Translate this page into:
Rhoifolin protects cisplatin mediated pulmonary toxicity via attenuation of oxidative stress, inflammatory response, apoptosis and histopathological damages
⁎Corresponding author. ali0703593@gmail.com (Ali Akbar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cisplatin (CP) is a ubiquitous antineoplastic medicine that has been recognized to have sever toxic effects on different organs including lungs. Rhoifolin (RHO) is a therapeutic compound with significant pharmacological activities. The present study was designed to evaluate the protective effect of RHO against CP induced pulmonary toxicity. Twenty-four rats were randomly divided into 4 groups: control group, CP treated group (20 mgkg−1), CP + RHO treated group (20 mgkg−1 + 10 mgkg−1) and RHO supplemented group (10 mgkg−1). Following 30 days of administration, our results showed that CP treatment decreased the activity of antioxidant enzymes such as glutathione (GSH), glutathione reductase (GSR), glutathione peroxidase (GPx), glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT) while elevated the level of malondialdehyde (MDA) along with reactive oxygen species (ROS). Furthermore, levels of inflammatory cytokines involving interleukin-6 (IL-6), nuclear factor-kappa B (NF-κB), interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α) and cyclo-oxygenase-2 (COX-2) activity were escalated. Besides, treatment with CP enhanced the activities of apoptotic proteins i.e., Bax, caspase-9 along with caspase-3 while reducing the activity of Bcl-2. Additionally, the histopathological examination revealed significant pulmonary tissue impairments in the CP exposed group. However, RHO treatment considerably (P < 0.05) recovered the abovementioned CP-induced toxic effects. Therefore, the current research demonstrated that RHO may be used as a promising pharmacological compound to cure CP-instigated pulmonary damages due to its antioxidant, anti-inflammatory, antiapoptotic and histo-protective properties.
Keywords
Rhoifolin
Antioxidant
Cisplatin
Pulmonary toxicity
Oxidative stress
1 Introduction
Cancer has emerged as an alarming health concern and a primary cause of mortalities worldwide (Aboubakr et al., 2023). Cisplatin (CP) is a platinum-derived antineoplastic drug that acts as a DNA alkylating compound and has been clinically applied in the management of a broad range of malignancies i.e., liver, kidney, ovarian, testicular, bladder as well as lung cancer (Elsherbiny et al., 2016). The chemotherapeutic action of CP is associated with its ability to provoke biochemical cross-linkage between DNA strands, obstructing DNA replication and impeding gene expression, ultimately leading to an apoptotic response in the body (Melnikov et al., 2016). Despite its remarkable clinical benefits, CP has been reported to have limited therapeutic index owing to its severe toxic effects including pulmonary toxicity (Afsar et al., 2018).
CP has been documented to induce pulmonary oxidative stress (OS) by increasing oxidant levels, decreasing antioxidant concentration and accelerating the secretion of proinflammatory cytokines (Unver et al., 2019). It also provokes amplification in H2O2 and MDA levels along with a significant increase in the apoptotic response in the lung tissue (Han et al., 2021). Furthermore, CP treatment also triggers an acute inflammatory condition in the biological system by reducing the activity of anti-inflammatory protein along with a substantial amplification in pro-inflammatory biomarkers (Ijaz et al., 2023a). Recent studies have reported that CP exposure can instigate histological abnormalities including edema, alveolar septal fibrosis and infiltration of polymorpho-nuclear leukocytes in lung tissue (Unver et al., 2019).
Flavonoids have garnered remarkable attention in current times due to their effective pharmacotherapeutic actions against various pathologies (Ferreira et al., 2015; Men et al., 2022). Rhoifolin (RHO) is an important flavonoid glycoside that has gained a substantial attention in recent years due to its broad range of pharmacological activities i.e., antioxidant, anti-inflammatory and hepatoprotective effects (Refaat et al., 2015). Regardless of these potential curative actions, the ameliorative effect of RHO to antagonize pulmonary toxicity is unascertained. Therefore, this study was designed to assess the alleviative potential of RHO against CP-induced pulmonary toxicity.
2 Materials and methods
2.1 Chemicals
CP (CAT No: 15663-27-1) & RHO (CAT No: 17306-46-6) were purchased from Sigma-Aldrich (Germany).
2.2 Animals
Twenty-four albino rats (age, 8–12 weeks; weight, 250 ± 20 g) were used for this study. Rats were confined in steel cages at the experimental facility of University of Agriculture, Faisalabad (UAF). They were maintained at 12-h of dark & light cycles, at a constant temperature (22–24 °C). Furthermore, tap water & food chaw was provided. Experiment was conducted in accordance with protocol (CEE Council 86/609) from European Union of Animal Care and Experimentation.
2.3 Experimental design
The rats were randomly divided into 4 groups (n = 6). The treatment regimens were as follows: control group, CP treated group (10 mgkg−1), CP + RHO administrated group (10 mgkg−1 + 20 mgkg−1) and only RHO supplemented group (20 mgkg−1) via oral gavage. The dosage of CP, 10 mgkg−1 and RHO, 20 mgkg−1 was administered in accordance with the previous study of Saher et al. (2023). On the 31st day of experiment, rats were anesthetized using ketamine (60 mgkg−1) and xylazine (6 mgkg−1), then decapitated & blood samples were taken for further analysis. The lungs were carefully removed, divided into two equal parts and cleaned with normal saline. One part was fixed in a formalin (10 %) solution for histological analysis. The second part was kept in plastic bag for biochemical evaluations.
2.4 Biochemical marker assessment
The activity of CAT was quantified in compliance with the protocol illustrated by Aebi (1984) while, SOD activity was measured by using the approach outlined Kakkar et al. (1984). GPx content was analyzed in accordance with the approach of Lawrence & Burk (1976). The activity of GSR was measured by employing the methodology explained by Carlberg & Mannervik (1975). The GSH activity was measured following the guidelines documented by Jollow et al. (1974). The quantification of GST activity was carried out as per the protocol illustrated by Younis et al. (2018). Hayashi et al. (2007) approach was followed to analyze ROS concentration. Ohkawa et al. (1979) procedure was considered for the assessment of the level of MDA.
2.5 Inflammatory markers assessment
For the evaluation of inflammatory markers in the lungs, commercially procured kits were employed. The quantification of NF-κB (CSB-E13148r), IL-6 (CSB-E04640r), TNF-α (CSB-E07379r), IL-1β (CSB-E08055r) along with COX-2 (CSB-E04640r) activity was performed with the help of ELISA kits specifically designed for rats, sourced from Shanghai, China. The evaluation was undertaken in compliance with the guidelines provided by the manufacturer.
2.6 Assessment of apoptotic markers
The quantification of Caspse-3 (CSB-E08857r), Bax (CSB-EL002573RA), Bcl-2 (CSB-E08854r) and caspase-9 (CSB-E08863r) was carried out using ELISA kits specifically designed for rats, procured from Cusabio Technology Llc, Houston, TX, USA.
2.7 Histological examination
The lungs samples were kept in formalin (10 %) solution and passed through dehydration process using ascending grades of ethanol. Lung tissues were encased in paraffin wax and sliced into small pieces by using microtome machine. These slices were stained with eosin following hematoxylin & analyzed using light microscope, Olympus BX51 (Imagetec Pvt. Ltd., Chennai, India), for histo-pathological examination.
2.8 Statistical evaluation
Data were depicted as Mean ± SEM. One-way analysis of variance (ANOVA) was performed, followed by the Tukey’s test. P < 0.05 was set as level of significance.
3 Results
3.1 Impact of RHO on antioxidant profile
CP exposure significantly increased (P < 0.05) the activities of CAT, GSR, SOD, GPx, GSH & GST as compared to control group. RHO co-administration with CP substantially restored the activities of aforementioned antioxidant enzymes as compared to CP exposed rats. Supplementation of RHO (alone) displayed mean values of these biochemical marker close to the control rats (Table 1). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
CP
CP + RHO
RHO
CAT (U/mg protein)
12.423 ± 1.26a
7.120 ± 0.43b
9.697 ± 0.55b
12.610 ± 1.36a
SOD (U/mg protein)
8.830 ± 1.17a
4.230 ± 0.39b
7.033 ± 0.29a
8.790 ± 1.16a
GSR (nM NADPH oxidized/min/mg tissue
6.587 ± 0.38a
2.013 ± 0.40c
5.100 ± 0.28b
6.663 ± 0.43a
GPx (U/mg protein)
19.043 ± 0.66a
5.600 ± 0.30c
14.677 ± 1.06b
19.323 ± 0.60a
GSH (U/mg protein)
32.38 ± 2.06a
11.480 ± 1.04c
25.187 ± 1.52b
32.46 ± 2.08a
GST (U/mg protein)
35.480 ± 1.72a
14.56 ± 1.88c
25.910 ± 1.52b
35.92 ± 2.22a
3.2 Impact of RHO on oxidant profile
The levels of ROS & MDA were markedly (P < 0.05) augmented in CP exposed animals as compared to control rats. Co-administration of RHO with CP remarkably lowered the ROS & MDA levels as compared to CP exposed rats. However, only RHO supplemented rats resulted in ROS & MDA levels close to control rats (Table 2). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
CP
CP + RHO
RHO
MDA (nmol/g)
0.86 ± 0.09c
6.52 ± 0.37a
2.56 ± 0.20b
0.83 ± 0.08c
ROS (nmol/g)
0.49 ± 0.22c
8.43 ± 0.44a
2.33 ± 0.27b
0.46 ± 0.23c
3.3 Impact of RHO on inflammatory biomarkers
CP intoxication remarkably (P < 0.05) upsurged the NF-κB, IL-1β, IL-6, TNF-α levels along with activity of COX-2 as compared to control rats. CP + RHO treatment led to a substantial restoration in the aforesaid cytokines in contrast to CP exposed rats. No significant difference was analyzed in these cytokines level between the RHO treated rats & the control (Table 3). Distinct superscripts on various values demonstrated discrepancies among other groups.
Parameters
Groups
Control
CP
CP + RHO
RHO
NF-kB (ng/g tissue)
23.99 ± 1.28c
88.60 ± 2.40a
47.60 ± 2.62b
23.81 ± 1.36c
TNFα (ng/g tissue)
9.84 ± 1.380c
37.49 ± 1.75a
19.56 ± 1.40b
9.72 ± 1.35c
IL-1ß (ng/g tissue)
25.02 ± 1.82c
83.97 ± 1.48a
52.92 ± 3.07b
24.38 ± 1.16c
IL-6 (ng/g tissue)
9.823 ± 1.29c
62.75 ± 2.84a
27.38 ± 3.65b
9.78 ± 1.29c
COX-2 (ng/g tissue)
24.99 ± 1.56c
73.52 ± 2.02a
39.10 ± 1.99b
24.86 ± 1.61c
3.4 Impact of RHO on apoptotic biomarkers
CP treatment significantly (P < 0.05) escalated the activities of Caspase-3, Bax, Caspase-9 while reduced the Bcl-2 activity as compared to control rats. However, the co-administration of RHO with CP culminated in a substantial restoration in the disturbed apoptotic biomarkers status as compared to rats treated with CP. Furthermore, supplementation of RHO resulted in mean values of these biomarkers close to the control animals (Fig. 1).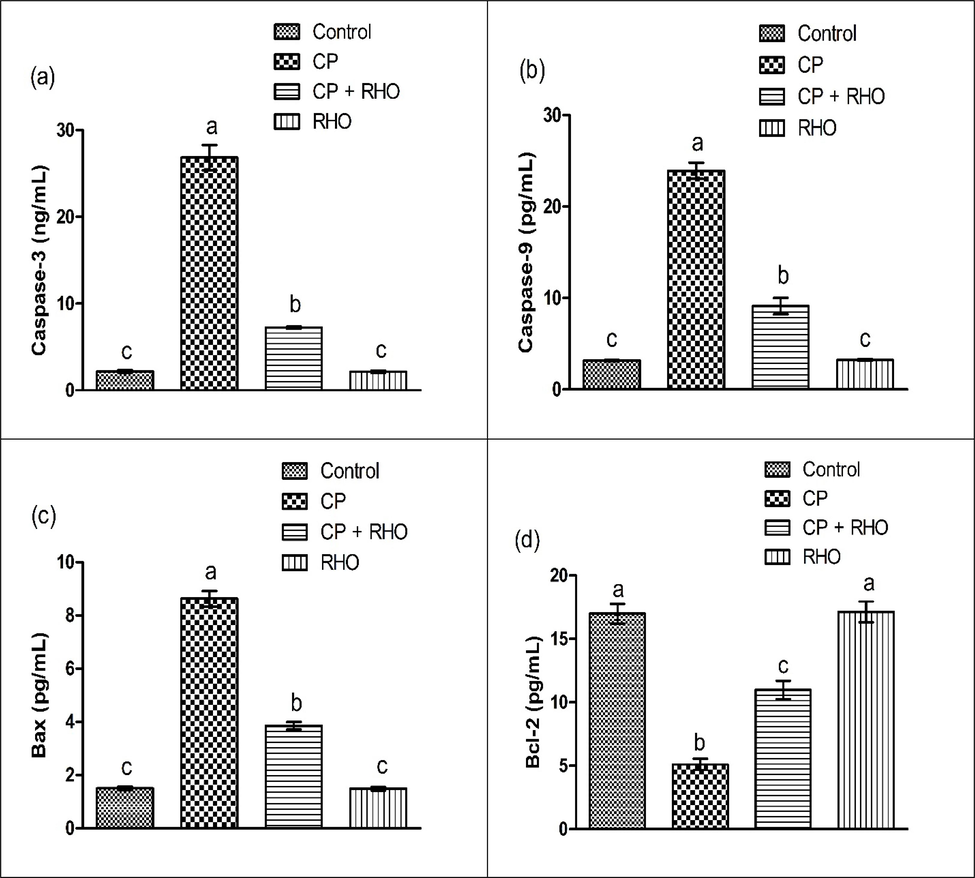
Effect of CP & RHO on activity of (a) Caspase-3 (b) Caspase-9 (c) Bax (d) Bcl-2. Values are depicted as Mean ± SEM. Significant differences displayed as (P < 0.05).
3.5 Impact of RHO on pulmonary histology
CP intoxication led to various histopathological disruptions in pulmonary tissues such as elevated interstitial cellularity, dilated inter-alveolar spaces, alveolar fibrosis, congested alveolar capillaries along with thickening of bronchiolar walls as compared to control group. Nevertheless, RHO supplementation remarkably (P < 0.05) mitigated abovementioned histopathological disruptions prompted by CP exposure. However, RHO only supplemented group showed normal histology as compared to control group.
4 Discussion
The antioxidant enzymes (GPx, GSR, SOD, GSH, GST & CAT) activities were remarkably decreased in CP supplemented rats, while MDA & ROS levels was notably increased. Antioxidant enzymes are the primary protective barrier that safeguards the biomolecules (lipids, DNA and proteins) from oxidative injury by decreasing ROS generation (Ighodaro and Akinloye, 2018). H2O2, OH, O−2 & NO are main reactive molecules that are engaged in cellular injury (Mijatović et al., 2020). SOD enzymatically mitigates the O−2 via catalyzing the conversion of O−2 into O2 & H2O2 (Xu et al., 2015), whereas H2O2 is transformed into H2O via the biocatalytic function of GPx & CAT (Weydert and Cullen, 2010). GSR maintains the level of GSH, which retains the uninterrupted GPx levels (Rojo et al., 2014). CP stimulates OS in the pulmonary tissues (Ali et al., 2015). OS is a state in which the elevated level of ROS detriments the cells, tissue & organ (Hajam et al., 2022). When the ROS contents in the tissues are upsurged, they attack PUFA in the cellular plasma membrane & brought on cascade of bio-catalytic process “lipid peroxidation (LP)” (Unver et al., 2019). The concentration of LP is positively correlated with the generation of superoxide radicals, as evidenced by the levels of MDA, the ultimate product of LP (Kaynar et al., 2005; Ehsan et al., 2023). Our findings are further supported by the study of Unver et al. (2019) who reported that CP treatment increased the levels of ROS & LP as well as decreased the levels of antioxidative enzymes in the lungs. However, CP induced OS was notably averted by RHO supplementation as evidenced by the escalated antioxidant enzymes levels, declined MDA & ROS level owing to its ROS neutralizing nature.
CP treatment culminated in a considerable augmentation in inflammatory biomarkers (IL-6, NF-κB, TNF-α, IL-1β levels & activity of COX-2). NF-κB is considered as an integral factor underlying initiation of inflammatory events in the cells (Dorrington and Fraser, 2019). NF-κB is an oxidation–reduction modulated transcriptional factor that has been documented as the mediator of OS (Sequeira, 2021). Augmented cellular ROS concentration subsequently amplify NF-κB initiation (Ijaz et al., 2020a). IL-6 is recognized as a pro-inflammatory biomarker that serves as an indispensable cytokine in the acceleration of inflammatory reaction (Hou et al., 2008). Besides, COX-2 is a principal cytokine that governs the systemic inflammatory condition (Wu et al., 2012). OS instigated on account of CP intoxication elicits NF-κB activation that accelerates the biosynthesis of aforesaid biomarkers (Ijaz et al., 2020b; Ijaz et al., 2023a). However, RHO supplementation notably restored the state of these inflammatory cytokines. Our outcomes confirm the hypothesis that RHO could suppress NF-κB initiation & declined levels of proinflammatory cytokines on account of its anti-inflammatory action & ROS neutralizing attributes. The fact that the anti-oxidants supplementation mitigates the initiation of NF-kB evident that ROS are engaged in NF-kB initiation, which trigger the inflammatory cascade (Elliott and Chithan, 2017).
In the present research, CP intoxication upsurged Caspase-9, Bax & Caspase-3, while it subsided Bcl-2 concentration. Bax & Bcl-2 are main cytokines of the Bcl-2 protein family. Bcl-2 (anti-apoptotic cytokine), restrains the process of cellular apoptosis, while Bax facilitate cellular apoptotic events (Santana, 2018). In addition to this, Caspase-3 & 9 are engaged in cleavage of intracellular enzymes, consequently provoking structural modifications in cells that elicit the mechanism of apoptotic (Uğuz et al., 2009). Any imbalance in these biomarkers changes permeability of mitochondrial membranous channels, which promotes cytoplasmic secretion of hemeprotein “cytochrome c” (Akao et al., 2001). The elevated concentration of this hemeprotein ultimately activates Caspase-3 which culminated in cellular apoptosis (Porter and Jänicke, 1999). Therefore, RHO treatment declined Bax along with Caspsse-3, while upregulated Bcl-2 concentration due to its anti-apoptotic effects. Present research results are in accordance with the findings of Saher et al. (2023), who also documented that RHO treatment suppresses apoptosis in neuronal cells by reducing Caspase-3 & Bax as well as enhancing Bcl-2 expression.
CP intoxication culminated in histopathological disruptions in pulmonary tissues i.e., elevated interstitial cellularity, dilated inter-alveolar spaces, alveolar fibrosis, congested alveolar capillaries along with thickening of bronchiolar walls. OS recognized as the key event underlying the structural irregularities in the pulmonary tissues. ROS instigate an inflammatory response, which culminates in enhanced interstitial cellularity (Ijaz et al., 2023b). Moreover, it prompts oxidative damage leading to tissue remodeling i.e., dilated inter-alveolar spaces and alveolar fibrosis, disrupts vascular function (congested inter-alveolar capillaries), and induces airway inflammation as well as thickening of bronchiolar walls (Fekri et al., 2018; Unver et al., 2019). Nevertheless, RHO effectively mitigated these adverse histological damages provoked by CP owing to its antioxidative attributes and its capability to neutralize augmented ROS concentration. Our results are corroborated by Unver et al., (2019) who revealed that flavonoids exert effective therapeutic action to antagonize OS provoked pulmonary histological impairments.
5 Conclusion
CP intoxication provoked pulmonary toxicity via augmenting concentration of oxidants “MDA & ROS” following a substantial decline in the antioxidant biomarkers levels. Besides, a profound upsurge was observed in proinflammatory cytokines concentration and apoptotic biomarkers levels were also scaled up. Additionally, histoarchitectural modifications were noticed in the pulmonary tissues on account of CP intoxication. Nevertheless, RHO effectively counteract the impairments in the pulmonary tissues of rats indicating that the RHO may be utilized as an important pharmacotherapeutic agent to rectify CP provoked pulmonary toxicity.
CRediT authorship contribution statement
Ali Akbar: Conceptualization, Writing – original draft, Investigation, Formal analysis. Rabia Azmat: Data curation, Writing – review & editing, Investigation. Moazama Batool: Methodology, Visualization, Formal analysis. Bader O. Almutairi: Funding acquisition, Project administration, Resources, Data curation. Mian Nadeem Riaz: Formal analysis and Software.
Acknowledgement
This work was funded by Researchers Supporting Project number (RSP2024R414), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Allicin and lycopene possesses a protective effect against methotrexate-induced testicular toxicity in rats. Pak. Vet. J.. 2023;43:559-566.
- [Google Scholar]
- Acacia hydaspica R. Parker ameliorates cisplatin induced oxidative stress, DNA damage and morphological alterations in rat pulmonary tissue. BMC Compl. Aler. Med. 2018;18:1-13.
- [Google Scholar]
- Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ. Res.. 2001;88:1267-1275.
- [Google Scholar]
- The effect of thymoquinone treatment on the combined renal and pulmonary toxicity of cisplatin and diesel exhaust particles. Exper. Bio. Med.. 2015;240:1698-1707.
- [Google Scholar]
- Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem.. 1975;250:5475-5480.
- [Google Scholar]
- NF-κB signaling in macrophages: dynamics, crosstalk, and signal integration. Front. 2019 immunol.10.705
- [Google Scholar]
- Attenuative Effects of Ginkgetin Against Polystyrene Microplastics-Induced Renal Toxicity in Rats. Pak. Vet. J.. 2023;43:819-823.
- [Google Scholar]
- The Impact of Plant Flavonoids on Mammalian Biology: Implications for Immunity, Inflammation and Cancer. Routledge; 2017.
- Renal protective effects of arjunolic acid in a cisplatin-induced nephrotoxicity model. Cytokine. 2016;77:26-34.
- [Google Scholar]
- Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin-induced pulmonary fibrosis: biochemical and histopathological study. Drug Chem Toxicol. 2018;41:408-414.
- [Google Scholar]
- Flavonoid compounds as reversal agents of the P-glycoprotein-mediated multidrug resistance: biology, chemistry and pharmacology. Phytochem. Rev.. 2015;14:233-272.
- [Google Scholar]
- Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells.. 2022;11
- [Google Scholar]
- Cisplatin induces lung cell cilia disruption and lung damage via oxidative stress. Free Radic. Biol. Med.. 2021;177:270-277.
- [Google Scholar]
- High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res.. 2007;631:55-61.
- [Google Scholar]
- Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev.. 2008;4:179.
- [Google Scholar]
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med.. 2018;54:287-293.
- [Google Scholar]
- Methanolic extract of Fraxinus xanthoxyloides attenuates cisplatin-induced reproductive toxicity in male albino rats. Pak. Vet. J.. 2020;40:489-493.
- [Google Scholar]
- Casticin Alleviates Testicular and Spermatological Damage Induced by Cisplatin in Rats. Pak. Vet. J.. 2020;40:234-238.
- [Google Scholar]
- Evaluation of possible palliative role of tamarixetin against cisplatin-induced renal toxicity by modulation of oxidative stress, inflammation and apoptosis in rats. J. King Saud Univ. Sci.. 2023;35:102787
- [Google Scholar]
- Sciadopitysin attenuates paraquat induced renal toxicity by modulating Nrf-2/Keap-1 pathway in male albino rats. Asian J. Agric. Biol. 2023;10:2023110.
- [Google Scholar]
- Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151-169.
- [Google Scholar]
- A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys.. 1984;21:130-132.
- [Google Scholar]
- Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu–Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett.. 2005;227:133-139.
- [Google Scholar]
- Glutathione peroxidase activity in seleniumdeficient rat liver. Biochem.. Biophys. Res. Commun.. 1976;71:952-958.
- [Google Scholar]
- Insights into RNA binding by the anticancer drug cisplatin from the crystal structure of cisplatin-modified ribosome. Nucleic Acids Res.. 2016;44:4978-4987.
- [Google Scholar]
- Men, T., Dinh Hai Yen, N., la Kim, Thi Kim Hue, N., 2022. Phytochemical constituents and antioxidant activity of some medicinal plants collected from the Mekong Delta, Vietnam. Asian J. Agric. Biol. 202105230.
- The double-faced role of nitric oxide and reactive oxygen species in solid tumors. Antioxidants. 2020;9:374.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Rhoifolin: a review of sources and biological activities. Int. J. Pharmacogn.. 2015;2:102-109.
- [Google Scholar]
- Redox control of microglial function: molecular mechanisms and functional significance. Antioxidants Redox Signaling. 2014;21:1766-1801.
- [Google Scholar]
- Saher, F., Ijaz, M.U., Hamza, A., Ain, Q.U., Hayat, M.F., Afsar, T., Almajwal, A., Shafique, H. and Razak, S., 2023. Mitigative potential of rhoifolin against cisplatin prompted testicular toxicity: biochemical, spermatogenic and histological based analysis. Toxicol. Res.,073.
- Sequeira, R.B., 2021. Mitochondrial and redox-based transcriptional changes in type-2 diabetes mellitus and periodontitis (Master's thesis).
- Unver, E., Tosun, M., Olmez, H., Kuzucu, M., Cimen, F.K. and Suleyman, Z., 2019. The effect of taxifolin on cisplatin-induced pulmonary damage in rats: A biochemical and histopathological evaluation. Mediators Inflamm.2019.
- Selenium modulates oxidative stress-induced cell apoptosis in human myeloid HL-60 cells through regulation of calcium release and caspase-3 and-9 activities. J. Membr. Biol. J. Membrane Biol.. 2009;232:15-23.
- [Google Scholar]
- Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc.. 2010;5:51-66.
- [Google Scholar]
- Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J. Neuroinflammation.. 2012;9:1-15.
- [Google Scholar]
- In situ OH generation from O2− and H2O2 plays a critical role in plasma-induced cell death. PLoS One.. 2015;10:e0128205.
- [Google Scholar]
- Ameliorating role of methanolic leaves extract of Fraxinus xanthoxyloides against CCl 4-challanged nephrotoxicity in rats. Pak. J. Pharm. Sci.. 2018;31:1475-1484.
- [Google Scholar]







