Translate this page into:
Risk assessment of malathion on health indicators of catfish: Food and water security prospective research
⁎Corresponding author. mushahid@ksu.edu.sa (S. Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Specimens of Heteropneustes fossilis were treated with various levels of malathion and its 96 h LC50 value was determined. The effects of sub-lethal concentrations of insecticide on hematological and biochemical profile of Heteropneustes fossilis were also registered. The 96 h LC50 value obtained was 8.81 mg/l. Exposure of sub-lethal doses (0.44, 0.88 and 1.76 mg/l which are 5, 10 and 20% of the LC50) of malathion to Heteropneustes fossilis for 3 weeks demonstrated that the toxicant had a detrimental effect on the blood profile of fish. Blood cell (Erythrocyte and leucocytes) counts (from 1.66 to 1.44 cell × 106 × mm3 and 37.93 to 30.24 cell × 103 × mm3; respectively), haemoglobin (Hb) level (from 6.21 to 3.54 g/dl) and haematocrit (Ht) estimates (from 34.26 to 30.52%) were reduced in the fishes exposed to malathion. Elevated level (from 46.25 to 92.45 mg/100 ml) of glucose and decreased quantity (29.95 to 24.25 g/dl) of protein in plasma was registered. Glycogen content in muscle and liver was also decreased. The activity of enzymes glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) were high in the fish treated with pesticide. The possible causes for variations in the different parameters were identified and discussed.
Keywords
Acute toxicity
Blood cell count
Enzymes
Heteropneustes fossilis
Malathion
Sub-lethal treatment
1 Introduction
In recent years almost whole world is using the pesticides to ameliorate human health by eliminating undesired living organisms like insects and disease vectors and also to improve the crop production by controlling undesirable plants and pests (Prakasam et al., 2001). In United States of America alone about two billion kilograms of pesticides were applied each year (Aspelin and Grube, 1999). Commonly organophosphorus (OP) compounds are used as insecticides. The malathion which is an organophosphorus compound is used to control the variety of insects including aphids, beetles, pill bugs and scales in agriculture and houses. The unsystematic use of toxicants greatly affects to non-target animals including fish. It appears that the fish deal with the toxic effects of endogenous and exogenous agents in similar biochemical pathways as mammals do (Lackner, 1998). To have the information regarding the noxious consequences of pesticides on fish is quite important because the fish establish a salient link in food chain.
The different blood factors like haemoglobin content, haematocrit values, erythrocyte and leucocyte counts, and blood glucose demonstrate the physiological feedback of a polluted environment (Dethloff et al., 2001). Venakataramana et al. (2006) opined that the application of a clinical diagnosis of fish physiology for the effects of pollutant may be based on the blood profile of fish. Activity of glutamic-oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) would signify the repercussions of pollutants on fish. Normally, the cells of the organs like liver, heart, gills and kidneys have these enzymes (Shalaby, 2009) and elevation of the activity of these enzymes in blood is the reflection of tissue dysfunction or tissue injury (Wells et al., 1996). “However, effects of different pollutants on the biochemical and hematological parameters of fish have been documented by many authors (Al-Attar, 2005; Ogueji and Auta, 2007; Shalaby, 2009; Abalaka et al., 2011; Alkahem Al-Balawi et al., 2011; Ahmad, 2012; Mahboob et al., 2014)”. Malathion delays the functioning of thyroid hormone in fish, Solea Senegalese (Ortiz-Delgado et al., 2019). The results presented by Kata (2020) indicates that malathion can induce the hepatic and renal toxicity in mice in only 6 days. Heteropneustes fossilis is native to Asia and is also introduced in other parts of the world also. This species is an economically important freshwater fish. The present study is focused on the acute and sub-acute effect of malathion to Heteropneustes fossilis. The death of fish was monitored after the exposure of fish with lethal concentrations. Variations in blood profile (haemoglobine, cell and haematocrit estimations), fluctuations in plasma chemistry (glucose, glycogen and protein content) and enzyme's activity (GOT and GPT) were observed after sub-lethal treatment of malathion.
2 Materials and methods
Live individuals of H. fossilis were managed to get from the freshwater fish pond. The length of fish ranged from 13 to 15 cm and the weight ranged from 60 to 70 g. The fishes were kept in glass aquaria (100 cm × 30 cm × 35 cm) for fifteen days for acclimatization to the conditions of the laboratory. In the adaptation period the fish were fed minced meat to satiety twice daily. Water of aquarium changed daily. The different physical and chemical parameters like temperature (25.5 ± 1. °C), dissolved oxygen (7.9 ± 0.4 mg/l), hardness mg/l as CaCO3 (47.5 ± 4.5) and pH (7.9 ± 0.5) of aquarium water were monitored weekly.
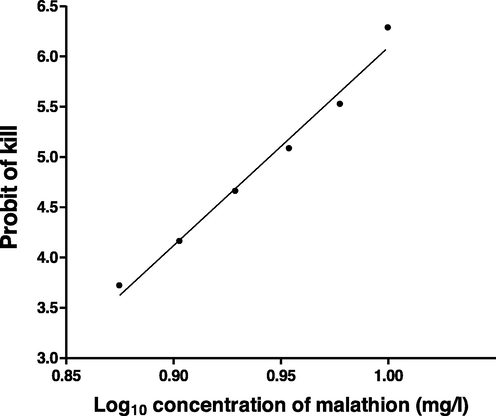
Ten fish individuals were kept in one aquarium (50 cm × 35 cm × 35 cm) containing 30 L of water after two weeks of acclimatization. A total of 330 fish were used in the present experiments. A solution of known concentration was made by adding the known quantity of original formulation in known volume of distilled water. Working solution (7.5, 8.0, 8.5, 9.0, 9.5 and 10.0 mg/l) of malathion were made by mixing needed volume from above solution. The active ingredient of malathion (MW: 330.4, CAS number: 121-75-5) in the compound used was 57%. The water quantity and counts of fish were same in control like the experimental group. Three sets of aquarium were used for the control and treated fish. The mechanical pump was used to aerate the aquarium water and the fish were unfed throughout the experiment. Dead fish counts were recorded and taken out from the aquarium after the death. The method described by Finney (1971) was employed to find the 96 h LC50. A graph was constructed using probit of kill at y axis and log10 concentration of malathion at x axis.
In another set of experiment, ten fishes kept in each aquarium were brought into contact with malathion for three weeks. The experiment was run in triplicates and in three different concentrations (0.44, 0.88 and 1.76 mg/l which are 5, 10 and 20% of LC50). Some important biochemical and physical parameters of blood of exposed specimens were analyzed. A control set was also run for the same period with same counts of fish. Fishes were fed to satiety two times daily. At the end of each week, one fish from each aquarium i.e. three fish from each group were removed and blood samples were obtained in heparinized vials by cutting the caudal peduncle. If the blood from one fish is not sufficient for measurement of all parameter, the blood of more fishes was combined. The hemoglobin was estimated by using the method of Blaxhall and Daisley (1973). The blood was centrifuged in micro-hematocrit centrifuge to determine the hematocrit. The Dace’s solution was used to dilute the blood for enumeration of erythrocytes and Turk’s solution was used to dilute the blood for counting of leucocytes. Neubar haemocytometer was used for the counting of cells.
The plasma from the blood was separated by centrifugation at 6000 rpm for 10 min at 4 °C and was stored at −20 °C for biochemical analysis. The biochemical constituents like Glucose, total protein, GOT and GPT were assayed by using kits (BIOMERIEUX, FRANCE).
The significance of difference among the control and treated values was tested applying one-way analysis of variance (ANOVA). The difference was considered statistically significant if the P values was <0.05.
3 Results and discussion
The mortality of fish after intoxication with various levels of malathion is tabulated in Table 1. The LC50 value for 96 h for Heteropneustes fossilis estimated from the diagram (Fig. 1) was expressed as 8.81 mg/l. Quite high values of the LC50 of malathion compared to present value (8.81 mg g/l) for Heteropneustes fossilis was reported by Durkin (2008) and Faria et al. (2010) for other fish species (9.14 mg/l for Ptychocheiilus lucius, 11700 µg/l for black bulhead, 15.3 mg/l for Gila elegance and 17.0 mg/l for Ictalurus furcatus). A high 96 h LC50 value (15.77 mg/l) for Colossoma macropomum was also reported by De Souza et al. (2020). The result presented by Jothigayathri et al. (2020) show a lower value (3.52 mg/l) of 96 h LC50 of malathion to Oreochromis mossambicus. The 96 h LC50 value (2.2 ppm) for Oreochromis niloticus was reported by Pathiratne and George (1998) which is quite low in comparison to present value. The LC50 values of malathion listed by Newhart (2006) for various fish species ranged between 0.06 and 7620 µg/l. Different authors like Durkin (2008); Patil and David (2008); Pugazhvendan et al. (2009) had reported that malathion was extremely toxic to walleye, brown trout, cutthroat trout; Labeo rohita and Opheocephalus punctatus. It was moderately toxic to minnows and murrels as summarized by Durkin (2008). The variations in the toxic power of Malathion exhibited by different species of fish may be ascribed to the differences in susceptibility and tolerance capacity of fish. Rate of biotransformation, accumulation and excretion of malathion may also play an important role causing the toxicity. Fish species may have discrepancies in metabolic pathways which may result in varied patterns of bio-transformation, producing more toxic or less toxic metabolites (Johnson and Toledo, 1993). Three factors such as varied inhibition of acetylcholinesterase, detoxification and absorption were reported by Oh et al. (1991) as causative factors for the selective toxicity of pesticides for various fish species. “In general the toxicity varied with respect to species and size of fish, and the duration of exposure (Oh et al., 1991).”
Concentrations (mg/l)
Time (hours)
24
48
72
96
Control (0.0)
–
–
–
–
7.5
–
–
1(3.33)
3 (10.00)
8.0
–
–
3 (10.00)
6 (20.0)
8.5
–
3 (10.00)
6 (20.00)
11 (36.66)
9.0
1 (3.33)
3 (10.00)
7 (23.31)
16 (53.28)
9.5
3 (10.00)
5 (16.66)
13 (43.29)
21 (69.93)
10.0
5 (16.66)
15 (49.99)
22 (73.26)
27 (90.00)
Relationship between log10 concentration of malathion and probit of kill.
It was found in the present investigation that the various blood parameters of Heteropneustes fossilis after the sub-lethal chronic treatment to malathion has been changed. The exposed fish manifested decline in haemoglobin concentration, haematocrit values and erythrocyte and leucocyte counts in comparison to untreated fish (Table 2). Alterations in MCV, MCH and MCHC were recorded in Heteropneustes fossilis due to malathion treatment.
Parameters
Concentrations (mg/l)
Exposure Time (Weeks)
1st
2nd
3rd
Erythrocytes (Cell × 106/mm3)
Control (0.0)
1.63 ± 0.06
1.66 ± 0.06
1.62 ± 0.045
0.44
1.54 ± 0.09
1.52 ± 0.05
1.45 ± 0.05*
0.88
1.50 ± 0.05*
1.51 ± 0.06*
1.50 ± 0.04*
1.76
1.48 ± 0.08
1.47 ± 0.12
1.44 ± 0.08
Leucocytes (Cell × 103/mm3)
Control (0.0)
37.51 ± 0.54
37.93 ± 0.68
37.1 ± 0.72
0.44
32.56 ± 0.54
31.29 ± 0.57*
31.01 ± 0.52*
0.88
31.05 ± 0.61*
31.01 ± 0.41*
30.05 ± 0.54*
1.76
30.24 ± 0.84
30.12 ± 0.95
30.24 ± 1.21
Hematocrit (%)
Control (0.0)
33.86 ± 0.62
34.26 ± 0.50
33.94 ± 0.92
0.44
31.85 ± 0.92
32.05 ± 0.80
32.01 ± 1.05
0.88
30.45 ± 0.74*
31.05 ± 0.92*
30.52 ± 0.75*
1.76
29.25 ± 0.98
29.15 ± 1.04
28.89 ± 0.95
Hemoglobin (g/dl)
Control (0.0)
5.85 ± 0.09
6.21 ± 0.12
6.35 ± 0.11
0.44
4.24 ± 0.14*
4.14 ± 0.13*
4.46 ± 0.09
0.88
4.04 ± 0.09*
4.09 ± 0.08*
4.02 ± 0.12*
1.76
3.75 ± 0.08*
3.45 ± 0.06*
3.54 ± 0.11*
MCV (fl/cell)
Control (0.0)
207.73 ± 4.75
206.96 ± 4.11
209.50 ± 4.58
0.44
206.81 ± 4.55
210.85 ± 4.56
220.75 ± 5.11
0.88
204.91 ± 4.33
204.87 ± 4.25
206.46 ± 5.25
1.76
197.63 ± 5.35
198.65 ± 6.24
200.6 ± 4.65
MCH (Pg/cell)
Control (0.0)
38.04 ± 2.75
36.95 ± 3.65
39.19 ± 2.16
0.44
27.93 ± 2.25
27.86 ± 2.85
30.87 ± 2.75
0.88
27.15 ± 2.32*
27.04 ± 1.45*
26.89 ± 2.45*
1.76
25.66 ± 2.65*
23.95 ± 2.05*
24.88 ± 2.67*
MCHC (%)
Control (0.0)
17.19 ± 1.25
18.29 ± 1.56
18.79 ± 1.25
0.44
13.90 ± 1.05
13.89 ± 1.36
14.47 ± 1.85
0.88
13.88 ± 1.15
13.97 ± 1.45
13.24 ± 1.45*
1.76
12.55 ± 1.56*
11.85 ± 1.35*
12.85 ± 1.65*
For the evaluation of the pollution effects of pollutants the haematological parameters are being considered as suitable tool. Cyriac et al. (1989) have considered the fluctuations in blood profile as an excellent indicator for pollutants exposure. Alterations in the various blood characteristic obtained in this investigation may be due to the malathion effects on the haematopoietic system of exposed fish causing malfunctioning of the system. The RBC counts, haemoglobin and haematocrit estimates of fish treated with diazinon were declined as documented by Banaee et al. (2008) and Banaee et al. (2011). The authors have attributed this depreciation to eradication of cells and/or reduce in the size of cells because of the inimical effects of pollutant. Zaki et al. (2009) and Adeyemo (2007) have registered subside RBC counts, haemoglobin levels and PCV evaluates in the fish exposed to malathion and lead nitrate, respectively. Analogous outcomes were documented by Sharmin et al. (2015) and Osman (2018) in Cyprinus carpio and Oreochromis niloticus after the treatment of pesticides. “Changes in the leukocyte system manifest in the form of leukocytosis with heterophilia and lymphopenia, which are characteristics of leukocytic response in animals exhibiting stress”. Fall in the number of WBC in the fish treated with chromium was reported by Alkahem (1995) and he has attributed this to the fallen number of lymphocytes. The decline of the leucocytes counts in the H. fossilis, treated with malathion may be ascribed to high rate of elimination from the blood or alternatively a curtailment in its creation or may be due to fast damage of white blood cells. Similar to present investigation, low leukocyte counts in freshwater fish, tilapia exposed to phosalone was registered by Jaffer Ali and Rani (2009). Reverse to the present results an elevated population of WBC was reported in Cyprinus carpio and Oreochromis niloticus after the treatment of pesticides by Sharmin et al. (2015) and Osman (2018), respectively. This elevation in the leucocytes counts was probably for the antibody production to act as defense line. “The mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) seem to be changes that are more sensitive and can cause reversible changes in the homeostatic system of fish” (Ahmad, 2012). Variations in these blood parameters are related to population of erythrocyte, haemoglobin level and haematocrit values. Rao (2010) has reported that the values of these blood indices were elevated in different fish species after acute treatment of various pesticides.
“The hyperglycemia in the fish exposed to malathion may be due to the mobilization of glycogen into glucose to meet the increased demand for energy. Glucocorticoids and catecholamines hormones are supposed to produce hyperglycemia in animals as stress stimuli elicit rapid secretion of these hormones in the fish” (Pickering, 1981). The gluconeogenesis in the toxicant exposed fish in their attempt to meet the increased demands of energy (Winkler et al., 2007) might be responsible for such elevation of glucose level. The hyper production of these hormones in the fish could be ascribed for the hyperglycemia observed in present study. The results reported by Abalaka et al. (2011), Alkahem Al-Balawi et al. (2011) and Ahmad (2012) support to the present findings.
The exposed fish exhibited significant hypoproteinaemia (declined from 29.5 to 24.22 g/dl) and hyperglycemia (increased from 25.01 to 92.45 mg/100 ml). These alterations were distinct in the elevated doses and in the prolonged time of exposure (Table 3). “The destruction of cells or necrosis and impairment in the machineries of protein synthesis may be responsible for the hypoproteinaemia in the fish treated with pesticide (Bradbury et al., 1987). The histological and pathological changes occurred in kidney causing the loss of proteins could be the other causative factor for hypoproteinaemia (Salah El-Deen et al., 1996). The aforementioned aspects may be reason for the hypoproteinaemia in the fish of the present investigation. Hypoproteinaemia in pollutants exposed fish was documented by Omoniyi et al. (2002) and Shalaby (2009). Total protein content was inhibited in the muscles of Oreochromis niloticus after the exposure of pesticide (Naqvi et al., 2017). Al-Attar (2005), Omitoyin (2007) and Abalaka et al. (2011) have reported hyperproteinaemia in fish exposed to toxicants. These authors have suggested that elevation of protein in treated fish may be the consequence of loss of water from blood, increased synthesis of protein or comparatively change in mobilization of protein in the blood. “They also mentioned that such observed hyperproteinaemia may be indicative of efficient immune response and physiological reaction of body to pollutants” (Al-Attar, 2005; Omitoyin, 2007; Abalaka et al., 2011).
Parameters
Concentrations (mg/l)
Exposure Times
1st
2nd
3rd
Total Protein (g/dl)
Control (0.0)
29.25 ± 1.76
29.95 ± 1.67
29.15 ± 1.39
0.44
26.98 ± 1.78
26.65 ± 1.12
26.15 ± 1.75
0.88
26.05 ± 1.88*
26.25 ± 1.55*
25.55 ± 1.86*
1.76
25.01 ± 1.24
24.98 ± 1.05
24.25 ± 1.12
Glucose (mg/100 ml)
Control (0.0)
46.25 ± 5.25
47.35 ± 5.68
47.52 ± 6.08
0.44
63.25 ± 6.68*
65.65 ± 8.25*
77.54 ± 6.75*
0.88
71.25 ± 7.26*
74.25 ± 5.54*
89.25 ± 6.25*
1.76
85.24 ± 4.56*
86.24 ± 6.25*
92.45 ± 7.25*
Liver glycogen (mg/g)
Control (0.0)
9.82 ± 0.32
9.92 ± 0.25
8.99 ± 0.27
0.44
7.05 ± 0.16*
7.12 ± 0.19*
6.95 ± 0.17*
0.88
6.86 ± 0.15*
6.52 ± 0.17*
6.35 ± 0.16*
1.76
5.65 ± 0.96*
5.12 ± 1.02*
5.05 ± 0.98*
Muscle glycogen (mg/g)
Control (0.0)
4.25 ± 0.08
4.05 ± 0.06
4.15 ± 0.06
0.44
2.55 ± 0.06*
2.32 ± 0.05*
2.11 ± 0.05*
0.88
2.15 ± 0.05*
2.02 ± 0.05*
2.01 ± 0.06*
1.76
2.02 ± 0.11*
1.95 ± 0.08*
1.96 ± 0.08*
PGOT (IU/l)
Control (0.0)
75.25 ± 11.2
80.32 ± 10.5
82.26 ± 9.4
0.44
88.25 ± 16.2
103.35 ± 14.3*
109.45 ± 10.5*
0.88
105.25 ± 11.2*
125.25 ± 13.2*
128.35 ± 12.2*
1.76
110.25 ± 10.2*
135.15 ± 12.5*
138.24 ± 12.5*
PGPT (IU/l)
Control (0.0)
63.12 ± 5.66
67.22 ± 8.11
66.21 ± 6.84
0.44
80.25 ± 11.3
88.25 ± 10.3*
85.25 ± 11.2*
0.88
97.25 ± 11.2*
95.35 ± 12.1*
112.25 ± 11.1*
1.76
105.24 ± 9.5*
108.23 ± 10.5*
124.28 ± 11.5*
The enzymes (GOT and GPT) activity had significantly (P < 0.05) enhanced (GPT from 67.22 to 124.28 IU/l and GOT from 82.26 to 138.24 IU/l) after first week at higher dose (2.0 mg/l) and in all malathion exposed groups (Table 3) in the last period of treatment. The GOP and GPT are alleged to be very easily affected by the environmental changes. Hence, the pollutant exposed fish expressed enhanced level of GOT and GPT activity. Jeney et al. (1991) have documented an increased activity of GOT and GPT in the serum of ammonia treated fish. They believed that enzyme SGPT is more easily affected by the alterations in the environmental conditions. Lemaire et al. (1991) have fed the fish with the diet without docosahexaenoic acid and registered that the activity of values of GOT was significantly high. The GPT activity remain unchanged. They concluded that the fish fed diet free of docosahexaenoic acid develop generalized massive steatosis, show necrosis centers in hepatic parenchyma. The fish, C. punctatus exposed to monocrotophos has increased activity of the two enzymes (Agrahari et al., 2007). Any damage to hepatic cell could cause release of tremendous amount of GOT and GPT enzymes into the blood circulation (Palanivelu et al, 2005). Hence, the variations in the level of the activity of these (PGOT and PGPT) are supposed to be a manifestation of cellular damage (Palanivelu et al., 2005; Alkahem Al-Balawi et al., 2011). The elevated values of the activity of enzymes recorded in this study can be ascribed to damage done by malathion to hepatic cells. Ibrahim (2019) has reported that malathion has altered the Biochemical and enzymological conditions in fish.
4 Conclusion
The LC50 value of malathion was registered as 8.81 mg/l. This value shows that malathion is moderately toxic to fish, Heteropneustes fossilis, and the value recorded are within the range registered for different fish species. The information regarding the variations in biochemical parameters and haematological profile in fish after the long term sub-lethal exposure of malathion would be used to manage the pesticide spray. An adverse effect on the metabolism of macromolecule and haematopoietic organs of fish are anticipated after the exposure of malathion. Therefore, the use of pesticide spray to control the pests undoubtedly affects the life of untargeted fauna of the aquatic environment and of course to human.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education“in Saudi Arabia for funding this research work through the project number IFKSURG-1435-012.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of biochemical changes in Clarias gariepinus adults exposed to aqueous and ethanolic extracts of Parkia biglobosa pods. Afr. J. Biotech.. 2011;10:234-240.
- [Google Scholar]
- Haematological profile of Clarias gariepinus (Burchell, 1822) exposed to lead. Turk. J. Fish. Aquat. Sci.. 2007;7:163-169.
- [Google Scholar]
- Biochemical alteration induced by monocrophos in the blood plasma of fish, Channa punctatus (Bloch) Pest. Biochem. Physiol.. 2007;88:268-272.
- [Google Scholar]
- Toxicity bioassay and effects of sub-lethal exposure of malathion on biochemical composition and haematological parameters of Clarias gariepinus. Afr. J. Biotech.. 2012;11:8578-8585.
- [Google Scholar]
- Biochemical effects of short-term cadmium exposure on the freshwater fish, Oreochromis niloticus. J. Biol. Sci.. 2005;5:260-265.
- [Google Scholar]
- Behavioral responses and changes in some haematological parameters of the cichlid fish, Oreochromis niloticus, exposed to trivalent chromium. J. King Abdul Aziz Univ. -Sci.. 1995;7:5-13.
- [Google Scholar]
- Toxicity bioassay of lead acetate and effects of sub-lethal exposure on growth, haematological parameters and reproduction in Clarias gariepinus. Afr. J. Biotech.. 2011;10:11039-11047.
- [Google Scholar]
- Pesticide Industry Sales and Usage 1996 and 1997 Market Estimates, EPA 733-R-99-001. Washington, DC: Office of pesticide programs; 1999.
- Effects of sub-lethal diazinon concentrations on blood plasma biochemistry of common carp. Int. J. Environ. Res.. 2008;2:189-198.
- [Google Scholar]
- Effects of diazinon on biochemical parameters of blood in rainbow trout (Onchorhynchus mykiss) Pest. Biochem. Physiol.. 2011;99:1-6.
- [Google Scholar]
- Routine haematological methods for use with fish blood. J. Fish Biol.. 1973;5:771-781.
- [Google Scholar]
- Toxicology of fenvalerete and its constituenyt isomers to the fathead minnow (Piephales promeos) and blue gill (Lepomis macrochirus) Bull. Environ. Cont. Toxicol.. 1987;38:727-735.
- [Google Scholar]
- Hemoglobin and hematocrit values in the fish Oreochromis mossambicus (Peters) after short term exposure to copper and mercury. Bull. Environ. Conta. Toxicol.. 1989;43:315-320.
- [Google Scholar]
- Severe damage caused by malathion exposure in Colossoma macropomum. Ecotoxicol. Environ. Saf 2020
- [CrossRef] [Google Scholar]
- Effect of dissolved copper on selected haematological, biochemical and immunological parameters of wild rainbow trout (Oncorhynchus mykiss) Archi. Environ. Contam. Toxicol.. 2001;40:371-380.
- [Google Scholar]
- Durkin, P.R., 2008. Malathion; Human Health and ecological risk assessment. Final report submitted to Paul Mistretta, PCR, USDA/Forest Service, Southern region, Atlanta Georgia. SERA TR-052-02-02c, pp325.
- Faria, I.R., Palumbo, A J., Fojut, T.L., Tjeerdema, R.S., 2010. Water quality criteria report for malathion. Phase III: Application of the pesticide water quality criteria methodology. UCDAVIS, pp vii + 64.
- Probit Analysis. Ram Nagar, Delhi: S. Chand and Company Ltd.; 1971.
- Biological and histopathological respons of Oreochromis niloticus malathion hepatotoxicity. J. R. Sci.. 2019;1:10-15.
- [Google Scholar]
- Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus. Turk. J. Vet. Anim. Sci.. 2009;33:407-411.
- [Google Scholar]
- Acute effect of sublethal ammonia concentrations on common carp (Cyprinus carpio L.). II. Effect of ammonia on blood plasma transaminase (GOT, GPT), GDH enzyme activity and ATP value. Aquaculture. 1991;104:149-156.
- [Google Scholar]
- Acute toxicity of endosulfan to the fish Hyphessobrycon bifasciatus and Brachydanio rerio. Arch. Environ. Contam. Toxicol.. 1993;24:151-155.
- [Google Scholar]
- Impact of neem oil on malathion in the fish, Oreochromis mossambicus. Curr. Environ.. 2020;15
- [CrossRef] [Google Scholar]
- Short-time effects of malathion pesticide on functional and histological changes of liver and kidney in female mice. Pak. J. Biol. Sci.. 2020;23:1103-1112.
- [Google Scholar]
- Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (Cholesterol, triglycerides) of sea- bass (Dicentrarchus labrax) Aquaculture. 1991;93:63-75.
- [Google Scholar]
- Fish cholinesterases as biomarker of sub-lethal effects of organophosphorus and carbamates in tissue of Labeo rohita. Pak. J. Zool. 2014:46.
- [Google Scholar]
- Pesticide impact on protein in fish (Oreochromis niloticus) tissue. Indian J. Geo. Mar. Sci.. 2017;46:1864-1868.
- [Google Scholar]
- Environmental Fate of Malathion. California Environmental protection Agency; 2006. p. :20.
- Investigations of biochemical effects of acute concentrations of Lamda-cyhalothrin on African catfish, Clarias gariepinus –Teugels. J. Fish. Int.. 2007;2:86-90.
- [Google Scholar]
- Mechanism of selective toxicity of diazinon to killifish (Oryzias latipes) and loach (Misgurnus anguillicaudatus) Aquat. Toxicol. Risk Assess.. 1991;14:343-353.
- [Google Scholar]
- Plasma biochemistry changes in Clarias gariepinus (Buchell, 18220 fed poultry litter. Asian J. Anim. Sci.. 2007;7:45-52.
- [Google Scholar]
- Effects of lethal and sub-lethal concentrations of Tobacco (Nicotiana tobaccum) leaf dust extract om weight and haematological changes in Clarias gariepinus (Burchell) J. Appl. Sci. Environ. Manage.. 2002;6:37-41.
- [Google Scholar]
- The organophosphate pesticide –OP – malathion inducing thyroid disruptions and failures in the metamorphosis of the Senegalese sole, Solea senegalensis. BMC Vet. Res.. 2019;15:57-82.
- [Google Scholar]
- Effect of Malafos (57 EC) Acute Toxicity on Fresh Water Fish Oreochromis Niloticus. J. Aquat. Sci. Mar. Biol.. 2018;1:26-30.
- [Google Scholar]
- Influence of insecticidal derivatives (Cartap Hydrochloride) from the marine polychaete on certain enzymes of the freshwater fish Oreochromis mossambicus. J. Environ. Biol.. 2005;26:191-196.
- [Google Scholar]
- Toxicity of malathion to Nile tilapia, Oreochromis niloticus and modulation by other environmental contaminants. Aquat. Toxicol.. 1998;43:261-271.
- [Google Scholar]
- Behaviour and respiratory dysfunction as an index of malathiom toxicity in the freshwater fish Labeo rohita (Hamilton) Turk. J. Fish. Aquat. Sci.. 2008;8:233-237.
- [Google Scholar]
- Stress and compensation in teleostean fishes: Response to social and physical factors. In: Pickering A.D., ed. Stress and fish. New York, USA: Academic Press; 1981. p. :295.
- [Google Scholar]
- Plasma and RBCs antioxidant status in occupational male pesticide sprayers. Clin. Chem. Acta. 2001;10:107-112.
- [Google Scholar]
- Effect of malathion toxicity in the freshwater fish Opheocephalus punctatus-A histological and histochemical study. World J. Fish Mar. Sci.. 2009;1:218-224.
- [Google Scholar]
- Carbaryl induced changes in the haematological, serum biochemical and immunological responses of common carp, Cyprinus carpio, (L.) with special emphasis on herbal extracts as immunomodulators. India: Andhra university; 2010. p. :235. Ph. D. Thesis
- Some metabolic alteration in grass carp (Ctenopharyngodon idella) induced by exposure to cadmium. J. Egypt. Ger. Soc. Zool.. 1996;21:441-457.
- [Google Scholar]
- Shalaby, A.M.E., 2009. The opposing effects ascorbic acid (Vitamin C) on Ochratoxin toxicity in Nile tilapia (Oreochromis niloticus). <http://www.ag.arizona.edu/ista/ista6web/pdf/209.pdf>. Rterieved:0 5-04 -09.
- Sharmin, S., Salam, M.A., Haque, M.A., Shahjahan, M., 2015. Toxicity bioassay of organophosphorous pesticide malathion in common carp, Cyprinus carpio. Proceedings of 5th International Conference on Environmental Aspects of Bangladesh [ICEAB 2015]. pp 99–100.
- Impact of malathion on the biochemical parameters of gobiid fish, Glossogobius giuris (Ham) J. Environ. Boil.. 2006;27:119-122.
- [Google Scholar]
- Physiological stress responses in big gamefish after exposure: Observations on plasma chemistry and blood factors Comp. Biochem. Physiol.. 1996;84:565-571.
- [Google Scholar]
- Acute lethal and sub-lethal effects of neem leaf extracts on neotrapical freshwater fish, Prochilodus lineatus. Comp. Biochem. Physiol. Part C. 2007;145:236-244.
- [Google Scholar]
- Zaki, M.S., Mostafa, S.O., Nasr, S., Noor El-deen, A.I., Ata, N.S., Awad, I.M., 2009. Biochemical, clinicopathological and microbial changes in Clarias gariepinus exposed to pesticide malathion and climate changes. Reports and opinion. pp. 6–11.







