the risk of CHD in the population (Seki et al., 2003). Law (2000) in his review has mentioned that margarines containing plant sterols and stanols play an important role in the prevention of ischaemic heart disease. In a meta-analysis it has been found that plant sterols/stanols added to fat spreads, mayonnaise, salad dressings, milk, or yogurt were more effective in reducing LDL cholesterol levels compared to when plant sterols/stanols were incorporated into other products, such as chocolate, juice, cheese and cereal bars (AbuMweis et al., 2008). Eussen et al. (2011) in his analysis found that phytosterol/-stanol-enriched margarines can modestly reduce serum total cholesterol in the community. The effectiveness of these margarines cannot equalize the effect of cholesterol lowering drugs, but they can act additively.
5.3 Depression
Depression is a condition in which the patient experiences a variety of symptoms such as feeling of worthlessness, helplessness or hopelessness, fatigue and even suicidal thoughts, and is a cause of concern which is increasing all over the world. According to WHO, depression affects 121 million people worldwide and is considered as the fourth largest contributor to global burden of disease (WHO, 2011). Fatty acids play an important role in brain cell structure as 36–60% of nervous tissue in brain are lipids which include glycerophospholipids rich in DHA and AA, sphingolipids, cholesterol and its esters (McNamara and Carlson, 2006) which influence the activities of membrane-linked functional molecules. In the brain DHA plays significant role in (1) membrane related events such as regulation of membrane bound enzymes and glucose uptake, signal transduction, regulation of dopaminergic and serotonergic neurotransmission, (2) metabolic events such as regulation of synthesis of eicosanoids derived from AA, (3) cellular events such as regulation of neuron size and nerve growth factor, stimulation of cell membrane expansion by action on syntaxin and stimulation of neurite growth in hippo campal cells and (4) regulation of gene expression (Sinclair et al., 2007). There are various epidemiological studies which show an inverse relationship between the consumption of ω-3 fatty acids and incidence of depression (Stahl et al., 2008). The proposed mechanism of action of ω-3 PUFA in mood disorders includes effects on the biophysical properties of synaptic membranes which affects neurotransmitter receptors, and effects on inflammatory responses involving eicosanoids (Sinclair et al., 2007; Stahl et al., 2008). Phospholipids are essential for neuronal and synaptic structures. It plays important roles in the signal transduction responses to dopamine, serotonin, acetyl choline and glutamate (Horrobin, 2001). The cyclic adenosine monophosphate (cAMP) signal transduction suggests that depression may be caused by an impaired phospholipids metabolism (Horrobin and Bennett, 1999). The first trial of EPA (purified ethyl eicosapentaenoate at a dose 4 g daily) showed marked benefits in treating depression (Puri, 2005). After 9 months of treatment, symptoms such as low esteem, insomnia, sadness, inner tension, poor appetite, suicidal thoughts had disappeared altogether. Low dietary intake and/or tissue levels of ω-3 PUFAs especially lower DHA is associated with both non-puerperal and post partum depression (Levant, 2010).
5.4 Alzheimer's disease
Alzheimer's disease is a progressive neurodegenerative disorder which is characterized clinically by the formation of amyloid plaque in the brain and nerve cell degeneration (Maccioni et al., 2001). Data from a cross sectional study among 1613 subjects aged 45 to 70 years showed that dietary consumption of marine ω-3 PUFA (EPA and DHA) was inversely related to the risk of impaired overall cognitive function (memory function, cognitive flexibility) and speed of cognitive process (Kalmijn et al., 2004). Epidemiological studies suggest a protective role of ω-3 PUFA against Alzheimer's disease (Morris et al., 2003; Barberger- Gateau et al., 2011). In humans, fish consumption or administration of DHA has been associated with cognitive improvement in some (Barberger-Gateau et al., 2002) but not all, studies (Morley and Banks, 2010). Fotuhi et al. (2009) conducted a systematic review of the literature to establish the association between eating fish (a source of long-chain ω-3 fatty acids) or taking long-chain ω-3 fatty acid supplements and the risk of cognitive decline or Alzheimer's disease. It has been reported in this review that long-chain ω-3 fatty acids play a role in slowing cognitive decline in elderly individuals without dementia, but not for the prevention or treatment of dementia (including Alzheimer's disease). No significant effect of EPA (500 mg for 12 weeks) was found on the cognition of patients with Alzheimer's disease (Boston et al., 2004). Several clinical trials investigating the effects of ω-3 fatty acid supplementation in Alzheimer's disease suggest that the beneficial effects of ω-3 fatty acid supplementation may depend on the dosages, the ratio of long-chain ω-3 to ω-6 fatty acids (Fotuhi et al., 2009), stage of disease and apolipoprotein E status (Jicha and Markesbery, 2010). More clinical studies are required to fully elucidate the roles and utility of EPA and DHA in the treatment of Alzheimer's disease.
5.5 Parkinson's disease
Parkinson's disease is a brain disorder that results from nerve damage in certain regions of the brain causing muscle rigidity, resting tremor, gait disturbance and postural instability (Olanow and Tatton, 1999; Losso, 2003). An immunohistological study found that a small synaptic protein called alpha-synuclein, responsible for neurodegeneration is the main component of Lewy bodies in the brain of Parkinson's disease patients (Spillantini et al., 1997). Oxidative stress and mitochondrial dysfunction are the contributing factors of Parkinson's disease (Miller et al., 2009; Su et al., 2010). De Lau et al. (2005) in the Rotterdam study suggested that high intake of unsaturated fatty acids might protect against Parkinson disease but Chen et al. (2003) did not find any association between ω-3 PUFA intake and Parkinson's disease.
5.6 Bone health
Bone is a living tissue which is constantly renewed and bone health plays a very important role in providing mobility and protection from falls and injury. Long chain PUFA have been found to be beneficial as they play an important role in bone metabolism and in bone health. Omega-3 fatty acids have been shown to enhance the activity of osteoblasts and to inhibit the activity of osteoclasts (Watkins et al., 2003; Sun et al., 2003). Osteoblasts produce and mineralize while osteoclasts cause bone resorption; both are involved in bone modelling and remodelling and function in a coordinated way throughout life. Activities of bone formation and bone resorption are locally regulated by eicosanoids, cytokines and growth factors and systemically through hormones such as thyroid stimulating hormone, estrogen, progesterone and testosterone (Ng et al., 1997; Hadjidakis and Androulakis, 2006). Leukotrienes are the stimulators of bone resorption (Gallwitz et al., 1993) and they inhibit osteoblast proliferation (Ren and Dziak, 1991). Numerous studies have shown that prostaglandins stimulate bone formation as well as bone resorption (Kawaguchi et al., 1995). PGE2 synthesized from AA is a potent stimulator of bone resorption and also influences the production and action of insulin-like growth factor which is responsible for new bone formation and matrix production during osteoclastic bone resorptive activity (Linkhart et al., 1996; Watkins et al., 2001). Some studies suggest that lower concentration of PGE2 promote bone formation while higher concentrations inhibit it (Raisz and Fall, 1990; Watkins et al., 1997). The ratio between ω-6 and ω-3 fatty acids also plays an important role in bone health (Watkins et al., 2006). Among 1532 community-dwelling men and women aged 45–90 years, a significant inverse association between the ratio of dietary LA to ALA and bone mineral density at the hip in men, in women not using hormone therapy, and in women using hormone therapy was found. A higher ratio of ω-6 to ω-3 fatty acids was associated with lower bone mineral density at the hip in both sexes (Weiss et al., 2005). A controlled feeding study in humans (n = 23) evaluating the effect of dietary plant-derived ω-3 PUFA on bone turnover, showed no difference in bone specific alkaline phosphatase (BSAP) in individuals consuming the ALA-rich diet and those consuming an American diet or a LA-rich diet (Griel et al., 2007). The concentration of N-telopeptides (NTx) was positively correlated with the pro-inflammatory cytokine tumour necrosis factor (TNF) alpha for all three diets. It was reported that the plant ω-3 PUFA ALA may have a protective effect on bone metabolism via a decrease in bone resorption in the presence of consistent levels of bone formation (Griel et al., 2007). Fish consumption was associated with very small differences in bone mineral density and had no association with hip fracture risk (Virtanen et al., 2010). CLA also has been reported to improve bone mass but available studies are inconsistent which might be due to interaction between CLA and dietary calcium (Li and Watkins, 1998; Rahman et al., 2007).
There are two major factors influencing the risk of the development of osteoporosis, firstly peak bone mass and secondly the rate at which bone loss occurs in later years. Calcium and magnesium are biologically interlocked with each other and their deficiency affects osteoclastic and osteoblastic activities (Thomson Healthcare, 2001). In animals contradictory results were found on the effect of PUFA on calcium absorption but in humans there are some older studies which showed positive effect of MCTs on calcium absorption (Kehayoglou et al., 1973; Tantibhedhyangkul and Hashim, 1978) although not all studies find this (Huston et al., 1983; Haderslev et al., 2000).
5.7 Atopic dermatitis
Atopic dermatitis (AD) is a chronic itchy, inflammatory skin disease (Vender, 2002) which may be due to a partial enzymatic defect in delta-6 desaturase (Kerscher and Korting, 1992) and is characterized by the symptoms like itching and dryness, redness, swelling, flaking, blistering, cracking, bleeding or skin discolouration. For the past decades, the prevalence of atopic dermatitis is on the rise (Willemaers et al., 1998). EFAs play a vital role in skin structure and physiology. Decreased concentrations of GLA and DGLA may lead to changed skin permeability and in turn contribute to AD (Horrobin, 1992, 2000). There are studies, which suggest that the ω-6 fatty acid GLA is effective for the treatment of atopic dermatitis. A review identified by 12 trials of oral or topical borage oil for the treatment of atopic dermatitis and one preventive trial and the majority of studies showed at least a small degree of efficacy and can be used as an alternative treatment (Foster et al., 2010). A randomized controlled trial of 500 mg/day evening primrose oil (a source of GLA) for 5 months in patients with atopic dermatitis reported that 24 (96%) in the GLA group and 8 (32%) patients of placebo group showed improvements in atopic dermatitis (Senapati et al., 2008). Dietary studies have found an inverse association of consumption of milk with the dermatitis symptoms (Perkin and Strachan, 2006; Kummelings et al., 2007). Pomegranate seed oil which also promotes regeneration and strengthening of epidermis by stimulating keratinocyte proliferation (Aslam et al., 2006) is also a good source of CLA (Hora et al., 2003; McFarlin et al., 2009) and associated with specific skin repair activity (Aslam et al., 2006).
6 Side effects
6.1 Omega-3 fatty acids
Omega-3 fatty acids presented as fish oil are generally well tolerated but may cause some side effects. These are usually mild and transient mostly including a fishy after taste and gastrointestinal disturbances. Other symptoms include nausea, heartburn or indigestion, and diarrhoea. These effects are mostly dose-dependent. Some concern has been raised that ω-3 fatty acids increase the risk of bleeding, although a number of studies suggest that this is unlikely to occur (Peet and Horrobin, 2002; Su et al., 2003) even at a high dosage and in combination with other anticoagulant medications (Eritsland et al., 1996). Nevertheless doses greater than 3 g/day of fish oil (or high dietary fish intake) may inhibit platelet aggregation and increase bleeding (Knapp, 1997), so care should be taken before the consumption of ω-3 fatty acids with antithrombotic therapies. DHA has six double bonds and five methylene carbons and it is highly prone to peroxidation (Cho et al., 1987; Kawai et al., 2006). Exposure to DHA is known to block several ionic currents. Peroxidation products of DHA change cell membrane fluidity and thereby modify the activity of ion channels (Jude et al., 2003). Meydani et al. (1991) indicated that although long-term (>3 months) fish oil supplementation may be beneficial in reducing plasma total triglycerides; susceptibility of plasma lipids to free radical attack is potentiated.
6.2 Omega-6 fatty acids
An improper ratio of ω-6 to ω-3 fatty acids may promote problems like inflammation as an increased amount of ω-6 fatty acids produces eicosanoids which are responsible for the formation of thrombus and atheromas and which play a role in inflammatory disorders (Simopoulos, 2008a,b).
6.3 Conjugated linoleic acid
Clarinol is a mixture of CLA isomers and is produced from sunflower oil; it includes some cis-9, trans-11 CLA. Clarinol was found to cause liver enlargement in female rats but this effect was reported to be reversible (Hagan, 2002) and no major liver abnormalities have been reported in humans consuming CLA although most of the studies were of short duration (Larsen et al., 2003). Insulin resistance and increased oxidative stress have been reported with the consumption of trans-10, cis-12 CLA but not with a mixed preparation of cis-9, trans-11 and trans-10, cis-12 CLA (Ritzenthaler et al., 2001; Riserus et al., 2004).
6.4 Medium chain triglycerides
MCTs are well tolerated with no serious demonstrable side effects. Ingestion of larger amounts (>25–30 g) of MCTs causes adverse gastrointestinal symptoms, including nausea, vomiting, abdominal cramps, gastrointestinal discomfort, bloating, and osmotic diarrhoea (Jeukendrup and Aldred, 2004). Due to hyperosmolarity, MCTs cause a large influx of fluid into the large intestines and initiate gastrointestinal problems (Bainbridge et al., 1999) and so it is generally advised to start MCTs treatment gradually and to increase the amount over time.
6.5 Phytosterols
Phytosterols are generally considered safe and the side effect associated with phytosterol and stanol is their interference with the absorption of beta-carotenoids, while levels of fat soluble vitamins A, D, K1 and E did not change (Hendriks et al., 2001; Judd et al., 2002). Since beta-carotenoids are precursors of vitamin A, reduced intake could affect vitamin A status. Thus it is advised that pregnant women and children do not consume supplemental phytosterols (ACNFP, 2002b). It seems unlikely that phytosterols induced beta carotenoid deficiency is of much concern in other subgroups.
7 Conclusion and future perspectives
Functional lipids are healthy dietary components with the potential for influencing human health, lowering risk of diseases and improving quality of life. As mentioned in this review, the health benefits could include reduced susceptibility to heart disease, obesity, depression, Alzheimer's disease, Parkinson's disease and atopic dermatitis. They have been proved to be effective and are easily available and economical to include in the food chain/diet. Nevertheless, more studies are required to investigate and fully elucidate the doses, dietary intake and the efficacy and mechanisms of various functional lipids discussed. An understanding of the development of valid disease biomarkers, clinical trials, and long-term effects of these lipids in humans awaits further study.
References
- Plant sterols/stanols as cholesterol lowering agents: a meta-analysis of randomized controlled trials. Food Nutr. Res. 2008
- [CrossRef] [Google Scholar]
- ACNFP, 2002a. Cholesterol lowering: foods with added plant sterols. London, pp. 1–2. Cited from <http://www.food.gov.uk/multimedia/pdfs/acnfpcholesterolfactsheet.pdf>.
- ACNFP, 2002b. Extension in the range of uses of phytosterol esters in: opinion on an application under the novel food regulation to extend the range of uses of phytosterol esters in food products. UK. pp. 1–10. Cited from <http://www.food.gov.uk/multimedia/pdfs/unilever.pdf>.
- N-3 polyunsaturated fatty acids and the cardiovascular system. Curr. Opin. Lipidol.. 2000;11(1):57-63.
- [Google Scholar]
- Pomegranate as a cosmeceutical source. Pomegranate fractions promote proliferation and procollagen synthesis and inhibit matrix metalloproteinase-1 production in human skin cells. J. Ethnopharmacol.. 2006;103:311-318.
- [Google Scholar]
- Sorghum phytochemicals and their potential impact on human health. Phytochemistry. 2004;65:1199-1221.
- [Google Scholar]
- Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with a diet containing medium chain triglycerides. Am. J. Clin. Nutr.. 1982;35:678-682.
- [Google Scholar]
- The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy. J. Lipid Res.. 1996;37:708-726.
- [Google Scholar]
- Dietary omega 3 polyunsaturated fatty acids and Alzheimer's disease: interaction with apolipoprotein E genotype. Curr. Alzheimer Res.. 2011;8:479-491.
- [Google Scholar]
- Omega-3 fatty acids: their role in the prevention and treatment of atherosclerosis related risk factors and complications. Int. J. Clin. Pract.. 2003;57:305-314.
- [Google Scholar]
- Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum. Dev.. 2007;83:279-284.
- [Google Scholar]
- Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr.. 2011;93:950-962.
- [Google Scholar]
- Ethyl-EPA in Alzheimer's disease – a pilot study. Prostaglandins Leukot. Essent. Fatty Acids. 2004;71:341-344.
- [Google Scholar]
- Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care. 2002;5:127-132.
- [Google Scholar]
- Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:137-144.
- [Google Scholar]
- Cater, N.B., Heller, H.J., Denk, M.A., l997. Comparison of the effects of medium-chain triacylglycerols palm oil, and high oleic acid sunflower oil on plasma triacylglycerol fatty acids and lipid and lipoprotein concentrations in humans. Am. J. Clin. Nutr. 65, 41–45.
- Dietary intakes of fat and risk of Parkinson's disease. Am. J. Epidemiol.. 2003;157:1007-1014.
- [Google Scholar]
- Alteration of prostaglandin and agonist responsiveness by n-6 polyunsaturated fatty acids in endometrial cells from late-gestation ewes. J. Endocrinol.. 2004;182:249-256.
- [Google Scholar]
- Autoxidation of ethyl eicosapentaenoate and docosahexaenoate. J. Am. Oil Chem. Soc.. 1987;64:876-879.
- [Google Scholar]
- Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040-2045.
- [Google Scholar]
- Dratz, E.A., Holte, L.L., 1993. The molecular spring model for the function of docosahexaenoic acid (22:6, ω-3) in biological membrane. In: Sinclair, A., Gibson, R. (Eds.), Essential Fatty Acids and Eicosanoids: Invited Papers from the Third International Congress. AOCS Press, Champaign, IL, pp. 122–127.
- Plant stanol ascorbate esters reduce body weight gain through decreased energy absorption in hamsters. Int. J. Obes.. 2006;30:751-757.
- [Google Scholar]
- Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot. Essent. Fatty Acids. 2007;76:189-203.
- [Google Scholar]
- Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am. J. Cardiol.. 1996;77:31-36.
- [Google Scholar]
- Dose-dependent cholesterol-lowering effects of phytosterol/phytostanol-enriched margarine in statin users and statin non-users under free-living conditions. Public Health Nutr.. 2011;14:1823-1832.
- [Google Scholar]
- FAO, 2008. Fats and fatty acids in human nutrition. Report of an expert consultation. Food and Nutrition paper, 10–14 November, Geneva. pp. 1–161.
- Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nat. Clin. Pract. Neurol.. 2009;5:140-152.
- [Google Scholar]
- 5-Lipoxygenase metabolites of arachidonic acid stimulate isolated osteoclasts to resorb calcified matrices. J. Biol. Chem.. 1993;268:10087-10094.
- [Google Scholar]
- An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. J. Nutr.. 2007;16:1-2.
- [Google Scholar]
- Absorption of calcium and magnesium in patients with intestinal resections treated with medium chain fatty acids. Gut. 2000;46:819-823.
- [Google Scholar]
- Hagan, S.O., 2002. Toxicology studies on clarinol. In: Perspectives on Conjugated Linoleic Acid Research, Current Status and Future Directions. Lister Hill Auditorium, Bethesda, Maryland, p. 14.
- N-3 fatty acids and serum lipoproteins: human studies. Am. J. Clin. Nutr.. 1997;65:1645S-1654S.
- [Google Scholar]
- One-year follow-up study on the use of a low fat spread enriched with plant sterol-esters. Ann. Nutr. Metab.. 2001;45:100.
- [Google Scholar]
- Metabolic effects of very low calorie weight reduction diets. J. Clin. Invest.. 1984;73:750-758.
- [Google Scholar]
- Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food. 2003;6:157-161.
- [Google Scholar]
- Nutritional and medical importance of gamma-linolenic acid. Prog. Lipid Res.. 1992;31:163-194.
- [Google Scholar]
- Essential fatty acid metabolism and its modification in atopic eczema. Am. J. Clin. Nutr.. 2000;71:367S-372S.
- [Google Scholar]
- Phospholipid metabolism and depression: the possible roles of phospholipase A2 and coenzyme A-independent transacylase. Hum. Psychopharmacol.. 2001;16:45-52.
- [Google Scholar]
- Depression and bipolar disorder: relationships to impaired fatty acid and phospholipids metabolism and to diabetes, cardiovascular disease, immunological abnormalities, cancer, ageing and osteoporosis. Possible candidate genes. Prostaglandins Leukot. Essent. Fatty Acids. 1999;60:217-234.
- [Google Scholar]
- Nutrient and mineral retention and vitamin D absorption in low-birth-weight infants: effect of medium-chain triglycerides. Pediatrics. 1983;72:44-48.
- [Google Scholar]
- Low liver conversion rate of α-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. J. Lipid Res.. 2006;47:1812-1822.
- [Google Scholar]
- Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr.. 2003;143:S1-S8.
- [Google Scholar]
- Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer. 1994;233:1050-1054.
- [Google Scholar]
- Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am. J. Clin. Nutr.. 2008;87:1981S-1990S.
- [Google Scholar]
- Fat supplementation, health, and endurance performance. Nutrition. 2004;20:678-688.
- [Google Scholar]
- Omega-3 fatty acids: potential role in the management of early Alzheimer's disease. Clin. Interv. Aging. 2010;5:45-61.
- [Google Scholar]
- Plant sterol esters lower plasma lipids and most carotenoids in mildly hypercholesterolemic adults. Lipids. 2002;37:33-42.
- [Google Scholar]
- Peroxidation of docosahexaenoic acid is responsible for its effects on ITO and ISS in rat ventricular myocytes. Br. J. Pharmacol.. 2003;139:816-822.
- [Google Scholar]
- Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Am. Acad. Neurol.. 2004;62:275-280.
- [Google Scholar]
- Effect of conjugated linoleic acid supplementation after weight loss on appetite and food intake in overweight subjects. Eur. J. Clin. Nutr.. 2003;57:1268-1274.
- [Google Scholar]
- Importance and production of omega-3 fatty acids from natural sources. Int. Food Res. J.. 2011;18:493-499.
- [Google Scholar]
- The role of prostaglandins in the regulation of bone metabolism. Clin. Orthop. Relat. Res.. 1995;313:36-46.
- [Google Scholar]
- Formation of Nepsilon-(succinyl) lysine in vivo: a novel marker for docosahexaenoic acid-derived protein modification. J. Lipid Res.. 2006;47:1386-1398.
- [Google Scholar]
- The effect of medium-chain triglyceride on 47 calcium absorption in patients with primary biliary cirrhosis. Gut. 1973;14:653-656.
- [Google Scholar]
- Treatment of atopic eczema with evening primrose oil: rationale and clinical results. Clin. Invest.. 1992;70:167-171.
- [Google Scholar]
- Kitts, D.D., 2005. Toxicity and safety of fats and oils. In: Fereidoon, Shahidi (Ed.), Bailey's Industrial Oil and Fat Products, sixth edition, six volume set. University of British Columbia, Vancouver, British Columbia, Canada, pp. 513–564.
- Dietary fatty acids in human thrombosis and haemostasis. Am. J. Clin. Nutr.. 1997;65:1687S-1698S.
- [Google Scholar]
- Resolvins and protectins: mediating solutions to inflammation. Br. J. Pharmacol.. 2009;158:960-971.
- [Google Scholar]
- Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747-2757.
- [Google Scholar]
- Value of VLCD supplementation with medium chain triglycerides. Int. J. Obes.. 2001;25:1393-1400.
- [Google Scholar]
- Consumption of organic foods and risk of atopic disease during the first two year of life in Netherland. Br. J. Nutr.. 2007;29:1-8.
- [Google Scholar]
- The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol. Res.. 2006;55:63-72.
- [Google Scholar]
- Efficacy and safety of dietary supplement containing Conjugated Linoleic Acid (CLA) for the treatment of obesity-evidence from animal and human studies. J. Lipid Res.. 2003;44:2234-2241.
- [Google Scholar]
- Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am. J. Clin. Nutr.. 2006;83:606-612.
- [Google Scholar]
- Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646-2652.
- [Google Scholar]
- The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. Q. J. Med.. 2003;96(7):465-480.
- [Google Scholar]
- N-3 (omega-3) fatty acids in postpartum depression: implications for prevention and treatment. Depress. Res. Treat.. 2010;2011:1-16.
- [Google Scholar]
- Conjugated linoleic acids alter bone fatty acids composition and reduce ex vivo prostaglandins E2 biosynthesis in rats fed n-6 and n-3 fatty acids. Lipids. 1998;33:417-425.
- [Google Scholar]
- Stanol/sterol ester-containing foods and blood cholesterol levels. Circulation. 2001;103:1177-1179.
- [Google Scholar]
- Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19:1-12.
- [Google Scholar]
- Targeting excessive angiogenesis with functional foods and nutraceuticals. Trends Food Sci. Technol.. 2003;14:455-468.
- [Google Scholar]
- The molecular bases of Alzheimer's disease and other neurodegenerative disorders. Arch. Med. Res.. 2001;32:367-381.
- [Google Scholar]
- Effects of two conjugated linoleic acid isomers on body fat mass in overweight humans. Obes. Res.. 2004;12:591-598.
- [Google Scholar]
- Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risk in CD-1 mice. Br. J. Nutr.. 2009;102:54-59.
- [Google Scholar]
- Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:329-349.
- [Google Scholar]
- Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am. J. Clin. Nutr.. 2003;77:1146-1155.
- [Google Scholar]
- Effect of long-term fish oil supplementation on vitamin E status and lipid peroxidation in women. J. Nutr.. 1991;121:484-491.
- [Google Scholar]
- Oxidative and inflammatory pathways in Parkinson's disease. Neurochem. Res.. 2009;34:55-65.
- [Google Scholar]
- Moreau, R.A., 2011. An overview of functional lipids. In: 102nd AOCS Annual Meeting and Expo, Duke Energy Center, Cincinnati, Ohio, USA.
- Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol.. 2003;60:940-946.
- [Google Scholar]
- Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J. Nutr. Biochem.. 2001;12:585-594.
- [Google Scholar]
- Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng.. 2005;100:152-157.
- [Google Scholar]
- Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci.. 1999;22:123-144.
- [Google Scholar]
- A diet rich in long chain omega 3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51:676-680.
- [Google Scholar]
- A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry. 2002;59:913-919.
- [Google Scholar]
- Which aspect of farming lifestyle explains the inverse association with childhood allergy. J. Allergy Clin. Immunol.. 2006;117:1374-1381.
- [Google Scholar]
- Review plant sterols: biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric.. 2000;80:939-966.
- [Google Scholar]
- Nutrients, Stress and Medical Disorder, Nutrition and Health: Lipids and Depression. Totowa, NJ: Human Press Inc.; 2005.
- Conjugated linolenic acid protects against age associated bone loss in C57BL/6 female mice. J. Nutr. Biochem.. 2007;18:467-474.
- [Google Scholar]
- Biphasic effects of prostaglandin E2 on bone formation in cultured fetal rat calvariae: interaction with cortisol. Endocrinology. 1990;126:1654-1659.
- [Google Scholar]
- Effects of leukotrienes on osteoblastic cell proliferation. Calcif. Tissue Int.. 1991;49:197-201.
- [Google Scholar]
- Metabolic effects of conjugated linoleic acid in humans: the Swedish experience. Am. J. Clin. Nutr.. 2004;79:1146S-1148S.
- [Google Scholar]
- Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J. Nutr.. 2001;131:1548-1554.
- [Google Scholar]
- Concepts and strategy of functional food science: the European perspective. Am. J. Clin. Nutr.. 2000;71:1660S-1665S.
- [Google Scholar]
- Lipid mediators of inflammatory and immune reactions. J. Parenter. Enteral Nutr.. 1988;12:S37-S42.
- [Google Scholar]
- Isomerspecific antidiabetic properties of CLA. Improved glucose tolerance, skeletal muscle insulin and VCP-2 gene expression. Diabetes. 2001;50:1149-1157.
- [Google Scholar]
- The role of omega-3 long chain polyunsaturated fatty acids in health and disease of retina. Prog. Retin. Eye Res.. 2005;24:87-138.
- [Google Scholar]
- Lipids of nervous tissue: composition and metabolism. Prog. Lipid Res.. 1985;24:69-176.
- [Google Scholar]
- Identification of conjugated linoleic acid isomers in cheese by gas chromatography, silver ion high performance liquid chromatography and mass spectral reconstructed ion profiles. Comparison of chromatographic elution sequences. Lipids. 1999;33:963-971.
- [Google Scholar]
- Effects of phytosterol ester-enriched vegetable oil on plasma lipoproteins in healthy men. Asia Pac. J. Clin. Nutr.. 2003;12:282-291.
- [Google Scholar]
- Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J. Dermatol. Venereol. Leprol.. 2008;74:447-452.
- [Google Scholar]
- Omega 3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr.. 1991;54:438-463.
- [Google Scholar]
- Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr.. 2002;21:495-505.
- [Google Scholar]
- Importance of the ratio of omega 6/omega 3 essential fatty acids: evolutionary aspects. World Rev. Nutr. Diet.. 2003;92:1-174.
- [Google Scholar]
- The omega 6/omega 3 fatty acids ratio, genetic variation, and cardiovascular disease. Asia Pac. J. Clin. Nutr.. 2008;17:131-134.
- [Google Scholar]
- The importance of omega 6/omega 3 fatty acids in cardiovascular diseases and other chronic diseases. Exp. Biol. Med.. 2008;233:674-688.
- [Google Scholar]
- Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Exp. Biol. Med.. 2010;235:785-795.
- [Google Scholar]
- Workshop statement on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:119-121.
- [Google Scholar]
- Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac. J. Clin. Nutr.. 2007;16:391-397.
- [Google Scholar]
- Ready absorption of medium chain triglyceride in the steatorrhoea syndrome. Gut. 1968;9:28-33.
- [Google Scholar]
- The role of omega-3 fatty acids in mood disorders. Curr. Opin. Investig. Drugs. 2008;9:57-64.
- [Google Scholar]
- Chemically defined structured lipids: current status and future directions in gastrointestinal diseases. Int. J. Colorectal Dis.. 1999;14:79-85.
- [Google Scholar]
- Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim. Biophys. Acta. 2010;1802:135-142.
- [Google Scholar]
- Omega-3 fatty acids in major depressive disorder: a preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharm.. 2003;13(4):267-271.
- [Google Scholar]
- Dietary n-3 fatty acids decreases osteoclastogenesis and loss of bone mass in ovariectomized mice. J. Bone Miner. Res.. 2003;18:1206-1216.
- [Google Scholar]
- The effect of n-3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am. J. Kidney Dis.. 2004;44:77-83.
- [Google Scholar]
- Medium-chain triglyceride feeding in premature infants: effects on calcium and magnesium absorption. Paediatrics. 1978;61:537-545.
- [Google Scholar]
- Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother.. 2002;56:215-222.
- [Google Scholar]
- The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J. Kidney Dis. Transplant.. 2007;18:571-576.
- [Google Scholar]
- Effect of conjugated linoleic acid on body composition and plasma lipids in humans: an overview of the literature. Am. J. Clin. Nutr.. 2004;79:352-361.
- [Google Scholar]
- Thomson Healthcare, 2001. PDR for Nutritional Supplements. Medical Economics Company Inc., Montvale, NJ.
- Dietary supplementation with phytosterol and ascorbic acid reduces body mass accumulation and alters food transit time in a diet-induced obesity mouse model. Lipids Health Dis.. 2011;28:107.
- [Google Scholar]
- Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int. J. Obes.. 2007;31:1560-1566.
- [Google Scholar]
- Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res.. 2009;50:1259-1268.
- [Google Scholar]
- Alternative treatments for atopic dermatitis: a selected review. Skin Therapy Lett.. 2002;7:1-8.
- [Google Scholar]
- Fish consumption, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J. Bone Miner. Res.. 2010;25:1972-1979.
- [Google Scholar]
- Omega-3 fatty acids and cardiovascular disease risk: Do we understand the relationship? Physiol. Res.. 2009;58:19-26.
- [Google Scholar]
- WHO, 2011. Depression. Mental Health, pp. 1–2.Cited from <http://www.who.int/mental_health/management/depression/definition/en/>.
- Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J. Nutr.. 1997;127:1084-1091.
- [Google Scholar]
- Bioactive fatty acids: role in bone biology and bone cell function. Prog. Lipid Res.. 2001;40:125-148.
- [Google Scholar]
- Modulatory effect of omega-3 polyunsaturated fatty acids on osteoblast function and bone metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 2003;6:387-398.
- [Google Scholar]
- Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats. J. Nutr. Biochem.. 2006;17:282-289.
- [Google Scholar]
- Ratio of n-6 to n-3 fatty acids and bone mineral density in older adults: the Rancho Bernardo Study. Am. J. Clin. Nutr.. 2005;81:934-938.
- [Google Scholar]
- Antagonistic effects of dietary arachidonic acid and n-3 polyunsaturated fatty acids. J. Nutr.. 1996;12:1086S-1091S.
- [Google Scholar]







