Translate this page into:
Role of aquaporin 9 in hyperglycaemia-induced testicular leydig cell apoptosis
⁎Corresponding authors. thiyagaramesh@gmail.com (Thiyagarajan Ramesh), prahalath@gmail.com (Chidambaram Prahalathan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Hyperglycaemia is an early symptom of diabetes mellitus (DM) and hyperglycaemia-induced apoptosis is the most widely accepted cause for diabetes induced male infertility/subfertility. Aquaporin (AQP) isoforms (AQP0-12), are water channels involved in maintaining water homeostasis and regulate fluid absorption and/or excretion; thus, play a key role in reproductive function. Further, AQPs regulate diabetic complications, apoptosis induced by hyperglycaemia/high glucose (HG) and also involved in insulin regulation. Herein, we investigated AQP 9 role in hyperglycaemia-induced testicular Leydig cell apoptosis. In-vivo and in-vitro role of AQP 9 on hyperglycaemia induced Leydig cell apoptosis was analysed in diabetic rat testis and LC-540 rat Leydig cells by Real Time-PCR, western blotting and siRNA knock-down experiments. In addition, HG effect on viability and morphological changes due to apoptosis in cells were assessed by MTT and fluorescence microscopy. In-vivo and in-vitro AQP 9 expressions were increased in diabetic testicular Leydig cells. Hyperglycaemia caused apoptosis in testicular Leydig cells by increasing Cytochrome c, Bax and decreasing Bcl-2. Furthermore, AQP 9 knock-down resulted in downregulation of pro-apoptotic proteins and in contrast, upregulated anti-apoptotic protein expressions; thereby decreased apoptosis in testicular Leydig cells under hyperglycemia. Our findings collectively suggest, AQP 9 role in hyperglycaemia-induced apoptosis in testicular Leydig cells.
Keywords
Aquaporin 9
Apoptosis
Leydig cells
Hyperglycaemia
Male infertility
- AQP
-
Aquaporin
- DM
-
Diabetes Mellitus
- STZ
-
Streptozotocin
- RT-PCR
-
Real Time Polymerase Chain Reaction
- ROS
-
Reactive Oxygen Species
- MMP
-
Mitochondrial Membrane Potential
- RNS
-
Reactive Nitrogen Species
Abbreviations
1 Introduction
Diabetes mellitus (DM) is a metabolic disorder increasing at an alarming rate over the last few decades with worldwide prevalence of 537 million adults aged from 20 to 79 years and International Diabetes Federation (IDF; Diabetic atlas, 10th Ed; 2021)) has predicted a rise in incidence of diabetes to 643 million by 2030 and 784 million by 2045. DM is characterized by high blood glucose levels (hyperglycaemia) affecting insulin secretion and/or action; persistent hyperglycaemia, either type 1 or 2 diabetes promotes micro or macro vascular complications, including reproductive dysfunction (Alves et al., 2013). However, reduced insulin sensitivity is involved in the pathogenesis leading to vascular complications and also acts as an important therapeutic target (Ormazabal et al., 2018). The key events in development of diabetic complications includes degeneration of lipids, reactive oxygen species (ROS) formation and oxidative stress associated with hyperglycaemia (Kiritoshi et al., 2003, Sureka et al., 2015, Ramesh 2021). Epidemiology of type 1 and 2 diabetes is high among people of reproductive age and is linked with reduction in birth rates and fertility (Kumar et al., 2021). Glucose metabolism in both human and animals is an important event in the process of reproductive function (Alves et al., 2013, Alkharfy et al., 2022, Zhang et al., 2022, Zheng et al., 2022). Dysregulation of blood glucose and hyperglycaemia caused by DM are closely associated with male subfertility leading to reproductive dysfunction (spermatogenesis and steroidogenesis), erectile dysfunction, decreased fertility, and deprived reproductive outcomes (Alves et al., 2013, Zatecka et al., 2021). In parallel, testosterone regulates genes involved in glucose uptake and insulin signalling. Increased GLUT4 expression in muscle cells, adipose tissue and testosterone deficiency may decrease glucose transport and insulin resistance, leading to progression of DM (Mattack et al., 2015). In the recent years, prevalence of testosterone deficiency is common among diabetic men but the exact mechanism of action remains debatable, however, poor diabetes control can lead to male sexual dysfunction.
Streptozotocin (STZ) induction in rats is an extensively used experimental model for type-1 DM. STZ induced chronic hyperglycemia; imbalance of ROS and RNS (Reactive Nitrogen Species) leads to glucotoxicity, also the main pathogenic mechanisms leading to diabetic complications (Li et al., 2018). The primary response to high glucose levels is production of ROS which leads to apoptotic cell death (Li et al., 2018). Hyperglycemia associated oxidative stress and apoptosis leads to the development of diabetic complications viz nephropathy, cardiomyopathy, neuropathy, endothelial and neurological dysfunctions (Alkharfy et al., 2022, Xiang et al., 2022, Zhang et al., 2022). Furthermore, chronic hyperglycemia is also a cause for reproductive complications; increasing apoptosis in testicular cells and oxidative stress leading to infertility/subfertility (Khalil et al., 2021). In testicular cells, HG treatment induced spermatogenic cell damage via increased apoptosis and in turn decreased cell viability (Zheng et al., 2022). Leydig cells treated with high glucose have shown to decrease cell proliferation, increase apoptosis, and cause decreased testosterone secretion (Hu et al., 2021, Wang et al., 2021a).
Aquaporins (AQPs) are water channels containing 13 isoforms (AQP 0–12), belongs to a transmembrane superfamily with differential expression in different cell types including Leydig cells, involved in fluid absorption and/or excretion (Kannan et al., 2022). Water movement across the reproductive tract in male and female is crucial in the maintenance of fertility. Hyperglycemia/high glucose associated diabetes complications, apoptosis, and insulin secretion are all regulated by AQP (Madonna et al., 2020, Aggeli et al., 2021). Under hyper-osmotic stress, a differential expression of AQP 1 and AQP 7 regulates apoptosis in cardio myoblasts (Aggeli et al., 2021). Interestingly, AQP 1 expression in endothelial cells suppressed hyperglycemia-induced apoptosis, increasing mitochondrial ROS (mtROS) (Sada et al., 2016). Furthermore, AQP 4 is involved in controlling neuronal apoptosis pathways in brain-related disorders and ischemic injury (Wang et al., 2021b). Earlier studies also suggests that, AQPs also inhibited cancer progression, migration, invasion and promote cell apoptosis (Liu et al., 2022). It is also interesting that, AQP 8 is involved follicular development of granulosa cells by regulating apoptosis and cell cycle progression (Cao et al., 2021).
Despite intensive research in the field of reproductive sciences in recent years, we are in need to search and identify unknown factors affecting male infertility. These new indicators/biomarkers will specifically allow us to determine male reproductive potential. Since, maintenance and development of the testes is mediated by Leydig cells, disruption of these crucial pathways contributes to testicular dysfunction. AQP 9 is reported to play an important role in cell proliferation and apoptosis. Earlier reports from our laboratory highlight the role of AQP 9 in male reproductive function under hyperglycaemia (Kannan et al., 2022). Hence, the present study is aimed at exploring the potential role of AQP 9 in hyperglycaemia induced testicular apoptosis.
2 Materials and methods
2.1 Chemicals
Streptozotocin (STZ), reagents and chemicals required for in-vivo and in-vitro experiments were procured from HiMedia, Sigma Chemicals and Sisco Research Laboratories.
2.2 In-vivo experimental design
In this study, male albino rats (Wistar strain) weighing 200 ± 25 g were maintained under standard humidity with temperature 25 ± 2 ⁰C and 12 hrs of dark and light cycle. Experimental rats were housed in large cages with free accesses to pelleted diet and water. Our animal experiments were approved by our institution ethical committee BDU/IAEC/P04/2018. Experimental design, duration and treatment is same as described earlier (Kannan et al., 2022). A schematic representation is as shown below in Fig. 1.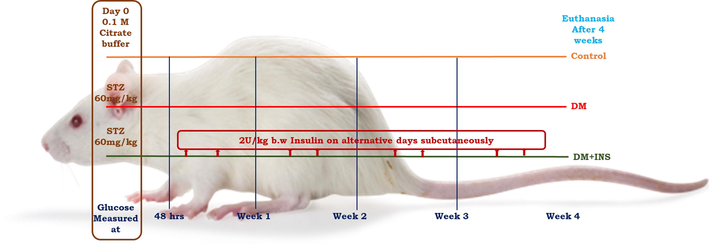
Schematic representation of experimental design and treatment.
Group I: (Controls) citrate buffer (0.1 M) treated animals.
Group II: (DM) animals received intraperitoneal injection of STZ (60 mg/kg; b.w)
Group III (DM + INS) diabetic animals (intraperitoneal injection of STZ (60 mg/kg; b.w) with 2 U/Kg b.w. of insulin
2.3 In-vitro experimental design and transfection
The LC-540 Leydig cells were cultured in Minimum Essential Medium (MEM) (AL047S) with antibiotics and serum; in a CO2 incubator. In-vitro expression of AQPs as a result of hyperglycemia were determined on LC-540 cells treated with different glucose (1 mM, 5.5 mM, 20 mM and 30 mM) concentrations for 24 hrs (5.5 mM as control) and 30 mM mannitol on a serum free medium for 24 hrs as osmotic control. Knock-down experiments on LC-540 cells were carried out with AQP 9 siRNA (Cat.no: SR-NP001-001; Eurogentec) and also NCsiRNA (Sigma Aldrich). The AQP 9 siRNA at a concentration of 60 nM was used to transfect LC-540 cells with RNAiMAX reagent as per the manual.
2.4 Cytotoxicity assay - MTT (3-[4,5-Dimethythiazol-2-yl]-2,5-diphenyltetrazolium bromide;thiazolyl blue)
MTT assay was performed to determine the cell viability of LC-540 cells grown in serum free medium; treated with low glucose (LG;1 mM), normal glucose (NG;5.5 mM), high glucose (HG;20 mM and 30 mM) and 30 mM of mannitol at different time points 12, 24 and 48 hrs. After incubation, 0.5 mg/ml concentration of MTT was added and left for 3–4 hrs. DMSO was added and the absorbance was read using an ELISA reader.
2.5 Dual acridine orange/ethidium bromide (AO/EB) fluorescence staining
AO/EB dual staining were performed to differentiate apoptotic, necrotic and normal cells. Briefly, LC-540 cells were grown in serum free medium were exposed to various glucose concentrations and mannitol for a period of 24 hrs and stained with 10 µM of AO/EB. The images were observed with fluorescence microscope.
2.6 Hoechst 33,258 nuclear damage staining
To assess the nuclear damage; LC-540 cells were grown in serum free medium with varied glucose concentrations (1 mM, 5.5 mM, 20 mM, 30 mM) and 30 mM mannitol for 24 hrs. After the treatment period 2 µg/ml of Hoechst 33,258 were added and observed for nuclear aggregation under fluorescence microscope.
2.7 DCF-DA cellular ROS detection assay
Briefly, LC-540 Leydig cells grown in an incomplete medium on 6-well plates were exposed to different glucose concentrations and mannitol for 24 hrs and exposed to 10 µM DCFDA and observed under fluorescence microscope.
2.8 Cell based mitochondrial membrane potential (MMP) assay
Rhodamine-123 was used at a concentration of 10 µg/ml in cell based MMP (ΔΨm) assay, LC-540 cells treated with glucose and mannitol for 24 hrs and after the treatment period cells were exposed to Rhodamine-123 and observed under fluorescence microscope.
2.9 Tissue and cell lysates preparation
Briefly, tissue homogenate from testes and cell lysates from LC-540 cells were prepared as described earlier (Kannan et al., 2022).
2.10 Isolation of RNA, cDNA synthesis and quantitative real time polymerase chain reaction (qRT-PCR) analysis
In-vivo and in-vitro RNA isolation, and cDNA synthesis were performed using One step RNA TRIzol Reagent and iScript cDNA synthesis kit (Bio-Rad Laboratories) following the manufacturer's protocol. The qRT-PCR was performed using SSO Advanced Universal SYBR Green Supermix according to the manufacturer's protocol (Bio-Rad Laboratories, Inc., USA). The real time gene expression was assessed on Himedia (Insta Q96™) PCR with specific primers for target genes as given in Table 1. Fold differences in target gene expression were calculated using the formula 2ΔΔCt. The gene expressions were normalized with corresponding internal control.
Gene
Forward Primer (5′-3′)
Reverse Primer (5′-3′)
Aquaporin 9
CTCAGTCCCAGGCTCTTCAC
ATGGCTCTGCCTTCATGTCT
Bax
GAGCTGCAGAGGATGATTGCT
GCAAAGTAGAAGAGGGCAACCA
Bcl-2
TGGGATGCCTTTGTGGAACT
CAGGTATGCACCCAGAGTGATG
β-actin
AAGATCATTGCTCCTCCTG
AAAGAAAGGGTGTAAAACGC
2.11 Western blot analysis
Proteins isolated from tissue and LC-540 cells were separated on SDS-PAGE and blotted onto a nitrocellulose membrane. The specific antibodies used are as shown in the Table 2. The membrane were visualised using BCIP/NBT substrate as described earlier (Kannan et al., 2022). The results were analysed using Lab image platform ver 2.1. The target protein expression was normalized with corresponding controls.
Antibody
Dilution
Catalogue.no
Aquaporin 9#
1:500
ABP57801
Cytochrome c (A-8)$
1:1000
SC13156
Bax (B-9)$
1:500
SC7480
Bcl-2 (C-2)$
1:1000
SC7382
β-actin (AC-15)$
1:500
SC69879
Secondary Anti-Mouse IgG*
1:5000
ab97020
Secondary Anti-Rabbit IgG*
1:2000
ab6722
2.12 Data analysis
The data values are expressed as mean ± standard deviation (SD). One-way ANOVA was used to assess the differences between the groups and post-hoc testing was performed for inter-group comparisons using Tukey’s multiple comparisons test with Graph pad Prism 9.0. Values were considered significant at p < 0.05.
3 Results
3.1 Glucose induces cell death in testicular Leydig cells
In this study, dose and time-dependent effect of varied glucose concentrations on cell viability were assessed in LC-540 Leydig cells. The results show a significant decrease in cell viability at high glucose concentrations 20 mM and 30 mM in comparison to control at both 24 and 48 hrs (Fig. 2). Further, the cell viability at 1 mM glucose concentration was reduced but was not significant compared to control. On the other hand, the LC-540 cells treated with 30 mM mannitol (osmotic control) compared to the control showed significant decrease in cell viability. The cell viability results were almost similar at 24 and 48 hrs, so we decided to use 24 hrs time period for our further experiments.
Effect of glucose at different concentrations on viability of LC-540 cells at various time intervals (12, 24, and 48 hrs). Significance at *p < 0.05, **p < 0.01, and ***p < 0.001(n = 3).
3.2 Morphological changes in LC-540 cells on glucose treatment by fluorescence microscopy
To identify, whether glucose treatment induces apoptosis-associated changes in LC-540 cells, cells were fluorescent stained with different dyes and observed under the fluorescent microscope. In our experiments, morphological changes like cell rounding, detachment, floating, and shrinkage on treatment with various concentrations of glucose were observed in LC-540 cells after 24 hrs treatment under inverted microscope. After 24 hrs of glucose/mannitol treatment LC-540 cells stained using AO/EB showed obvious morphological changes with viable cells stained with AO, while green and orange cells indicate early apoptosis with condensed chromatin, nuclei impaction, and fragmentation at HG concentrations and these changes were more obvious in mannitol compared to the control cells showing normal morphology with uniform fluorescence in nuclei and cytoplasm as shown in the Fig. 3 (A-E). Similarly, to identify if glucose treatment induces nuclear damage, LC-540 cells after 24 hrs of treatment were stained with the nuclear stain Hoechst 33,258 dye. The high glucose and mannitol treated cells showed increased nuclear aggregation compared to controls indicating glucose induced nuclear damage (Fig. 3 (F-J). DCFHDA dye to assess ROS production in LC-540 Leydig cells treated with glucose. The LC-540 cells exposed to high-glucose/mannitol showed higher ROS generation. The cells treated with 1 mM glucose has increased ROS generation compared to control but lesser compared to high glucose and mannitol treatment as observed in the microscopic images Fig. 3 (K-O). Mitochondrial membrane potential was assessed using Rhodamine-123 stain in LC-540 rat Leydig cells treated with glucose/mannitol the control cells exhibited increased fluorescence whereas high glucose and mannitol fluorescence intensity was reduced indicating decreased membrane potential (Fig. 3 (P-T). These findings substantiate our hypothesis that, glucose induces apoptosis, increasing nuclear damage, generation of ROS and loss of membrane integrity but also the effect of osmotic stress cannot be denied.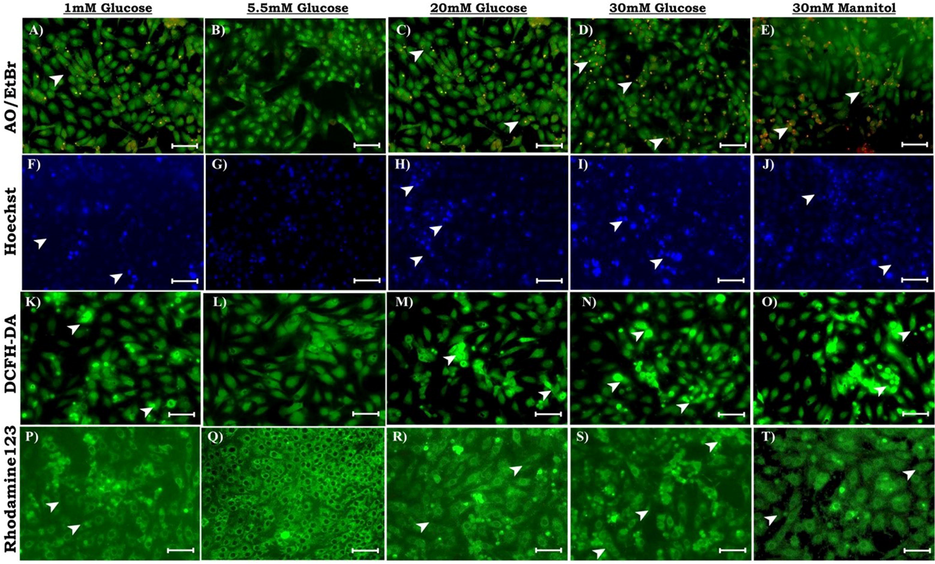
Effect of glucose on morphological changes in LC-540 cells after 24 hrs; observed under fluorescence microscope. (A-E: AO/EB staining to analyze cellular damage and apoptosis differentiating live and dead cells and also early apoptotic cells; F-J: Hoechst staining for assessing nuclear damage with increased fluorescence indicating nuclear damage; K-O: ROS production were analyzed using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA); P-T: Mitochondrial membrane potential (ΔΨm) was assessed with Rhodamine-123). Scale-100 µm; 20X Magnification.
3.3 Effect of hyperglycaemia on anti-apoptotic and pro-apoptotic expression in-vivo and in-vitro
It is well-established that, prolonged hyperglycemia induces dysfunction of several organ systems in diabetic patients via apoptosis. In-vivo and in-vitro apoptotic gene expression were analysed using real-time PCR. In-vivo analysis on diabetic rat testis showed significant increase in Bax expression along with decreased Bcl-2 in comparison to control. Interestingly, insulin treatment significantly decreased Bax gene expression and increased Bcl-2 expression in when compared to diabetic testis Fig. 4 (A). In-vitro analysis on effect of hyperglycaemia were assessed in LC-540 rat Leydig cells with various glucose concentrations. The pro-apoptotic gene Bax was significantly upregulated and Bcl-2 was downregulated on HG treatment compared to normal and low glucose treated cells. However, mannitol treated LC-540 Leydig cells also showed significant increase in Bax along with decreased Bcl-2 in comparison to control Fig. 4 (B). These results all collectively suggests that, hyperglycaemia induces apoptosis in both in-vivo and in-vitro. Furthermore, to confirm the same we analysed the protein expression by western blotting experiments.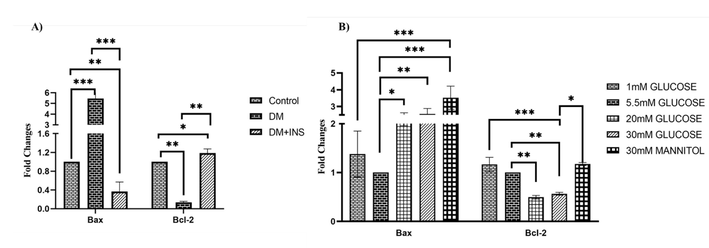
Hyperglycaemia effect on apoptosis using real time PCR (qRT-PCR). A) In-vivo study on expression of apoptotic markers in diabetic rat testis (n = 6). B) In-vitro analysis of on effect of hyperglycaemia on pro- and anti-apoptotic genes in LC-540 cells treated with varied glucose concentrations (1 mM, 5.5 mM, 20 mM, 30 mM) and 30 mM mannitol (osmotic control) for 24 hrs (n = 3). Significance at *p < 0.05, **p < 0.01, and ***p < 0.001.
In-vivo protein expression showed a significant increase in Cytochrome c on diabetic rat testis in comparison with control. Insulin treatment significantly decreased the expression of Cytochrome c compared to the diabetic testis. Similar to gene expression results, the Bax protein expression was significantly increased along with a significant decrease Bcl-2 expression in diabetic rat testis. On the other hand, insulin treatment significantly upregulated Bcl-2 protein expression along with a significant downregulation in Bax expression in comparison with diabetic testis Fig. 5 (A). Similarly, in-vitro analysis on pro-apoptotic and anti-apoptotic protein expression was also analysed in LC-540 rat Leydig cells after treatment with glucose and mannitol. The Cytochrome c protein expression was significantly increased on HG and mannitol treated cells compared to control. HG and mannitol treatment significantly increased the Bax protein expression and Bcl-2 was significantly downregulated in HG treated cells compared to control; along with increased expression on low glucose treatment compared to high glucose as indicated in Fig. 5 (B).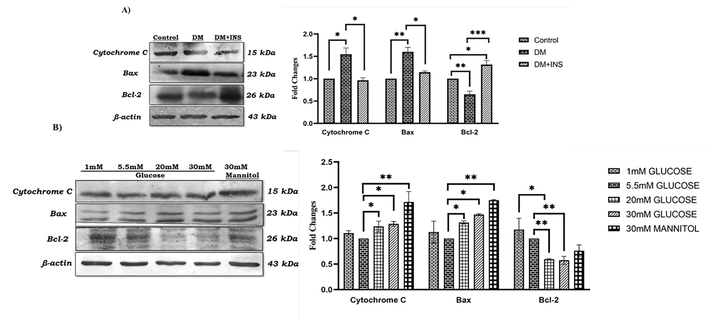
Hyperglycaemia effect on anti- and pro-apoptotic protein expression using western blot. A) In-vivo studies on Cytochrome c, Bax and Bcl-2 protein expression in diabetic rat testis (n = 6). B) In-vitro studies on Cytochrome c, Bax and Bcl-2 protein expression in LC-540 Leydig cells on glucose (1 mM, 5.5 mM, 20 mM, & 30 mM) and 30 mM mannitol treatment (n = 3). Significance at *p < 0.05, **p < 0.01, and ***p < 0.001.
3.4 Effect of AQP 9 knockdown on Leydig cell apoptosis under hyperglycaemia
Our earlier reports on expression of AQPs isoforms revealed that, AQP isoforms 0, 1, 3, 4, 5, 6,7, 8, 9,11, and 12 were expressed in testis and Leydig cells and AQP2 was below detection limit and AQP 10 was not analyzed; further, hyperglycaemia significantly increased AQP 9 expression in testis and Leydig cells (Kannan et al., 2022). In this present study, in-vivo AQP 9 gene and protein expression were significantly increased under hyperglycaemia as reported earlier. On insulin treatment diabetic testis showed significant decrease in AQP 9 gene and protein expression compared to diabetic rat testis Fig. 6 (A). In-vitro analysis showed a significant increase in AQP 9 gene and protein expression on HG treatment compared to 5.5 mM glucose as shown in Fig. 6 (B). Surprisingly, on mannitol treatment AQP 9 gene and protein expression were decreased compared to HG treated cells. Further, AQP 9 knockdown and its effect on apoptotic protein expression were studied. LC-540 cells transfected with AQP 9 siRNA decreased expression by almost 50% as shown in the Fig. 7 (A). Further, to evaluate the effect of AQP 9 on apoptosis, NC siRNA and AQP 9 siRNA transfected LC-540 cells treated with HG (30 mM) and the protein expressions of Cytochrome c, Bax and Bcl-2 were analysed by western blotting. Interestingly, AQP 9 knockdown significantly downregulated Cytochrome c, Bax and upregulated Bcl-2 protein levels in HG treated LC-540 cells as shown in Fig. 7 (B). These findings confirms that AQP 9 knockdown ameliorates the apoptotic index in high-glucose treated LC-540 cells indicating its role in diabetic apoptosis.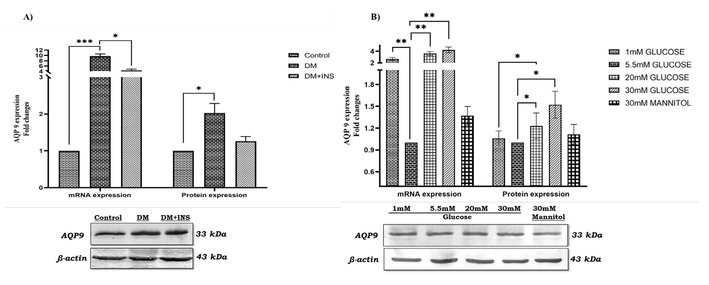
Hyperglycaemia/high glucose effect on Aquaporin 9 expression. A) In-vivo studies on AQP 9 gene and protein expression in diabetic rat testis (n = 6). B) In-vitro studies on expression of AQP 9 gene and protein in LC-540 Leydig cells upon glucose (1 mM, 5.5 mM, 20 mM, & 30 mM) and 30 mM mannitol treatment (n = 3). Significance at *p < 0.05, **p < 0.01, and ***p < 0.001.
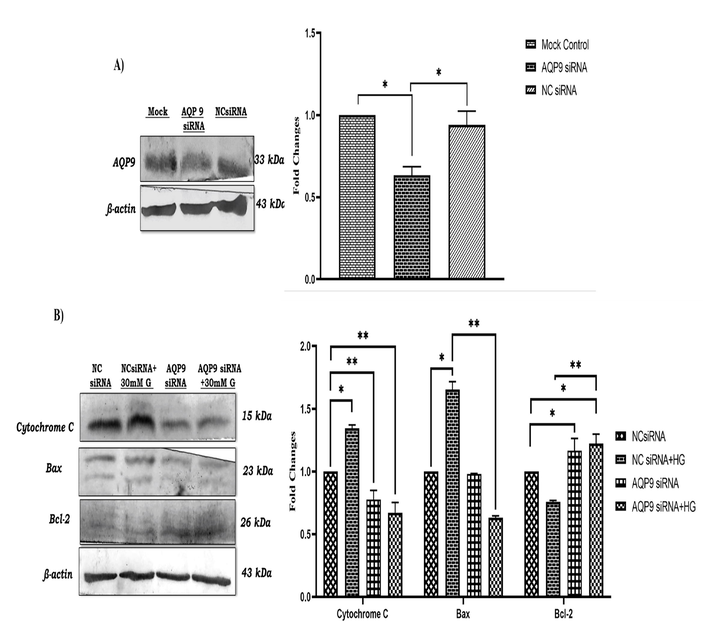
Knockdown of AQP 9 and its effect on anti- and pro-apoptotic protein expression in LC-540 cells. (A) knockdown of AQP 9 in LC-540 cells. (B) Knockdown of AQP 9 and its effect on expression of Cytochrome c, Bax and Bcl-2 (n = 3). Data represents mean ± SD (*p < 0.05, **p < 0.01, and ***p < 0.001).
4 Discussion
Infertility is a complex problem dependent on several factors that affects couples worldwide and are often diagnosed with unexplained or idiopathic infertility. Diabetes affects spermatogenesis and/or steroidogenesis altering cellular proliferation and apoptosis; thereby leads to male sexual dysfunction. The prevalence of DM is increased among males of childbearing age and is closely associated with the decline of fertility (Kumar et al., 2021). Diabetic complications have been linked to diabetes-induced persistent hyperglycemia as a result of glucose dysmetabolism caused by insulin resistance or pancreatic dysfunction. (Mattack et al., 2015, Ormazabal et al., 2018). Hyperglycemia’s detrimental effects on male reproductive dysfunction has been well documented. The transport of glucose from blood-to-germ cell and other metabolic intermediates in testicular cells are tightly regulated by several metabolic features. Moreover, DM affects glucose metabolism in testicular cells, various studies are focussed on understanding the molecular mechanisms responsible for the alterations induced in male reproductive potential. STZ induced diabetic model has been widely used to study DM induced testicular dysfunctions leading to male infertility/subfertility (He et al., 2021). In this study, the pro- and anti-apoptotic genes were evaluated, Bax was significantly increased and Bcl-2 was significantly decreased in diabetic testis, on insulin treatment the pro-and anti-apoptotic expression were restored. Zha et al. also have reported testicular apoptosis with increased Bax/ Bcl-2 ratio in diabetic testes (Zha et al., 2018). Moreover, our findings are in coherence with the earlier studies that, hyperglycaemia induces apoptosis in diabetic testis (Zha et al., 2018, Khalil et al., 2021). The proper functioning of hypothalamic-pituitary–gonadal axis and proper insulin regulation helps in maintenance of fertility (Schoeller et al., 2012) and also high insulin levels or insulin resistance play a key role in male infertility (Yan et al., 2015). Earlier studies suggests that, hyperglycemia and/or lack of insulin contributes to Leydig cell dysfunction and apoptosis. Hence, this could be the possible explanation that insulin restored the pro- and anti- apoptotic genes in our study (Wagner et al., 2021). Insulin treatment prevented hyperglycemia, thus decreased apoptotic index in diabetic rat testis. This decrease in apoptotic index is the result of reduced insulin secretion and not resistance.
Leydig cells are important for the growth and maintenance of the testes, and their improper functioning contributes significantly to testicular dysfunction. Our in-vivo findings also indicates that, hyperglycaemia induces apoptosis in diabetic rat testis. To further confirm our results, we studied the effect of hyperglycaemia in-vitro using LC-540 cells treated with varied glucose levels. Firstly, MTT assay showed decreased cell viability on glucose and mannitol treatment in LC-540 Leydig cells. However, the drastic decrease in cell viability was observed in mannitol treatment and this might be due to hyperosmotic stress, and hence, we speculate that hyperosmolar effect created upon high glucose treatment in LC-540 cells could also play a possible role in cell viability along with hyperglycaemia leading to apoptosis (Lakshmanan et al., 2013). Our data also suggests that high glucose induced apoptosis in LC-540 cells and could also possibly play a role in diabetes induced male infertility/subfertility and our results are in par with earlier findings reported (Wang et al., 2021a). Furthermore, LC-540 cells treated with glucose/mannitol were stained for AO/EtBr, Hoechst, DCFDA, and Rhodamine-123, these microscopic staining revealed morphological changes such as condensed chromatin, nuclei impaction, fragmentation, decreased membrane potential, decreased mitochondrial function, along with increased nuclear aggregation, nuclear damage, and ROS generation upon high glucose treatment and these changes were obvious in mannitol. Our findings suggests that, HG induce apoptosis in Leydig cells and the findings are similar to the earlier reports (Hu et al., 2021, Wagner et al., 2021).
Apoptosis is necessary for the development and maturation of testicular Leydig cells. The proper functioning of testes is well maintained by apoptosis and oxidative damage in Leydig cells; any changes might lead to male infertility (Wang et al., 2019). Apoptosis is considered as one of the important factors in the aetiology of testicular dysfunction associated with DM (Hu et al., 2021). The pro-apoptotic Bax and antiapoptotic Bcl-2 are implicated in cell death, and the ratio of Bax/ Bcl-2determines the fate of cells. Therefore, an intricate balance exists between these factors that induce or counteract apoptosis. However, to further understand the molecular mechanisms behind hyperglycaemia induced apoptosis, the upstream events were analysed in-vitro on LC-540 cells. The Bax gene expression was significantly increased, while the level of Bcl-2 was decreased, in the HG treated cells. In addition, the protein expression of Cytochrome c and Bax were increased, while the anti-apoptotic factor Bcl-2 was decreased in parallel, the in-vivo results indicated the same. Collectively these results demonstrate that, hyperglycaemia-induced apoptosis in both in-vivo and in-vitro. It has also been reported that, increased apoptosis may lead to decline in testosterone produced by Leydig cells and thus disrupts male reproductive functions (Chen et al., 2018). Bcl-2, an antiapoptotic protein, supresses Bax oligomerization and inhibits Cytochrome c release and thus inhibits mitochondrial apoptosis. Diabetes induces Leydig cell apoptosis via downregulation of Bcl-2 family proteins and our results are in par with the earlier reports (He et al., 2021, Hu et al., 2021). Nevertheless, the upstream pathway of Bax induction and Bcl-2 inhibition remain to be defined in hyperglycaemia induced apoptosis.
Water movement in cells alters the intracellular environment leading to cell shrinkage and initiates apoptosis; thus, indicating the importance of water homeostasis in apoptosis and maintenance of male reproductive function (Delpire and Gagnon 2018, Bortner and Cidlowski 2020). Alterations in AQPs expression, function and/or regulation impacts on the pathophysiology of various clinical conditions viz cancer, diabetes, metabolic disorders, apoptosis and reproductive function (Ribeiro et al., 2021). AQPs are involved in seminiferous tubule lumen fluid secretion during testis development, as well as fluid movements during spermatogenesis, sperm concentration, and maturation (Kannan et al., 2022). In this study, we speculate the role of AQPs in hyperglycaemia-induced apoptosis in diabetic infertility. Earlier in our laboratory, we have demonstrated that, hyperglycaemia induced AQP 9 expression inhibits Leydig cell steroidogenesis (Kannan et al., 2022). AQP 9 expression was found to be increased in both type 1 and type 2 diabetic liver, but the expression was decreased with the circulating insulin levels and knockdown of AQP 9 is found to decrease hyperglycaemia in diabetic and obese mice (Spegel et al., 2015). Recently, it has also been reported that insulin downregulates AQP 9 in a concentration-dependent manner in human placenta (Castro Parodi et al., 2011). In-vitro treatment with mannitol increased expression of AQP 9, our results are in agreement with that of a previous study, though augmentation by mannitol was not statistically significant compared with high glucose treatment. Osmotic stress may damage DNA and proteins, resulting in impairment of cell function and also in initiation of repair and protection processes. Recent reports indicates that, the expression of AQPs is induced in mammalian cells by hyperosmotic stress, in brain cortex and astrocytes after brain injury or ischemia, though hyperosmotic mannitol solution increases the expression of AQP4 and AQP 9 (Liu et al., 2012, Salman et al., 2022). Surprisingly, AQP 4 expression was found to be decreased in the ARPE-19 retinal cells under osmotic stress, resulting in pathophysiological mechanisms leading to the formation of macular oedema (Willermain et al., 2014). The differences in the expression of brain and testis might be due to an adaptation to hyperosmotic stress for AQP 9 in different environment. Hence, we investigated the role of AQP 9 in testicular diabetic apoptosis, knockdown of AQP 9 in LC-540 Leydig cells decreased Cytochrome c, pro-apoptotic and increased anti-apoptotic protein expression. Similarly, it has been reported that knockdown of AQP 9 decreased apoptosis in diabetic myocardial cells (Zhao and Sun 2015). Thus, our findings confirm that AQP 9 plays a pivotal role in hyperglycaemia induced apoptosis in Leydig cells, and might inhibit cell proliferation leading to male infertility/subfertility.
5 Conclusion
Diabetes associated hyperglycaemia-induced apoptosis is a proven cause for male infertility. Our findings suggest that, hyperglycaemia-induced AQP 9 expression plays a key role in Leydig cell apoptosis. The effects of hyperglycaemia-induced apoptosis are also mediated by hyperosmotic stress; since similar effects were observed on mannitol treated cells except for the slight increase in AQP 9. Further, the knock down of AQP 9 ameliorated apoptotic index by increasing Bcl-2 expression, decreasing Cytochrome c and apoptotic Bax expression in Leydig cells under hyperglycaemia. Hence, we conclude from our findings that AQP 9 regulates hyperglycaemia-induced apoptosis leading to male infertility/subfertility and also could be a potential therapeutic target for hyperglycaemia-induced testicular dysfunction.
Acknowledgement
AK acknowledges Senior Research Fellowship [RBMH/FW/2018/13] from Indian Council for Medical Research (ICMR), New Delhi. CP acknowledges RUSA-MHRD, DST-PURSE and DST-FIST for the infrastructure provided to the Department of Biochemistry, Bharathidasan University, Tiruchirappalli.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Differential response of cardiac aquaporins to hyperosmotic stress; salutary role of AQP1 against the induced apoptosis. Eur Rev Med Pharmacol Sci.. 2021;25(1):313-325.
- [Google Scholar]
- Thymoquinone Attenuates Retinal Expression of Mediators and Markers of Neurodegeneration in a Diabetic Animal Model. Curr Mol Pharmacol. 2022;15
- [CrossRef] [Google Scholar]
- Diabetes, insulin-mediated glucose metabolism and Sertoli/blood-testis barrier function. Tissue Barriers.. 2013;1(2)
- [CrossRef] [Google Scholar]
- Ions, the Movement of Water and the Apoptotic Volume Decrease. Front Cell Dev Biol.. 2020;8:611211
- [CrossRef] [Google Scholar]
- AQP8 participates in oestrogen-mediated buffalo follicular development by regulating apoptosis of granulosa cells. Reprod Domest Anim.. 2021;56(5):812-820.
- [Google Scholar]
- Evidence for insulin-mediated control of AQP9 expression in human placenta. Placenta.. 2011;32(12):1050-1056.
- [Google Scholar]
- Microcystin-leucine arginine mediates apoptosis and engulfment of Leydig cell by testicular macrophages resulting in reduced serum testosterone levels. Aquat Toxicol.. 2018;199:116-126.
- [Google Scholar]
- Water Homeostasis and Cell Volume Maintenance and Regulation. Curr Top Membr.. 2018;81:3-52.
- [CrossRef] [Google Scholar]
- Icariin improves testicular dysfunction via enhancing proliferation and inhibiting mitochondria-dependent apoptosis pathway in high-fat diet and streptozotocin-induced diabetic rats. Reprod Biol Endocrin.. 2021;19(1)
- [Google Scholar]
- MicroRNA regulation of the proliferation and apoptosis of Leydig cells in diabetes. Mol Med.. 2021;27(1)
- [CrossRef] [Google Scholar]
- Aquaporin 9 regulates Leydig cell steroidogenesis in diabetes. Syst Biol Reprod Med. 2022;68(3):213-226.
- [Google Scholar]
- Myristic acid defends against testicular oxidative stress, inflammation, apoptosis: Restoration of spermatogenesis, steroidogenesis in diabetic rats. Life Sci.. 2021;278
- [CrossRef] [Google Scholar]
- Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes.. 2003;52(10):2570-2577.
- [Google Scholar]
- Prevalence of pre-diabetes/type 2 diabetes among adolescents (10–19 years) and its association with different measures of overweight/obesity in India: a gendered perspective. BMC Endocr Disord.. 2021;21(1):146.
- [CrossRef] [Google Scholar]
- The hyperglycemia stimulated myocardial endoplasmic reticulum (ER) stress contributes to diabetic cardiomyopathy in the transgenic non-obese type 2 diabetic rats: a differential role of unfolded protein response (UPR) signaling proteins. Int J Biochem Cell Biol.. 2013;45(2):438-447.
- [Google Scholar]
- Inhibition of autophagy promoted high glucose/ROS-mediated apoptosis in ADSCs. Stem Cell Res Ther.. 2018;9(1)
- [CrossRef] [Google Scholar]
- Aquaporin 9 in rat brain after severe traumatic brain injury. Arq Neuropsiquiatr.. 2012;70(3):214-220.
- [Google Scholar]
- Circular RNA Circ-STIL Contributes to Cell Growth and Metastasis in Hepatocellular Carcinoma via Regulating miR-345-5p/AQP3 Axis. Dig Dis Sci. 2022;67(6):2269-2282.
- [Google Scholar]
- Simulated hyperglycemia impairs insulin signaling in endothelial cells through a hyperosmolar mechanism. Vascul Pharmacol.. 2020;130
- [CrossRef] [Google Scholar]
- The evaluation of serum levels of testosterone in type 2 diabetic men and its relation with lipid profile. J Clin Diagn Res.. 2015;9(1):BC04-07.
- [CrossRef] [Google Scholar]
- Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol.. 2018;17(1)
- [CrossRef] [Google Scholar]
- Oxidative stress and hepatocellular mitochondrial dysfunction attenuated by asiatic acid in streptozotocin-induced diabetic rats. Journal of King Saud University - Science. 2021;33(3):101369.
- [Google Scholar]
- Aquaporins and (in)fertility: More than just water transport. Biochim Biophys Acta Mol Basis Dis.. 2021;1867(3)
- [CrossRef] [Google Scholar]
- K. Sada T. Nishikawa D. Kukidome T. Yoshinaga N. Kajihara K. Sonoda T. Senokuchi H. Motoshima T. Matsumura E. Araki M.F. Essop Hyperglycemia Induces Cellular Hypoxia through Production of Mitochondrial ROS Followed by Suppression of Aquaporin-1 PLoS One. 11 7 2016 10.1371/journal.pone.0158619 e0158619 e0158619.
- Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2022;145(1):64-75.
- [Google Scholar]
- Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes.. 2012;61(7):1869-1878.
- [CrossRef] [Google Scholar]
- Deletion of glycerol channel aquaporin-9 (Aqp9) impairs long-term blood glucose control in C57BL/6 leptin receptor-deficient (db/db) obese mice. Physiol Rep.. 2015;3(9):e12538.
- [Google Scholar]
- Attenuation of erythrocyte membrane oxidative stress by Sesbania grandiflora in streptozotocin-induced diabetic rats. Biochem Cell Biol.. 2015;93(4):385-395.
- [CrossRef] [Google Scholar]
- I.V. Wagner N. Klöting I. Savchuk L. Eifler A. Kulle S. Kralisch-Jäcklein J. Dötsch O. Hiort K. Svechnikov O. Söder Diabetes Type 1 Negatively Influences Leydig Cell Function in Rats, Which is Partially Reversible By Insulin Treatment Endocrinology. 162 4 2021 2021 10.1210/endocr/bqab017.
- Quercetin ameliorates testosterone secretion disorder by inhibiting endoplasmic reticulum stress through the miR-1306-5p/HSD17B7 axis in diabetic rats. Bosn J Basic Med Sci. https:// 2021
- [CrossRef] [Google Scholar]
- Effects of interventional therapy on AQP4 gene expression and neuron apoptosis in rabbits with ischemic brain injury caused by carotid artery stenosis. Int J Clin Exp Pathol.. 2021;14(6):786-793.
- [Google Scholar]
- Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells. Toxicol Appl Pharmacol.. 2019;368:37-48.
- [Google Scholar]
- Osmotic stress decreases aquaporin-4 expression in the human retinal pigment epithelial cell line, ARPE-19. Int J Mol Med.. 2014;34(2):533-538.
- [Google Scholar]
- Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells. Bioengineered.. 2022;13(2):3486-3502.
- [Google Scholar]
- Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J Assist Reprod Genet.. 2015;32(7):1097-1104.
- [Google Scholar]
- The Transgenerational Transmission of the Paternal Type 2 Diabetes-Induced Subfertility Phenotype. Front Endocrinol (Lausanne).. 2021;12:763863
- [CrossRef] [Google Scholar]
- Curcumin Attenuates Testicular Injury in Rats with Streptozotocin-Induced Diabetes. Biomed Res Int.. 2018;2018:1-10.
- [Google Scholar]
- Ginsenoside Rb1 Protects Against Diabetic Cardiomyopathy by Regulating the Adipocytokine Pathway. J Inflamm Res.. 2022;Volume 15:71-83.
- [Google Scholar]
- Aquaporin in the proliferation and apoptosis of diabetic myocardial cells. Genet Mol Res.. 2015;14(4):17366-17372.
- [CrossRef] [Google Scholar]
- H. Zheng J. Huang M. Zhang H.-J. Zhao P. Chen Z.-H. Zeng miR-27b-3p Improved High Glucose-Induced Spermatogenic Cell Damage via Regulating Gfpt1/HBP Signaling Eur Surg Res. 63 2 2022 64 76.







