Rheological behavior of three polymers and their hybrid composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN)
⁎Corresponding author. r.hsissou@gmail.com (Rachid Hsissou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Our work is to compare the rheological behaviors of the three synthesized multifunctional epoxy resins: triglycidyl ether tribisphenol A of ethylene (TGEEBA), hexaglycidyl ethylene of trimethylene dianiline (HGEMDA) and nanoglycidyl trihydrazine 4.4.4-tripropoxy tribisphenol A of ethylene (NGHPBAE). As a first step, we crosslinked and formulated these three composite by means of methylene dianiline in the presence of different percentages (5%, 10% and 15%) of the natural phosphate as filler. In the second stage, we made a comparative study of the rheological properties of these three synthesized epoxy matrices. The latter plays a major role in the phenomenon of their flow and their implementation. Then, we carried out a comparative study of the thermodynamic parameters, namely: glass transition temperature, activation energy, enthalpy variation and entropy variation.
Keywords
Rheological behavior
Epoxy resins
Composite
Crosslinking
Formulation
1 Introduction
The behavior of viscoelastic materials is linear and their states are intermediate between that of the ideal elastic materials and that of the viscous liquid materials. On the one hand, the elasticity of these materials is reflected in their capacity of the energy to be preserved and to restore after its deformation. Their viscosity as well is expressed by the capacity of the energy to be dissipated in the form of heat (Zhang and Park, 2017; Sedlacek et al., 2014; Chen et al. 2010). In addition, rheology is a discipline that deals with the flow and application of materials under the action of applied stress (Bharadwaj et al. 2014; Lim et al., 2013; Aho et al., 2010). Indeed, epoxy resins are thermosetting multifunctional macromolecular matrices (Hsissou et al., 2014) and they affect several industrial domains such as: aeronautics (Gohardani et al., 2012), space construction (Panchavarnam et al., 2016; Sungsanit et al., 2010) and having many properties: thermal (Zhang et al., 2017), mechanical (Alvaro et al., 2016; Vadivelan et al., 2015), electrical (Sharaf et al., 2016; Shahenoor et al., 2016; Kota et al., 2007) dielectric (Vijayalakshmi et al., 2015), viscosimetric (Hsissou et al., 2016), viscoelastic and rheological (Hsissou et al., 2017). Our objective therefore is to carry out a comparative study of the synthesized multifunctional molecular architectures: the triglycidyl ether tribisphenol A of ethylene (TGEEBA), hexaglycidyl ethylene of trimethylene dianiline (HGEMDA) and nanoglycidyl trihydrazine 4, 4,4-tripropoxy tribisphenol A of ethylene (NGHPBAE) with respect to the viscoelastic and rheological behaviors over a wide temperature range and the control of the storage and loss conditions (Fernandez et al., 2010; Rodriguez et al., 2016). The incorporation of the dispersed additives into the composite is used to improve the rheological properties (Grich et al., 2014; Rami et al., 2008; Canales et al., 2015). It is very important to specify the influence of natural phosphate in composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN) in order to optimize and control the system dispersed in the matrices and in order to quantify its viscoelastic and rheological performances (Fan et al., 2007; Chen et al., 2015).
2 Experimental
2.1 Used products
We used three multifunctional epoxy resins in this work: triglycidyl ether tribisphenol A of ethylene (TGEEBA), hexaglycidyl ethylene of trimethylene dianiline (HGEMDA) and nanoglycidyl trihydrazine 4,4,4-tripropoxy tribisphenol A of ethylene (NGHPBAE). The molecular structures of the non-crosslinked epoxy matrices TGEEBA, HGEMDA, NGHPBAE which are synthesized in our laboratory are shown in Figs. 1, 2 and 3 while the crosslinked structures are shown in Figs. 4, 5 and 6. Methylene dianiline and natural phosphate have been marketed by Aldrich Chemical Co.
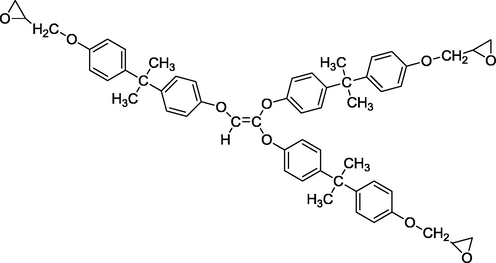
- Semi-developed formula of triglycidyl ether tribisphenol A of ethylene.
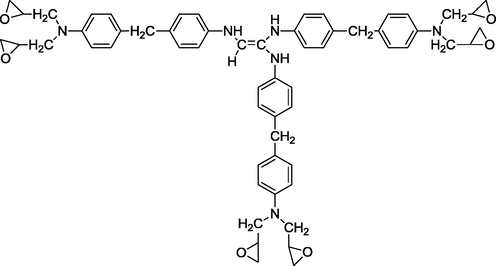
- Semi-developed formula of hexaglycidyl ethylene of trimethylene dianiline.
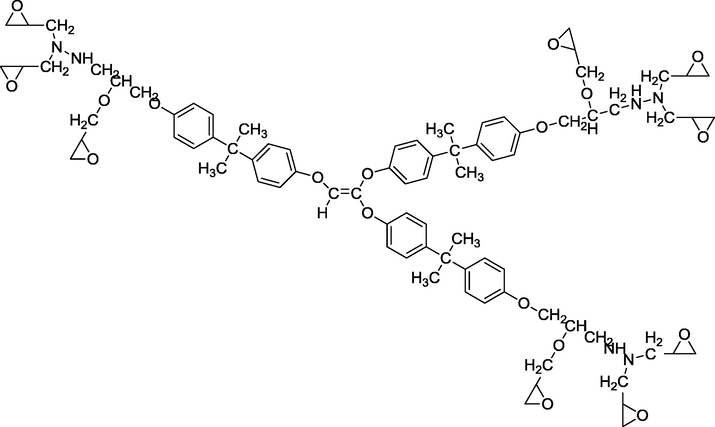
- Semi-developed formula of nanoglycidyl trihydrazine 4,4,4-tripropoxy tribisphenol A of ethylene.
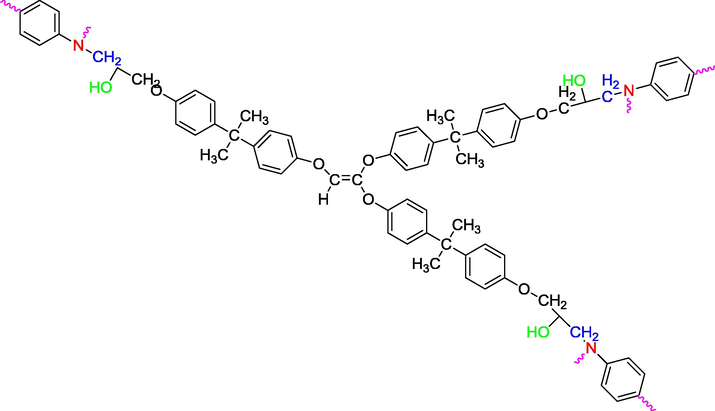
- Scheme of triglycidyl ether tribisphenol A of ethylene crosslinked with methylene dianiline.
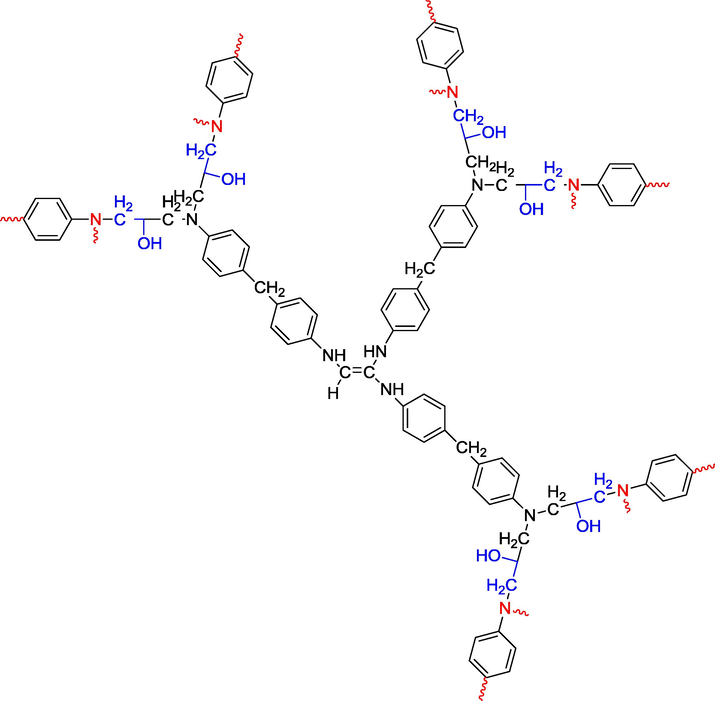
- Scheme of hexaglycidyl ethylene of trimethylene dianiline crosslinked with methylene dianiline.
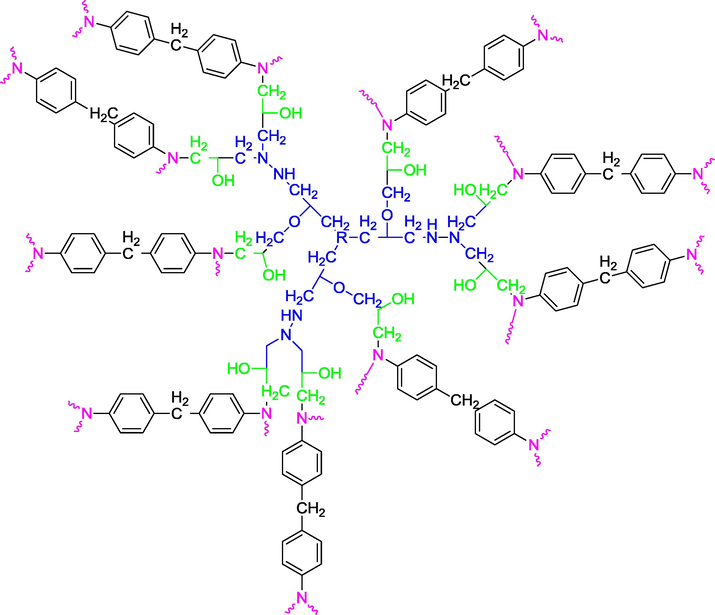
- Scheme of nanoglycidyl trihydrazine 4,4,4-tripropoxy tribisphenol A of ethylene crosslinked with methylene dianiline.
The semi-developed formulas of the polymers shown in Figs. 1, 2 and 3 have good viscosimetric properties. Moreover, the three-dimensional structures illustrated in Figs. 4, 5 and 6 show excellent rheological properties.
2.2 Viscoelastic and the rheological behaviors
The analyzes of the viscoelastic and rheological behaviors of the new standard synthesized, crosslinked and formulated epoxy resins were followed through RHM01-RD HAAKE type rheometer on the viscous samples.
3 Results and discussions
3.1 Viscoelastic behavior of standard resins
The viscoelasticity relates to the study of the flow, the deformation, the elasticity and the viscosity of the materials under consideration. We are interested in the viscoelastic behavior of the standard resins TGEEBA, HGEMDA and NGHPBAE since the latter play a primordial role in the phenomena of the flows of macromolecular matrices. Fig. 7 shows the different viscoelastic states of the standard macromolecular matrices TGEEBA, HGEMDA and NGHPBAE according to temperature.
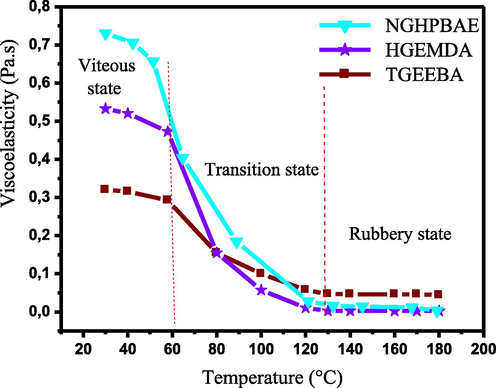
- Viscoelasticity of the three resins TGEEBA, HGEMDA and NGHPBAE according to temperature.
Fig. 7 it was showed that the different viscoelastic states of the standard multifunctional polymers in view of temperature. For each multifunctional epoxy resin in this figure, we noticed the plot of three phases, namely: (vitreous state, transition state and rubbery state). The density of the viscosity decreases with increasing temperature, which is probably due to the supplied heat since it accelerates the degradation process of the polyepoxide resins. Moreover, viscoelasticity depends strongly on the number of epoxide groups formed in the macromolecular matrix, since the latter increases with the increase in the number of epoxide groups.
3.2 Experimental determination of the glass transitions temperatures Tg
The thermal behavior of the different viscoelasticities clearly shows the existence of the transitions of the phases, that is to say the transformation of the viscous states into the rubbery states. We have deduced, from Fig. 7, the different glass transition temperatures of the three macromolecular matrices: TGEEBA, HGEMDA and NGHPBAE whose results are grouped in Table 1.
| Resins | TGEEGA | HGEMDA | NGHPBAE |
|---|---|---|---|
| Glass transition temperatures Tg (°C) | 120 | 125 | 128 |
This result seems to be very logical since the polyepoxide matrices TGEEGA, HGEMDA, NGHPBAE present respectively three, six and nine functional epoxide nuclei in their structures.
3.3 Experimental determination of activation energies Ea
Activation energy is a concept which was introduced in 1889 by the Swedish scientist Svante August Arrhenius who observed it empirical law and described the evolutions of viscosity with temperature. In this law, a term is called activation energy and the Arrhenius equation Ƞ=Ƞ0 exp (−Ea/RT). Therefore, allowed us to calculate the activation energies’ values from the Arrhenius slope, in Fig. 8.
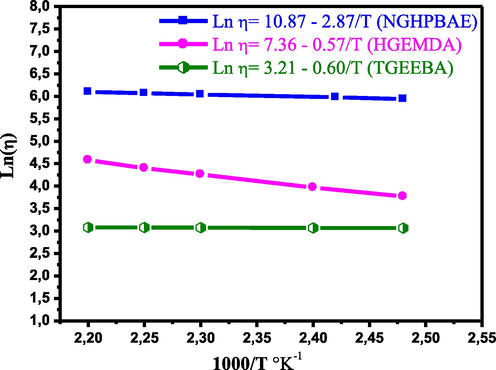
- Variation of the logarithm of the absolute viscosity in view of the inverse of the temperature of the resins TGEEBA, HGEMDA and NGHPBAE.
Ƞ: Viscosity (Pa); Ƞ0: Viscosity obtained whose ordinate at the origin (Pa); Ea: Activation energy (kJ/mole); R: Perfect gas constant (8.314 J.mol−1 K−1); T: Temperature (Kelvin).
Fig. 8 it was showed that the variation in the logarithm of the absolute viscosity in view of the inverse of the temperature of the various resins TGEEBA, HGEMDA and NGHPBAE. These variations are straight lines, starting from the Arrhenius relation. We have therefore calculated the activation energies, which are obtained from straight lines whose slopes (−Ea/R) are respectively equal to −0.60, −0.57 and −2.87. These energies are then grouped together in Table 2.
| Resins | TGEEGA | HGEMDA | NGHPBAE |
|---|---|---|---|
| Ea (kJ/mole) | 0.432 | 4.738 | 23.861 |
We notice that this energy increases with the number of epoxide groups for the polymers TGEEGA, HGEMDA and NGHPBAE having respectively three, six and nine epoxide nuclei. This result seemed to us to be in full agreement with our expectations.
3.4 Determinations of the thermodynamic parameters
The alternative formula of the Arrhenius equation thus makes it possible to determine the variation of enthalpy of activation and the variation of activation entropy according to the following equation (1) and (2).
N: Number of Avogadro (6.023 × 1023 mol−1), h: Planck constant (6.62 × 10–34 J.S.), ΔHa: Enthalpy variation of activation, ΔSa: Entropy variation of activation.
Fig. 9 shows the variation of the Ln (Ƞ/T) in view of the inverse of the temperature of the different polymers TGEEBA, HGEMDA and NGHPBAE. We obtained straight lines whose intercept is equal to (Ln(R/Nh) + (ΔSa/R)) and slopes equal to (ΔHa/R).
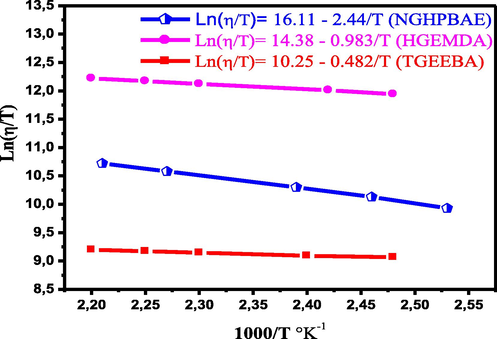
- Variation of Ln (Ƞ/T) in view of the inverse of the temperature of the different polymers TGEEBA, HGEMDA and NGHPBAE.
From Fig. 9 it was deduced that the values of the activation enthalpy variation ΔHa and the activation entropy variation ΔSa. Table 3 therefore groups together the values of ΔHa and ΔSa of the different macromolecular architectures TGEEBA, HGEMDA and NGHPBAE respectively.
| Matrices | TGEEGA | HGEMDA | NGHPBAE |
|---|---|---|---|
| ΔHa (kJ/mole) | −4.007 | −8.172 | −20.286 |
| ΔSa (J/mole) | −11,23,227 | −77,990 | −63,607 |
For the three tri, hexa- and nine functional polymers, their negative values of the enthalpy variation of activation ΔHa mean that the reactions are exothermic on the one hand. This value decreases respectively according to the increase of the epoxy core, which is explained by the released heat. This accelerates the process of degradation and multifunctional resins, on the other hand. As for the negative values of the activation entropy variation, it respectively increase according to the decrease of the disorder during the degradation of the multifunctional resins, which increases respectively according to the multifunctional number of the epoxide groups in the macromolecular matrix. This is consistent with the previous results.
3.5 Behavior of the complex viscosity of the polymers
Fig. 10 shows the evolution of the complex viscosity of the various epoxy resin TGEEBA, HGEMDA and NGHPBAE according to the angular velocity. Then, the obtained structures can evolve as function of the angular velocity under flow conditions.
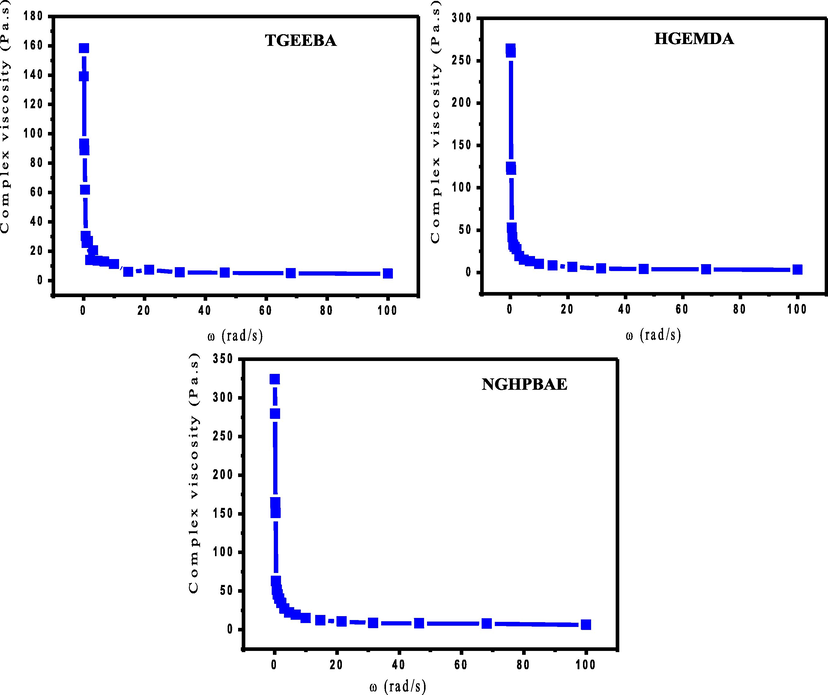
- Complex viscosity behavior according to the angular velocity.
Fig. 10 it was presented that the behavior of the complex viscosity show on the one hand, that the complex viscosity of different epoxy resins decreases with increasing in the angular velocity. On the other, the increase in angular velocity implies that the TGEEBA, HGEMDA and NGHPBAE resins pass from a viscous state to a liquid state. These results confirm that the viscoelasticity as a function of temperature. Consequently, the resins synthesized at low temperature must be stored.
3.6 Elastic and vitreous rheological behavior of the crosslinked and formulated polymers
The literature of the rheological behaviors of composites leads to a better understanding of the structures of the resin reinforcement by natural phosphate as load (Chen et al., 2015; Chen et al., 2015).
In this part of the work, we concentrated on the study of the rheological behavior respectively the elastic behavior G′ and the vitreous behavior G′′. The composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN) formulated by natural phosphate at different percentage (5%, 10% and 15%) were introduced into the rheometric cell RHM01-RD HAAKE.
3.7 Elastic and vitreous rheological behaviors according to the temperature
Fig. 11 shows respectively the elastic behaviors G′ and the vitreous behaviors G′′ of three new ethylene epoxy polymers (TGEEBA, HGEMDA and NGHPBAE) as a function of temperature. The rheological analyzes of the polymers were carried out between 20 and 180 °C.
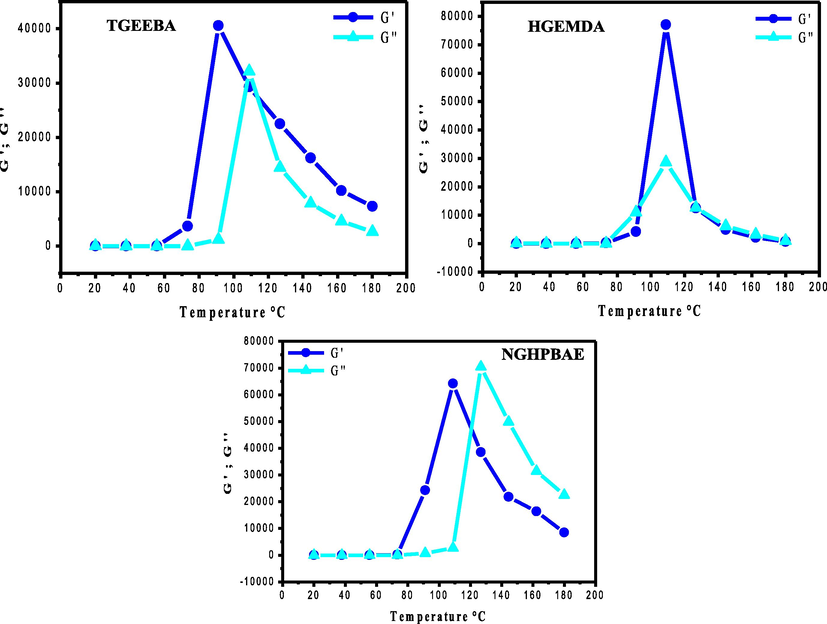
- Elastic G′ and vitreous G“ behaviors as a function of the temperature of the three polymers.
From Fig. 11 it was observed that the variation in elastic behaviors and vitreous behaviors for the three ethylene epoxy polymers increases with the increase in temperature to the phase transition temperature. From this last, the elastic and vitreous behaviors decrease. Above the temperature of the glass transitions the responses of the polymers are of the liquid type and below which they are of the gel type. The rheological measurements clearly show that the increase of the temperature induces transitions of the behaviors gels towards liquid behaviors.
3.8 Determination of glass transition temperatures
From Fig. 11 it was showed that the variation of elastic behavior G′ and vitreous G“ as a function of the temperature of the three epoxy macromolecular architectures. We have seen the existence of phase transition temperatures respectively tri, hexa and nine functional polymers (TGEEBA, HGEMDA and NGHPBAE). Finally, the glass transition temperatures respectively of the elastic behaviors G′ and glassy behaviors G′′ are grouped together in Table 4.
| Polymers | Tg (G′) (°C) | Tv (G′′) (°C) |
|---|---|---|
| TGEEBA | 91.110 | 108.869 |
| HGEMDA | 115 | 115 |
| NGHPBAE | 114 | 128 |
3.9 Experimental determination of activation energies Ea
Another method was used to estimate the activation energy associated with the rheological behavior process. This consists in determining the activation energy from Eq. (3).
Where G is the rheological behavior (Pa), G0 the obtained rheological behavior whose intercept (Pa), Ea the activation energy (kJ/mole), R the perfect gas constant (8.314 J. mol−1, K−1) and T temperature (Kelvin).
Fig. 12 presents respectively the variation of the logarithm of the elastic behaviors as a function of the inverse of the temperature and the variation of the logarithm of the vitreous behaviors as a function of the inverse of the temperature of new multifunctional polymeric architectures.
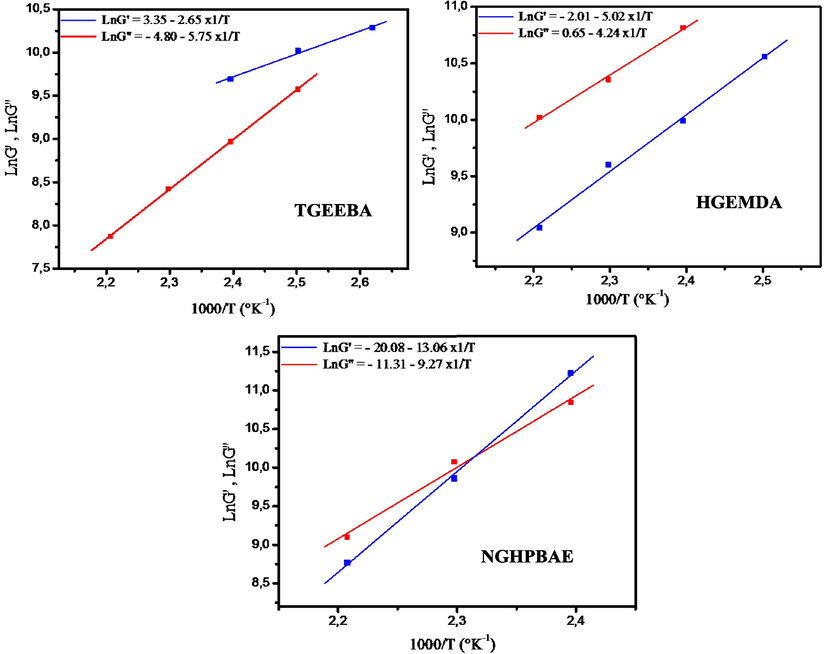
- Variation of Ln (G′) and Ln (G′′) as a function of the inverse of the temperature.
From Fig. 12 it was observed that the energy of activation of the elastic behaviors and vitreous behaviors respectively of the three new tri, hexa and nine-functional epoxy architectures. Table 5 summarizes the different energies of activations of three resins found from elastic behaviors and vitreous behaviors.
| Polymers | Ea (G′) (kJ/mole) | Ea (G′′) (kJ/mole) |
|---|---|---|
| TGEEBA | −22.032 | −47.805 |
| HGEMDA | −41.736 | −35.251 |
| NGHPBAE | −108.580 | −77.070 |
3.10 Determinations of thermodynamic parameters
Another Arrhenius law allows the determination of the activation enthalpy variation and the activation entropy variation. It is written according to Eqs. 4 and 5.
Fig. 13 shows respectively the variation of Ln (G′/T) and Ln (G′′/T) as a function of the temperature inverse of three epoxy polymer. (TGEEBA, HGEMDA and NGHPBAE). We have obtained lines whose intercept is equal to (Ln(R/Nh) + ΔSa/R) and equal slopes (−ΔHa/R).
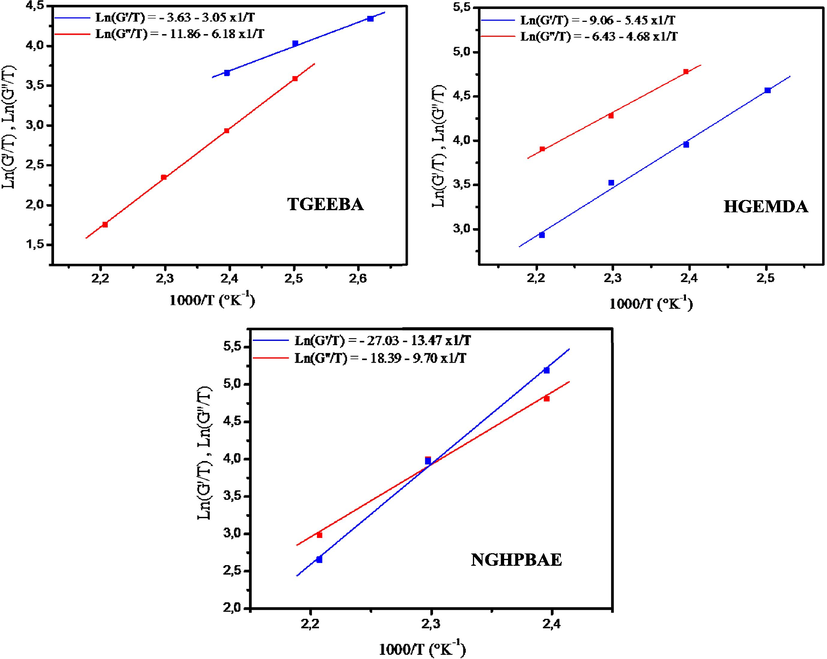
- Variation of Ln (G′/T) and Ln (G′′/T) as a function of the inverse of the temperature of three ethylene resins studied.
From Fig. 13 and Eq. 5 it was determined that the values of the activation enthalpy variation ΔHa and the activation entropy variation ΔSa of the new multifunctional macromolecular resins respectively tri, hexa and nine-functional (TGEEBA, HGEMDA and NGHPBAE) from elastic behaviors G′ and vitreous behaviors G′′. These values are grouped in Table 6.
| Behaviors | G′ | G′′ | ||
|---|---|---|---|---|
| Thermodynamic parameters | ΔHa (kJ/mole) | ΔSa (kJ/mole) | ΔHa (kJ/mole) | ΔSa (J/mole) |
| TGEEBA | −25.357 | −277.803 | −51.380 | −296.277 |
| HGEMDA | −45.311 | −272.948 | −38.909 | −251.082 |
| NGHPBAE | −111.989 | −422.351 | −80.645 | −348.855 |
Negative values of ΔHa activation enthalpy variation of elastic behaviors and vitreous behaviors mean that the reactions are exothermic. This is due to the heat provided by the apparatus which accelerates the process of the degradation of the multifunctional resins studied (TGEEBA, HGEMDA and NGHPBAE).
3.11 Elastic behavior G′ and vitreous G′′ according to the angular velocity
The rheological behavior of composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN) according to the angular velocity are presented in the following Figs. 14 and 15. Indeed, we performed rheological analyzes of the composites at 80 °C under a range of frequencies varying from 0.1 to 100 rad/s.
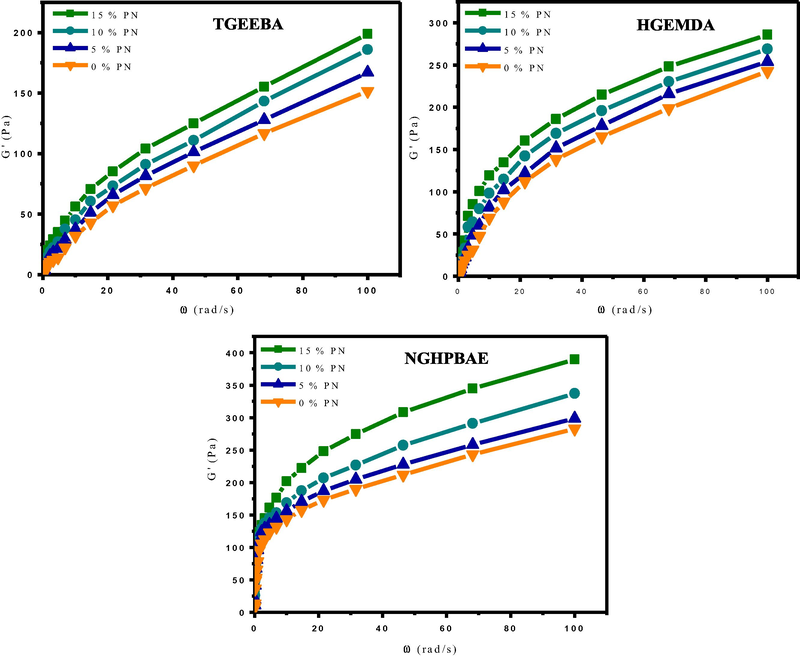
- Elastic behavior G′ of the composites according to the angular velocity at different formulations.
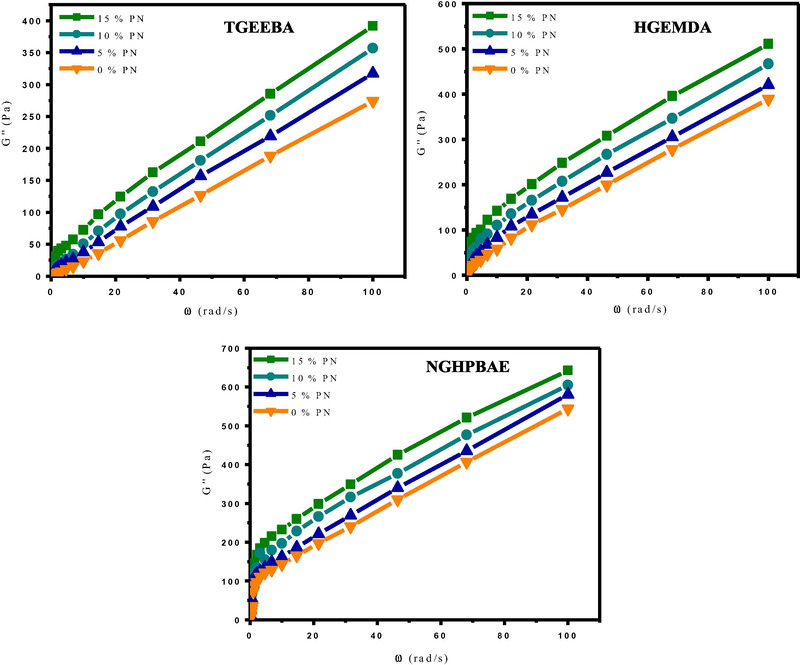
- Vitreous behavior G′′ of the composites according to the angular velocity at different formulations.
Figs. 14 and 15, its were showed that the storage modulus G′ and the loss modulus G′′ of three composites (TGEEBA/MDA/PN), (HGEMDA/MDA/PN) and (NGHPBAE/MDA/PN) with different formulations clearly show the increase of the latter as a function of the angular velocity. On the one hand, we observed a regular increase in the elastic and the loss behaviors at low frequency, thus reflecting the progressive reorganization of the composites. We have observed that the variation of the elastic and the vitreous behaviors increase with the percentage of the natural phosphate load incorporated in the composites for the same matrix and for the same family of multifunctional epoxy resins.
4 Conclusions
We have studied the comparison of viscoelasticity, thermodynamic parameters, complex viscosity, elastic behavior and vitreous behavior of the three new resins of different architectures TGEEBA, HGEMDA and NGHPBAE. The viscoelastic and rheological studies of the polymers in the standard and formulated state were carried out, through these structures, by using an RHM01-RD HAAKE rheometer. We first compared the viscoelastic states of the thermal behavior of standard polymers according to temperature whose result allowed us to notice that viscoelasticity increases with the number of epoxide groups. We, then, determined the thermodynamic parameters (Tg, Ea, ΔHa and ΔSa). The values which we found were in agreement with the structures of the synthesized resins. We secondly compared the complex viscosity of the three resins tri-, hexa- and nano-functional matrices with as function of the angular velocity. The obtained results describe that the elastic and vitreous behavior of three types of tri-, hexa- and nano-functional polymers respectively decreases according to the number of the epoxy. In a third step, the elastic and the vitreous behaviors according to the angular velocity, were carried out on the three crosslinked and formulated composites. Then, the study of the elastic and vitreous behaviors of the composites showed us that the behaviors increases as function of the angular velocity and also as in view of the numbers of epoxy groups constituting the standard matrices. This study allowed us finally to conclude that the rheological behaviors decrease for the same matrix in case of low charge rate for the same family of polymers.
Acknowledgements
I would like to thank Professor Ahmed ELHARFI, responsible for the Laboratory of Agricultural Resources, Polymers and Process Engineering (LARPPE), Team of Polymers and Organic Chemistry (TPOC), Department of Chemistry, Faculty of Science, Ibn Tofail University; Atiqa BEKHTA and Mehdi EL BOUCHTI who collaborated to the success of this paper.
References
- Measurements of the pressure dependence of viscosity of polymer melts using a back pressure regulated capillary rheometer. J. Appl. Polym. Sci.. 2010;117:1076-1084.
- [Google Scholar]
- Acevedo R.R. Mechanical Properties of Laminate of Residual Polyester Resin Reinforced with Recycled Newspaper. Inter. J. ChemTech Resea.. 2016;9:257-262.
- [Google Scholar]
- The general low-frequency prediction for asymptotically nonlinear material functions in oscillatory shear. J. Rheol.. 2014;58:891-910.
- [Google Scholar]
- Santamaria A. Rheological methods to investigate Graphene/amorphous polyamide nanocomposites: aspect ratio, processing and crystallization. Polym. Eng. Sci.. 2015;55:1142-1151.
- [Google Scholar]
- Capillary rheology of polystyrene, polypropylene and linear low density polyethylene melts. Polym. Eng. Sci.. 2015;55:506-512.
- [Google Scholar]
- Viscoelastic Mode Distribution of Moderately Entangled Linear Polymers. Nihon Reoroji Gakkaishi. 2010;38:187-193.
- [Google Scholar]
- Santamaria A. Thermal and viscoelastic features of new nanocomposites based on a hot melt adhesive polyurethane and multi-walled carbon nanotubes. Macromol. Mater. Eng.. 2010;295:1031-1041.
- [Google Scholar]
- Multiple liquid impacts on polymeric matrix composites reinforced with carbon nanotubes. J. Wear.. 2012;294–295:336-346.
- [Google Scholar]
- Thermal and rheological study of blended carbon nanotube/epoxy resin nanocomposites. J. Mater Environ. Sci.. 2014;5(2):374-379.
- [Google Scholar]
- Viscosimetric and rheological studies of a new trifunctional epoxy pre-polymer with noyan ethylene: Triglycidyl Ether of Ethylene of Bisphenol A (TGEEBA) J. Mater. Environ. Sci.. 2017;8:603-610.
- [Google Scholar]
- Theoretical, experimental and viscometric studies of a new phosphorus trifonctionnel epoxy polymer: Triglycidyl Dihydroxy Diphenyl Ether Phosphoric Ester (TGDHDPEPE) Mor. J. Chem.. 2016;4:315-323.
- [Google Scholar]
- Synthesis and characterization of a new epoxy resin homologous to DGEBA (diglycidyl 3-Aminopropyl Triethyl Silane): a study of thermal properties. Int. J. Innovation Appl. Stud.. 2014;7:674-682.
- [Google Scholar]
- Electrical and rheological percolation in polystyrene/MWCNT nanocomposites. Macromolecules. 2007;40:7400-7406.
- [Google Scholar]
- Nonlinear viscoelasticity of polymer nanocomposites under large amplitude oscillatory shear flow. J. Rheol.. 2013;57:767-789.
- [Google Scholar]
- Comparative Study on the Properties Of ZnO And ZnS Nanoparticles. Inter. J. ChemTech Resea.. 2016;9:308-315.
- [Google Scholar]
- Thermal and dielectric properties of an epoxy resin based on tetraglycidyl sulfonylamide (TGABSA) cured with aromatic diamines. Ann. Chim. Sci. Mat.. 2008;33:479-492.
- [Google Scholar]
- The effect of pressure on the viscosity of two different nanocomposites based on a PS matrix: a case of piezorheological complexity. J. Rheol.. 2016;60:1199-1210.
- [Google Scholar]
- Optical, Thermal and Electrical studies of PVP based solid Polymer electrolyte For Solid state battery applications. Inter. J. ChemTech Resea.. 2016;9:165-175.
- [Google Scholar]
- Novel conductive textile fabric based on polyaniline and CuO Nanoparticles. Inter. J. PharmTech Resea.. 2016;9:461-472.
- [Google Scholar]
- An evaluation of the pressure dependent melt viscosity of polyphenyl sulphone. Polym. Eng. Sci.. 2014;54:711-715.
- [Google Scholar]
- Physical and rheological properties of plasticized linear and branched PLA, Korea. Aust. Rheol. J.. 2010;22:187-195.
- [Google Scholar]
- Investigation of Structural, Thermal and Magnetic properties of Strontium substituted Barium Hexaferrite Synthesized via co-precipitation Method. Inter. J. ChemTech Resea.. 2015;8:404-410.
- [Google Scholar]
- Synthesis, Growth, Optical, Thermal and Dielectric studies of Lead Boro Glutamate (PbBG) Inter. J. ChemTech Resea.. 2015;8:638-642.
- [Google Scholar]
- Enhanced interfacial interaction by grafting carboxylated-macromolecular chains on nanodiamond surfaces for epoxy-based thermosets. J. Polym. Sci. Part B Polym. Phys.. 2017;55:1890-1898.
- [Google Scholar]
- Thermal conductivity and thermo-physical properties of nanodiamond-attached exfoliated hexagonal boron nitride/epoxy nanocomposites for microelectronics. Compos. Part A Appl. Sci. Manuf.. 2017;101:227-236.
- [Google Scholar]







