Translate this page into:
Revolutionizing therapeutics: The dazzling world of plant lectins
⁎Corresponding author at: Biomedical and Clinical Research Centre, School of Health and Allied Sciences, University of Cape Coast, Cape Coast, Ghana. ehkonozy@ucc.edu.gh (Emadeldin Hassan E. Konozy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Plant lectins, a diverse group of carbohydrate-binding proteins, have garnered significant interest for their potential biomedical applications. This review explores their multifaceted functionalities, highlighting their historical significance, unique carbohydrate recognition properties, and therapeutic potential in various medical conditions. We discuss their efficacy against microbes, cancer cells, and inflammation, as well as their roles in blood typing, diagnostics, wound healing, and neuroprotection. Additionally, we explored the potential of lectin-based drug delivery systems and lectin-nanoparticle conjugates for targeted therapeutic interventions. However, challenges such as lectin specificity, toxicity, production methods, and clinical validation are acknowledged. Overcoming these hurdles is crucial to unlocking the full potential of plant lectins and paving the way for advancements in medicine.
Keywords
Plant lectins
Future potential
Antimicrobial activities
Antiviral activity
Anticancer
Antiulcer
Drug delivery systems
Lectin nanoparticles
Data availability
All data obtained in this study are available in public databases.
1 Introduction
Ever since Peter Hermann Stillmark’s early discovery in 1888 that the extract of castor beans agglutinates red blood cells (RBCs), numerous lectins—carbohydrate-binding proteins—have been identified and thoroughly characterized across a wide array of living cells (Cummings et al., 2022). Research on these proteins, which were initially focused on their erythrocyte agglutination properties, has progressed to unveil their impact on various other biological processes. Remarkably, these effects consistently hinge on carbohydrate-binding pockets (Gabius et al., 2004). Due to their ability to agglutinate red blood cells (RBCs), lectins were initially named hemagglutinins. Research on plant lectins has progressed rapidly compared to that on other plant species, such as animals and microbes, following their first identification and characterization in plants (Sharon and Lis, 2004). This swift advancement led to the rapid isolation and characterization of lectins from various plant parts, including seeds, leaves, bark, flowers, and fruits. Collectively, these findings indicate that these lectins play physiological roles primarily associated with defense, although additional functions, such as storage, signalling, and the stabilization of other proteins, have been extensively documented (Rüdiger, 1998, Kestwal et al., 2007). Over the years, research has unveiled the multifaceted nature of plant lectins, revealing their potential applications in biomedical sciences (Sharon and Lis, 1972). They possess unique carbohydrate recognition domains (CBDs) that enable them to selectively interact with glycoconjugates. The finding that most of the properties of lectins are connected to their CBD has led to a growing interest in exploring their therapeutic and diagnostic applications in the field of medicine. Their ability to recognize and bind to specific cell surface carbohydrates has paved the way for potential applications in targeted drug delivery, cancer therapy and follow-up, wound healing, neuroprotection, antinociception, and diagnostic tools (Christie et al., 2014). The use of lectins in drug delivery systems has gained attention, with researchers investigating their potential to enhance the targeted delivery of therapeutic agents to specific cells or tissues (Konozy and Osman, 2022). Moreover, plant lectins have shown promise in the field of immunotherapy. Their ability to modulate the immune response by interacting with immune cells and influencing cytokine production suggests potential applications in the treatment of autoimmune diseases and infectious disorders (Majee and Biswas, 2013). Although the medical applications of plant lectins are promising, it is crucial to consider the potential challenges and safety concerns associated with their use. Further research is needed to unravel the full therapeutic potential of plant lectins and to address questions related to their efficacy, safety, and optimal delivery methods (Konozy and Osman, 2022). In this review, we will look at the significant potential and projected future uses of plant lectins using examples from recent studies. To illustrates these points, Table 1 highlights recently published articles on plant lectins (from 2022 to 2023) that show antimicrobial, anti-inflammatory, antinociceptive, and gastroprotective properties. Special emphasis will be placed on the most recent potential applications that show substantial promise (Fig. 1).
Plant species
Lectin
M.Wt. (kDa)
Biological activity
Refs
Canavalia
ensiformis
Con A
26
Act as drug delivery system (Con A coated chitosan nanoparticles act as a targeted delivery system for short antimicrobial peptide (CM11) against gastric infection by Helicobacter pylori).
(Moosazadeh Moghaddam et al., 2023)
Cratylia mollis
Cramoll
NA*
Acts as immunomodulatory and wound-healing agent in mice models with wounds induced by Staphylococcus aureus.
(Suarez Carneiro et al., 2021)
Dioclea violacea
DVL
25.5
Acts as Anti-candida (C. albicans, C. krusei, and C. parapsilosis) acts by inducing membrane and cell wall damage, redox system imbalance, inhibition of ergosterol biosynthesis, and the inducing of cytochrome c release from the mitochondrial membrane.
(Silva et al., 2023)
Has a synergistic effect when combined with neomycin against S. aureus and P. aeruginosa
(Santos et al., 2023)
Moringa oleifera
WSMoL
14
Acts as nematocidal and anthelmintic effect against the gastrointestinal parasite Haemonchus contortus-infected rodents
(Medeiros et al., 2023)
Reduces the tumor weight of sarcoma 180-bearing mice and increases the profiles of pro-inflammatory (TNF-α, IFN-γ, IL-2, IL-6, and IL-17) and anti-inflammatory (IL-4 and IL-10) cytokines.
(Brito et al., 2023)
Musa acuminata
BanLec
15
Acts as an anti-biofilm and synergistic effect with Enterococcus species (E. faecium (LCM002), E. lactis (LCM003) and E durans (LCM004 and LCM005) against Methicillin-resistant Staphylococcus aureus.
(Ahmed et al., 2023)
Parkia platycephala
PPL
47
Mediates orofacial antinociceptive effect in adult zebrafish ad rodents through TRPV1 channels by inhibiting capsazepine and capsaicin-induced desensitization.
(de Oliveira Leite et al., 2022)
Portulaca elatior
PeLL
20
Act as antibacterial against Pectobacterium strains (MIC 0.185 & MBC 0.74 μg/mL), fungicidal against C. albicans, C. tropicalis, C. krusei, and C. parapsilosis.
(da Silva et al., 2023)
Punica Granatum
PgL
28
Inhibites Ehrlich ascites carcinoma (EAC) growth in a dose-dependent manner in vitro and in an in vivo mice model bearing EAC
(Nurujjaman et al., 2023)
Schinus terebinthifolia
SteLL
12.4
Acts as antimicrobial, immunomodulatory, antitumour, antinociceptive, and functioning through modulation of depression through monoaminergic and nitric oxide signalling.
(Raíssa Ferreira de Lima et al., 2023)
Mediates antinociceptive effects through central and peripheral route.
(Marinho et al., 2023)
Reduces the S. aureus inflammatory burden of infected wounds and promotes skin repair in mice
(Nunes et al., 2022)
Urtica dioica
UDA
8.7
Protects against rabies virus (RABV) infection in muscle explant model
(Wang et al., 2023)
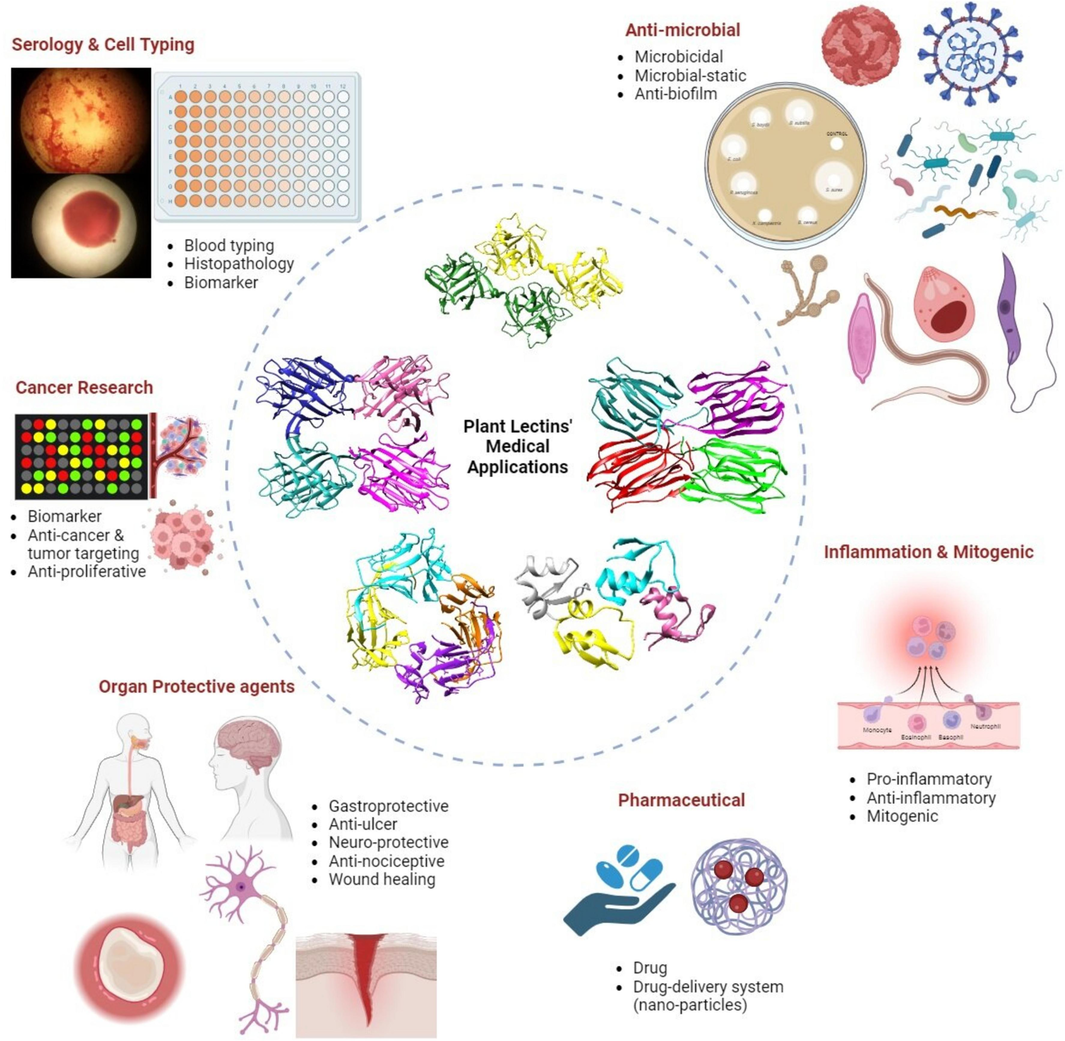
Potential applications of plant lectins in medical and pharmaceutical fields.
2 Antimicrobial properties of plant lectins
Plant lectins have antibacterial properties because they bind to carbohydrate structures on microbial surfaces, disrupting their physiological processes. Their specificity allows them to recognize and connect to the complex glycocalyx surface of bacteria, viruses, and fungi. By binding to these surfaces, they block adhesion and subsequent infection, making them potential candidates for antimicrobial therapies (Gupta and Gupta, 2012). Furthermore, certain plant lectins exhibit direct antimicrobial activity by disrupting microbial membranes or inhibiting key enzymatic processes essential for microbial survival. These properties make plant lectins potential candidates for developing novel antimicrobial agents. Research in this area has demonstrated the efficacy of various plant lectins against a range of pathogens, highlighting their potential in combating microbial infections (Konozy et al., 2022a, 2022b) (Table 1).
2.1 Antibacterial properties of plant lectins
Upon conducting a comprehensive examination of the robust antimicrobial properties exhibited by plant lectins, it becomes evident that these proteins serve as highly effective antibacterial agents. In vitro studies have consistently shown their ability to not only inhibit bacterial growth but also alter morphology, thereby reinforcing their efficacy in combating microbial pathogens. This understanding provides valuable insights into the potential utilization of plant lectins in antibacterial therapy, underscoring their versatility in targeting various bacterial strains. Concanavalin A, purified from jack bean (Canavalia ensiformis), is a widely recognized lectin. Studies have demonstrated its antibacterial efficacy through binding to bacterial surfaces, leading to interference with bacterial growth (da Silva Junior et al., 2021). Wheat germ agglutinin (WGA), derived from wheat germ, is a lectin that has been investigated for its antibacterial effects. Studies have revealed its ability to impede the growth of specific bacteria by binding to their surfaces (Khin et al., 2000). Lectin from the seeds of Eugenia uniflora L. (EuniSL) exhibited remarkable and broad-spectrum antibacterial activity. It effectively inhibited the growth of Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella sp. at a minimum inhibitory concentration (MIC) of 1.5 μg/mL. Additionally, it demonstrated a moderate inhibitory effect on the growth of Bacillus subtilis, Streptococcus sp., and Escherichia coli, with an MIC of 16.5 μg/mL (Oliveira et al., 2008). In a recent investigation conducted by Inoma et al. (2023), the antimicrobial potential of purified Erythrina senegalensis lectin (ESL) was explored. ESL demonstrated notable efficacy against pathogenic bacteria and fungi, with inhibition zones ranging from 18 to 24 mm and minimum inhibitory concentrations ranging from 50 to 400 μg/ml. The authors propose that these findings on the antimicrobial properties of ESL could pave the way for the development of lectin-based antimicrobial agents applicable in both agricultural and healthcare settings (Enoma et al., 2023). A recent study conducted by Podder and collaborators focused on purifying a seed lectin from Manilkara zapota (MZSL) and explored its antimicrobial properties. MZSL, weighing 33.0 ± 1 kDa, exhibited bacteriostatic effects against gram-positive bacteria, agglutinated Staphylococcus aureus and Shigella dysenteriae and demonstrated fungistatic activity against Aspergillus niger. Furthermore, MZSL reduced biofilm formation by E. coli and exhibited antioxidant activity (Podder et al., 2024). Evaluation of purified Portulaca elatior leaf lectin (PeLL) revealed compelling antimicrobial properties. PeLL, obtained through chromatography on a chitin column, displayed resilience to heat and exhibited heightened activity in acidic pH environments. Notably, PeLL exhibited significant antimicrobial efficacy against Pectobacterium strains and Candida species, with minimal inhibitory concentrations ranging from 0.185 to 1.48 μg/mL. There were no instances of hemolytic activity or acute toxicity in the mice. These findings underscore PeLL as a promising, low-toxicity, heat-resistant lectin that shows good antibacterial efficacy against Pectobacterium for the first time (da Silva et al., 2023).
2.2 How do plant lectins exert their antibacterial activity?
Some plant lectins exhibit antibacterial properties by interfering with the growth and survival of bacteria in different ways, as will be described here:
-
Binding to Bacterial Surfaces: Lectins from plants can bind specifically to bacteria through the carbohydrates that are found on bacterial surfaces, especially on their cell walls. Once bound to the bacterial surface, lectins can exert their antibacterial effects through several mechanisms. One common mechanism involves disrupting the bacterial cell membrane. Lectins may disrupt the integrity of the membrane, leading to leakage of cellular contents. This leakage can include essential components of the bacterial cell, such as ions and proteins, which are necessary for its survival and function (Konozy et al., 2022a, 2022b). In addition to membrane disruption, lectin binding can also lead to alterations in the structural properties of bacterial cells. Lectins may induce changes in the conformation or arrangement of cell wall components, affecting the overall stability and integrity of microbes. These structural alterations can compromise the ability of the bacterium to maintain its shape and function properly (Breitenbach Barroso Coelho et al., 2018). The combined effects of lectin binding, membrane disruption, and structural alterations can ultimately result in the collapse of the entire bacterial organism. This collapse may involve a loss of structural integrity, metabolic dysfunction, and ultimately, cell death.
-
Agglutination and Precipitation: Lectins can cause bacterial cells to cluster together (agglutination) or to produce insoluble compounds (precipitation). This makes it challenging for bacteria to colonize and invade host tissues (Khin et al., 2000).
-
Activation of Defense Responses: Lectin binding to bacterial surfaces can trigger plant defense responses. This process may involve the activation of signalling pathways that result in the production of antimicrobial compounds, thereby reinforcing the plant's defense against invading bacteria (Hwang and Hwang, 2011, Osman et al., 2023).
2.3 Antiviral properties of plant lectins
Various plant lectins can bind to glycoproteins on the surface of viruses. The interaction between plant lectins and viral glycoproteins can interfere with the early stages of viral infection, such as viral attachment and entry into host cells. Some plant lectins have been shown to have inhibitory effects on a variety of viruses, including influenza viruses, human immunodeficiency virus (HIV), and herpes simplex virus (HSV) (Konozy et al., 2022a, 2022b). The antiviral mechanisms employed by plant lectins may involve blocking viral entry, disrupting viral replication, or modulating the host immune response (Konozy et al., 2022a, 2022b).
2.4 Plant lectins as inhibitors of SARS-CoV-2 and its variants
Multidisciplinary research on potential COVID-19 vaccines and therapeutic candidates has intensified due to the SARS-CoV-2 pandemic. A study exploring bioactive molecules from plant sources focused on ten novel chitin-specific Hevein-like lectins derived from the Selaginella moellendorffii v1.0 genome and showed potential as an anti-SARS-CoV-2 agent. Molecular docking revealed interactions with the receptor binding domain of the spike protein, particularly Smo446851, Smo125663, and Smo99732, which bind to N-glycan (GlcNAc) and receptor binding domain (RBD) residues with binding free energies of −17.5, −13.0, and −26.5 kcal/mol, respectively. Molecular dynamics simulations highlighted the stability of the Smo99732-RBD complex. Lectins also showed preferential interactions with major SARS-CoV-2 mutant variants, suggesting that they are potential candidates for targeting key N-glycan sites that are crucial for virus virulence and infection (Alsolami et al., 2023).
In Wang and his colleagues’ study, 12 plant lectins with distinct sugar specificities were tested on various spike proteins from mutated SARS-CoV-2 variants. Lentil lectin derived from Lens culinaris, which binds to oligomannose-type glycans and GlcNAc, exhibited potent antiviral activity against a range of mutants and variants, including B.1.1.7, B.1.351, and P.1. Lentil lectin impeded angiotensin-converting enzyme 2 (ACE2) binding to the S trimer and hindered the early stages of SARS-CoV-2 infection, demonstrating broad-spectrum activity without causing cytotoxicity. These findings position lentil lectin as a promising candidate for future anti-SARS-CoV-2 strategies (Wang et al., 2021).
NTL-125, a unique mannose-binding plant lectin derived from Narcissus tazetta bulb, inhibits SARS-CoV-2 replication in the Vero-E6 cell line. In silico docking studies revealed that NTL-125 strongly binds to the viral spike RBD protein, preventing its attachment to the hACE2 receptor, which is crucial for cellular entry. Surprisingly, NTL-125 has a stronger affinity for all mutant forms of the spike protein than hACE2 (Sarkar et al., 2022). Another reported strong anti-SARS-CoV-2 candidate is Urtica diotica agglutinin (UDA) which has been supported by in silico and experimental studies. UDA exhibits a unique ability to block the RBD of spike proteins not only through carbohydrate-protein interactions but also through direct protein–protein interactions (Sabzian-Molaei et al., 2023).
2.5 Antifungal properties of plant lectins
Numerous plant lectins exhibit antifungal properties, and their mode of action closely resembles their impact on bacteria. In this context, these lectins typically initiate their antifungal effects by binding to receptors on fungal surface glycoconjugates. This binding initiates a cascade of actions that ultimately culminate in inhibiting fungal growth or causing fungal cell death. For a more in-depth examination of this topic and examples, please refer to the comprehensive review by Konozy et al. (2022a, 2022b).
3 Plant lectins’ antibiotic synergistic activities
Due to the evolution of defense mechanisms and the potential consequences of antibiotic misuse, antibiotic resistance poses a serious threat to the effectiveness of existing antibiotic treatments. Consequently, there is currently a robust and active pursuit of new antibiotics to address this pressing issue. Another avenue of research involves the repurposing of existing antibiotics. This approach explores alternative uses for antibiotics that may extend beyond their originally intended applications. Repurposing efforts aim to find novel therapeutic uses for these antibiotics, either by identifying different bacterial targets or by discovering additional properties that could be beneficial in the context of antimicrobial treatment (Miethke et al., 2021). The earlier part of the discussion thoroughly delves into the well-established antimicrobial attributes associated with plant lectins. Despite this, there is a recent and captivating line of research that focuses on exploring the synergistic impact of combining plant lectins with antibiotics that may have experienced a decrease in effectiveness or become inactive. This evolving field aims to uncover the potential cooperative mechanisms between plant lectins and antibiotics, especially in situations where antibiotics alone may have diminished efficacy. This research is driven by the idea that the combined action of lectins and antibiotics could offer a renewed and enhanced antimicrobial effect, addressing challenges posed by antibiotic resistance or diminished antibiotic potency. This exploration may open up new possibilities for overcoming microbial resistance and optimizing therapeutic strategies in scenarios where traditional antibiotics may no longer be as potent as needed (Santos et al., 2021, Costa et al., 2022).
Alpinia purpurata, commonly referred to as red ginger, is a readily available plant with significant medicinal value and properties. It is recognized for harboring numerous secondary metabolites, such as flavonoids, phenolic acids, and essential oils, and has shown preliminary evidence of antibacterial activity in research studies (Chan and Wong, 2015). The plant boasts environmental benefits like low maintenance, drought tolerance, soil stabilization, and wildlife attraction, making it an appealing plant for sustainable landscaping and conservation efforts. The lectin ApuL, derived from Alpinia purpurata, demonstrated antibacterial effects against human bacterial pathogens. It displayed bacteriostatic activity against Staphylococcus aureus isolates, including an oxacillin-resistant strain. ApuL impaired microorganism viability, as confirmed by propidium iodide staining and supported by various analyses, indicating distinct mechanisms of action. Synergistic effects were observed when ApuL was combined with antibiotics, such as oxacillin and ceftazidime, showing effectiveness against resistant isolates. Additionally, ApuL inhibited biofilm formation by S. aureus, demonstrating its potential as a therapeutic agent and exhibiting synergistic effects when combined with antibiotics (Ferreira et al., 2018). This research explored how a lectin from Parkia platycephala seeds (PPL), which binds to glucose/mannose, could increase the effectiveness of antibiotics against bacterial strains resistant to multiple drugs. While PPL demonstrated modest antibacterial activity on its own, its synergy with gentamicin notably improved the efficacy of the antibiotic against multidrug-resistant strains of S. aureus and Escherichia coli. The enhanced activity is believed to result from the interaction between gentamicin and the carbohydrate-binding site of the PPL (Silva et al., 2019). A compelling investigation led by Teixeira's team revealed that the combination of Dioclea violacea lectin (DVL) with neomycin significantly augmented the antibiotic activity against multidrug-resistant strains. Interestingly, DVL alone had no effect on S. aureus or P. aeruginosa. Preincubation of neomycin with DVL prevented DVL-induced hemagglutination, indicating an interaction between the antibiotic and the lectin through the carbohydrate binding sites of the latter (Santos et al., 2023).
4 Plant lectin in cancer therapy
Plant lectins have emerged as promising agents in anticancer therapy due to their ability to selectively recognize and bind to cancer cells, sparing normal cells from adverse effects. Some plant lectins exhibit inherent anticancer properties by inducing apoptosis or inhibiting the proliferation of cancer cells. Additionally, certain lectins serve as potential standalone drugs that can directly target cancer cells while minimizing damage to healthy tissues. Moreover, plant lectins have been investigated for their role as drug carriers in cancer therapy. They can be utilized to enhance targeted drug delivery systems, ensuring that therapeutic agents reach specific cancer cells with precision. In clinical trials, some plant lectins are being evaluated for their anticancer potential. For example, mistletoe lectin, a component of Viscum album extracts, has undergone clinical trials for its effects on various cancers, including breast and pancreatic cancer, demonstrating its potential as an anticancer agent (Kienle and Kiene, 2010). Another example is Abrin, a lectin from the Abrus precatorius plant, which has shown anticancer activity in preclinical studies, prompting further investigation for clinical applications (Hirschberger et al., 2018). These examples underscore the potential of plant lectins for diverse anticancer applications ranging from direct therapeutic agents to drug carriers in clinical settings (Table 1).
5 Plant lectins in blood typing and histopathology
Plant lectins have significant applications in blood typing and histopathology due to their specific carbohydrate-binding properties. In blood typing, lectins are utilized to distinguish between different blood types based on the presence or absence of specific blood group antigens. For instance, lectins from plants such as Dolichos biflorus and Ulex europaeus agglutinate with distinct blood group antigens, aiding in the accurate classification of blood types (Khan et al., 2002, Rudrappan and Veeran, 2016). In histopathology, plant lectins serve as valuable diagnostic tools for identifying and characterizing specific glycosylation patterns on cell surfaces. This is particularly relevant in cancer research, where alterations in glycosylation are associated with pathological conditions. Wheat germ agglutinin (WGA) is an example of a plant lectin commonly used in histopathology to identify changes in cell surface glycosylation associated with cancer (Silveyra et al., 2021).
6 Plant lectin as an anti-inflammatory
Plant lectins have garnered attention for their potential anti-inflammatory properties, showcasing their ability to modulate immune responses and mitigate inflammatory processes. For instance, pharmacological studies have examined the anti-inflammatory and antinociceptive impacts of a monocot lectin from Canna limbata seeds (CLL) using pharmacological assessments. Findings revealed nociceptive activity in the acetic acid test, a peripheral antinociceptive response, and anti-inflammatory effects, as evidenced by reduced inflammation and diminished neutrophil migration in the formalin and zymosan A-induced peritonitis tests, respectively (Araújo et al., 2013). Soybean agglutinin (SBA) is another lectin that has exhibited anti-inflammatory properties (Wang et al., 1997). Furthermore, lectins from the mistletoe have demonstrated antimetastatic effects and potential anti-inflammatory activity (Lim et al., 2023). The exploration of lectins as anti-inflammatory agents has extended to diverse sources, including natural products such as artocarpin from jackfruit (Artocarpus heterophyllus) (Souza et al., 2013). These findings highlight the multifaceted potential of plant lectins in modulating inflammation, paving the way for further research into their therapeutic applications.
7 Plant lectins as anti-nociceptive agents
Nociception is the sensory process that encodes noxious stimuli, frequently leading to the perception of pain. Extensive research has explored the capacity of plant lectins to alleviate nociception, indicating their potential role as analgesic or pain-relieving agents. The mechanisms underlying this potential role may include interactions with cell surface receptors, modulation of inflammation, and interference with pain signalling pathways (Campos et al., 2016). In a study conducted by Dafalla et al., the analgesic and antinociceptive properties of Ocimum basilicum seed lectin (OBSL) were examined in a Swiss rat model. Two pain stimulus methods, thermal and variable doses of OBSL, were employed for the study. The findings revealed that the intraperitoneal administration of OBSL notably prolonged the thermal threshold latency and diminished acetic acid-induced writhing. The conclusion drawn was that OBSL exhibits antinociceptive activity, suggesting the involvement of both peripheral and potentially central mechanisms. This finding supports the traditional use of OBSL as an analgesic (Dafalla et al., 2022) (for further examples, see reference (Konozy et al., 2022a, 2022b) and Table 1).
8 Plant lectins as gastroprotective agents
The potential of plant lectins as antiulcer agents is an area of emerging research, and although it is still in the early stages, the results are indeed promising. There are several examples of plant lectins with potential antiulcer effects. A lectin with antiulcer properties was extracted from the seeds of Phaseolus lunatus L. var. cascavel (PLUN). The isolated lectin exhibited significant gastroprotective effects, reducing the damaged area of the stomach by up to 63 % in response to ethanol-induced injury (e Lacerda et al., 2017).
The hemagglutinating activity originating from Calotropis procera leaves demonstrated a strong gastroprotective impact against ethanol-induced gastric lesions without observable toxicity to the kidneys and liver. Analysis of the gastric juice pH in lectin-treated animals suggested a potential role for lectin in maintaining stomach acidity within normal ranges, in contrast to the gastric juice pH of animals administered only ethanol (Al-Thobaiti and Konozy, 2022). While these examples showcase the potential of plant lectins as antiulcer agents, it is important to note that research in this field is ongoing. Further investigations are needed to fully understand the mechanisms of action, optimize the dosage, and evaluate the safety and efficacy of these lectins in clinical settings. These promising early results, however, suggest that plant lectins could become valuable components in the development of novel antiulcer therapies.
9 Properties of plant lectins in wound healing
Plant lectins exhibit potential in promoting wound healing by impacting vital cellular processes. They achieve this by stimulating cell proliferation, boosting fibroblast migration, and regulating the expression of growth factors and cytokines crucial for tissue repair. These lectins play a role in fostering the formation of new tissue by encouraging the synthesis and organization of extracellular matrix components, such as collagen (Lal et al., 2023). Neto and colleagues have conducted research on Centrolobium microchaete seed lectin, demonstrating its potential in promoting cutaneous wound healing in mice. Lectin exhibited noncytotoxic effects on murine dermal fibroblasts and significantly reduced the wound area within 12 days. The treated wounds showed complete restructuring of the epithelial layer and connective tissue, in contrast to the control groups, which exhibited incomplete re-epithelialization. These findings suggest that Centrolobium microchaete seed lectin expedites wound closure through the wound contraction process, leading to the efficient restructuring of skin layers. This indicates its potential as a promising therapeutic tool for the rapid and effective healing of acute wounds (do Nascimento Neto et al., 2021). The potential for healing exhibited by Bauhinia variegata lectin (nBVL) was investigated in a study in which mice subjected to surgical dorsal skin wounds were treated with nBVL. The fastest wound closure was observed in animals treated with nBVL, surpassing the effectiveness of the control group. Reconstruction of all skin layers and an increase in keratin deposition were noted. These findings suggest that the lectin from Bauhinia variegata possesses properties conducive to the healing process and could be used for the treatment of acute skin wounds (Neto et al., 2011).
While numerous studies have highlighted promising results regarding the wound-healing potential of various plant lectins, their practical medical application may still be in the early stages, and further extensive research is essential to rule out any potential side effects.
10 Plant lectins as neuroprotective agents
As the exploration of the potential neuroprotective effects of plant lectins continues, mounting evidence indicates that certain plant lectins indeed exhibit neuroprotective effects. A noteworthy example is ConBr, a lectin extracted from Canavalia brasiliensis seeds. It has been shown to be effective in multiple studies. Although the exact mechanism is not fully understood, ConBr seems to influence L-type voltage-gated Ca2+ channels. This influence offers neuroprotection under conditions of oxygen and glucose deprivation. This effect was observed in a model using rat organotypic hippocampal cultures (Rieger et al., 2017). According to another study on the same protein, early protein kinase activation may trigger CREB-dependent brain-derived neurotrophic factor (BDNF) transcription, leading to a subsequent increase in BDNF protein levels. Additionally, Akt phosphorylation increased 24 h after ConBr administration, possibly due to BDNF/TrkB-dependent activation of Akt. This finding suggested that ConBr has multiple functions, activating signalling pathways associated with neuroplasticity and neuroprotection (Rieger et al., 2014).
11 Plant lectins as a shuttle for drug delivery and targeting
Plant lectins are increasingly acknowledged for their potential in drug delivery due to their unique ability to target specific glycan structures on cell surfaces. This is particularly promising because many diseased cells exhibit altered glycan structures. Plant lectins can be tailored to target these unique glycan patterns, enabling precise drug delivery to affected cells. This targeted approach shows promise for developing more effective therapeutic interventions. The ability of plant lectins to selectively recognize and bind to altered glycan structures on diseased cells opens avenues for advanced drug delivery systems with enhanced specificity and efficacy (Bies et al., 2004).
12 Plant lectin nanoparticles: Another approach for precise drug targeting
Nanotechnology surfaced towards the end of the 20th century, capturing interest during the 1980s and 1990s owing to significant advancements in microscopy and the manipulation of materials at the nanoscale. Therefore, biomedical applications have flourished, employing nanoparticles either independently as a biomedical tool (Anju et al., 2019) or in conjunction with plant compounds to increase their effectiveness in combating pathogenic microorganisms or pinpointing cancer cells for eradication (Maria Magdalane et al., 2017; Mani et al., 2021; Nithiyavathi et al., 2021).
Lectin nanoparticles refer to nanoparticles that incorporate lectins. These nanoparticles are designed to leverage the specific binding properties of lectins for various applications, particularly in the fields of drug delivery and diagnostics. In drug delivery, lectin nanoparticles can be engineered to target specific cells or tissues by recognizing and binding to surface glycan structures (Bhardwaj et al., 2005). This targeted approach enhances the accuracy of drug administration, diminishing unintended effects and enhancing treatment results. The distinctive ability of lectins to engage with cell surface carbohydrates renders them valuable for crafting drug delivery systems and serving as antimicrobial agents independently. For instance, JAgNPs, created by coupling jacalin (a lectin sourced from Artocarpus integrifolia seeds) with silver nanoparticles, efficiently combat drug-resistant S. aureus pathogens. They demonstrate significantly lower minimum inhibitory concentrations than AgNPs, swiftly eradicating bacteria through oxidative stress and membrane disruption. Furthermore, JAgNPs show superior efficacy in hindering biofilm formation, leading to substantial reductions in both volume and thickness (Subramaniyan et al., 2021). In a separate study conducted by Yasin et al., Lepidium sativum lectin protein-loaded chitosan-TPP nanoparticles were engineered for potential cancer treatment. These nanoparticles successfully decreased proliferation markers and increased the expression of apoptotic genes in HepG2 cells, showing potential as anticancer agents (Yasin et al., 2020). Another intriguing method of utilizing plant lectins in drug targeting involves combining them with sugar-stabilized nanoparticles, which hold promise in cancer therapy by specifically targeting cancer cells. Momordica charantia seed lectin (MCL), which is renowned for its antitumour properties, effectively interacts with sugar-stabilized silver nanoparticles (AgNPs). Even in the presence of specific sugars, this interaction remains robust, as verified by fluorescence spectroscopy analysis. Importantly, MCL maintains its unique sugar-binding site, which is distinct from the nanoparticle-binding site, ensuring the preservation of cell recognition. These findings suggest exciting opportunities for developing stable MCL-AgNP conjugates for cancer diagnosis and treatment (Kenoth et al., 2023). Beyond their prior uses, lectin nanoparticles have found utility in diagnostic endeavors. By coupling lectins with nanoparticles, scientists can fabricate probes adept at selectively adhering to particular carbohydrates linked to diseases. This capability enables the identification and visualization of afflicted cells or tissues, furnishing crucial insights for early detection and surveillance across diverse conditions (Kekki et al., 2017). The development of lectin nanoparticles represents an innovative and evolving area of research at the intersection of nanotechnology and biomedicine, holding promise for advancements in targeted drug delivery and diagnostic technologies.
13 Current constraints hindering the broader utilization of plant lectins in human therapy
Although plant lectins have therapeutic potential, several challenges must be overcome for their successful integration into medical practice. These obstacles include the following:
-
Specificity and Selectivity: Plant lectins often exhibit varying degrees of specificity and selectivity in their interactions with target molecules. This variability can make it challenging to predict and control its effects in therapeutic settings.
-
Immune Response to Plant Lectins: Plant lectins, while offering a range of therapeutic possibilities, can interact with the immune system in ways that pose potential hazards. Their ability to bind specifically to carbohydrates on cell surfaces extends to immune cells as well. This binding can trigger an immune response, leading to unintended consequences such as inflammation, autoimmunity (Vojdani et al., 2020) and allergic reactions (Smart et al., 1999).
-
Toxicity Concerns: Some plant lectins exhibit inherent toxicity, which can limit their clinical application (Vasconcelos and Oliveira, 2004). Identifying lectins with minimal toxicity profiles suitable for therapeutic use requires thorough evaluation and testing.
-
Standardization and Production: Standardizing the production of plant lectins to ensure consistent quality and potency is another obstacle. Large-scale production methods must be developed to meet the demands of therapeutic applications.
-
Clinical Validation: Despite promising preclinical research, the clinical efficacy of plant lectins in treating human diseases requires rigorous validation through well-designed clinical trials. Conducting such trials presents logistical and financial challenges (Konozy and Osman, 2022).
14 Conclusion
With their diverse carbohydrate-binding abilities, plant lectins have emerged as promising candidates for a wide range of medical applications. From antimicrobial and antiviral activities to potential roles in cancer therapy, wound healing, and neuroprotection, these proteins offer exciting possibilities for future medical advancements. However, challenges remain in terms of specificity, potential toxicity, and the need for standardized production and rigorous clinical validation. Overcoming these hurdles will be crucial in unlocking the full potential of plant lectins and translating their promise into tangible therapeutic solutions. Further research focusing on optimizing lectin properties, developing targeted delivery systems such as lectin-nanoparticle conjugates, and conducting comprehensive clinical trials will be essential for facilitating the successful integration of plant lectins into the medical landscape.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
CRediT authorship contribution statement
Emadeldin Hassan E. Konozy: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Supervision. Makarim Elfadil M. Osman: Data curation, Visualization, Writing – original draft, Writing – review & editing. Amina I. Dirar: Data curation, Writing – review & editing. Rieham Sallah H. Osman: Data curation, Writing – review & editing.
Acknowledgment
Not available.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation of lectin from Musa acuminata for its antibiofilm potential against Methicillin-resistant Staphylococcus aureus and its synergistic effect with Enterococcus species. Arch. Microbiol.. 2023;205(5):181
- [CrossRef] [Google Scholar]
- Genome-wide mining of Selaginella moellendorffii for Hevein-like lectins and their potential molecular mimicry with SARS-CoV-2 spike glycoprotein. Curr. Issues Mol. Biol.. 2023;45(7):5879-5901.
- [CrossRef] [Google Scholar]
- Purification, partial characterization, and evaluation of the antiulcer activity of Calotropis procera leaf lectin. Protein Pept. Lett.. 2022;29(9):775-787.
- [CrossRef] [Google Scholar]
- Antimicrobial photodynamic activity of toluidine blue-carbon nanotube conjugate against Pseudomonas aeruginosa and Staphylococcus aureus - Understanding the mechanism of action. Photodiagn. Photodyn. Ther.. 2019;27:305-316.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and antinociceptive activity of chitin-binding lectin from Canna limbata seeds. Appl. Biochem. Biotechnol.. 2013;171(8):1944-1955.
- [CrossRef] [Google Scholar]
- Pharmaceutical aspects of polymeric nanoparticles for oral drug delivery. J. Biomed. Nanotechnol.. 2005;1(3):235-258.
- [Google Scholar]
- Lectin-mediated drug targeting: history and applications. Adv. Drug Deliv. Rev.. 2004;56(4):425-435.
- [CrossRef] [Google Scholar]
- Toxicity and antitumor activity of the water-soluble lectin from Moringa oleifera Lam. Seeds (WSMoL) in sarcoma 180-bearing mice. Toxicon. 2023;234:107306
- [CrossRef] [Google Scholar]
- Anti-inflammatory and antinociceptive activities of Bauhinia monandra leaf lectin. Biochim. Open. 2016;2:62-68.
- [Google Scholar]
- Phytochemistry and pharmacology of ornamental gingers, Hedychium coronarium and Alpinia purpurata: a review. J. Integr. Med.. 2015;13(6):368-379.
- [Google Scholar]
- Biophysical characterization of lectin–glycan interactions for therapeutics, vaccines and targeted drug-delivery. Fut. Med. Chem.. 2014;6(18):2113-2129.
- [Google Scholar]
- Plant lectins: a review on their biotechnological potential toward human pathogens. Curr. Protein Pept. Sci.. 2022;23(12):851-861.
- [Google Scholar]
- Cummings, R.D., Chiffoleau, E., van Kooyk, Y., et al., 2022. C-type lectins. Essentials of Glycobiology [Internet]. 4th edition.
- Purification, characterization, and assessment of antimicrobial activity and toxicity of Portulaca elatior leaf lectin (PeLL) Probiotics Antimicrob. Proteins. 2023;15(2):287-299.
- [CrossRef] [Google Scholar]
- Concanavalin A differentiates gram-positive bacteria through hierarchized nanostructured transducer. Microbiol. Res.. 2021;251:126834
- [CrossRef] [Google Scholar]
- Ocimum basilicum seeds lectin (OBSL) relieves pain through central and probably peripheral antinociceptive mechanisms. Neelain J. Sci. Technol. NJST. 2022;6(2):1-6.
- [Google Scholar]
- Parkia platycephala lectin (PPL) inhibits orofacial nociception responses via TRPV1 modulation. Molecules. 2022;27(21)
- [CrossRef] [Google Scholar]
- Wound healing activity of lectin isolated from seeds of Centrolobium microchaete Mart. ex Benth. on cutaneous wounds in mice. Nat. Prod. Res.. 2021;36(18):4734-4739.
- [CrossRef] [Google Scholar]
- Lectin from seeds of a Brazilian lima bean variety (Phaseolus lunatus L. var. cascavel) presents antioxidant, antitumour and gastroprotective activities. Int. J. Biol. Macromol.. 2017;95:1072-1081.
- [CrossRef] [Google Scholar]
- Antimicrobial activities and phylogenetic study of Erythrina senegalensis DC (Fabaceae) seed lectin. Biotechnologia. 2023;104(1):21
- [Google Scholar]
- Antimicrobial potential of Alpinia purpurata lectin (ApuL): growth inhibitory action, synergistic effects in combination with antibiotics, and antibiofilm activity. Microb. Pathog.. 2018;124:152-162.
- [CrossRef] [Google Scholar]
- Gupta, G., Gupta, G., 2012. Lectins: an overview. Animal lectins: Form, function and clinical applications. pp. 3–25.
- Exploring cytotoxic mRNAs as a novel class of anti-cancer biotherapeutics. Mol. Ther.-Methods Clin. Dev.. 2018;8:141-151.
- [Google Scholar]
- The pepper mannose-binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiol.. 2011;155(1):447-463.
- [CrossRef] [Google Scholar]
- Improved cancer specificity in PSA assay using Aleuria aurantia lectin coated Eu-nanoparticles for detection. Clin. Biochem.. 2017;50(1–2):54-61.
- [Google Scholar]
- Interaction of sugar stabilised silver nanoparticles with Momordica charantia seed lectin, a type II ribosome inactivating protein. Glycoconj. J.. 2023;40(2):179-189.
- [CrossRef] [Google Scholar]
- Characterization of alpha-mannosidase from Erythrina indica seeds and influence of endogenous lectin on its activity. BBA. 2007;1770(1):24-28.
- [CrossRef] [Google Scholar]
- Agglutination of Helicobacter pylori coccoids by lectins. World J. Gastroenterol.. 2000;6(2):202
- [Google Scholar]
- Influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr. Cancer Ther.. 2010;9(2):142-157.
- [Google Scholar]
- Plant lectins as potent anti-coronaviruses, anti-inflammatory, antinociceptive and antiulcer agents. Saudi J. Biol. Sci.. 2022;29(6):103301
- [Google Scholar]
- Lectin: a carbohydrate binding glyoprotein and its potential in wound healing. Bioact. Carbohydr. Diet. Fibre 2023100379
- [Google Scholar]
- Immuno-modulatory effects of Korean mistletoe in MDA-MB-231 breast cancer cells and THP-1 macrophages. Scientia Pharmaceutica.. 2023;91(4):48
- [Google Scholar]
- Exploring plant lectins in diagnosis, prophylaxis and therapy. J. Med. Plants Res.. 2013;7(47):3444-3451.
- [Google Scholar]
- A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ. Res.. 2021;198:111199
- [CrossRef] [Google Scholar]
- Photocatalytic degradation effect of malachite green and catalytic hydrogenation by UV–illuminated CeO2/CdO multilayered nanoplatelet arrays: Investigation of antifungal and antimicrobial activities. J. Photochem. Photobiol. B Biol.. 2017;169:110-123.
- [CrossRef] [Google Scholar]
- Schinus terebinthifolia leaf lectin has central and peripheral antinociceptive action mediated by its carbohydrate-recognition domain and delta-opioid receptors. J. Ethnopharmacol.. 2023;301:115817
- [CrossRef] [Google Scholar]
- Anthelmintic effect of a water soluble Moringa oleifera lectin in rodents experimentally infected with Haemonchus contortus. Parasitol. Int.. 2023;92:102656
- [CrossRef] [Google Scholar]
- Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem.. 2021;5(10):726-749.
- [CrossRef] [Google Scholar]
- Targeted delivery of a short antimicrobial peptide (CM11) against Helicobacter pylori gastric infection using concanavalin A-coated chitosan nanoparticles. J. Mater. Sci. - Mater. Med.. 2023;34(9):44.
- [CrossRef] [Google Scholar]
- Effect of the lectin of Bauhinia variegata and its recombinant isoform on surgically induced skin wounds in a murine model. Molecules. 2011;16(11):9298-9315.
- [Google Scholar]
- Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J. Infect. Public Health. 2021;14(12):1893-1902.
- [Google Scholar]
- Schinus terebinthifolius Leaf Lectin (SteLL) Reduces the bacterial and inflammatory burden of wounds infected by Staphylococcus aureus promoting skin repair. Pharmaceuticals (Basel). 2022;15(11)
- [CrossRef] [Google Scholar]
- Antitumor activity of a lectin purified from Punica granatum pulps against Ehrlich ascites carcinoma (EAC) cells. Anticancer Agents Med. Chem. 2023
- [CrossRef] [Google Scholar]
- Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol.. 2008;46(3):371-376.
- [CrossRef] [Google Scholar]
- Plant lectins: implications in tolerance and resistance. Ann. Plant Rev. Online 2023:31-55.
- [Google Scholar]
- Antimicrobial, antioxidant and antiproliferative activities of a galactose-binding seed lectin from Manilkara zapota. Heliyon. 2024;10(2):e24592
- [Google Scholar]
- The lectin from Schinus terebinthifolia leaf (SteLL) reduces immobility of mice on the tail suspension test dependent on the monoaminergic and nitric oxide signaling. Neurosci. Lett.. 2023;801:137092
- [CrossRef] [Google Scholar]
- ConBr, a lectin from Canavalia brasiliensis seeds, modulates signaling pathways and increases BDNF expression probably via a glycosylated target. J. Mol. Recognit.. 2014;27(12):746-754.
- [CrossRef] [Google Scholar]
- ConBr, a lectin purified from the seeds of Canavalia brasiliensis, protects against ischemia in organotypic culture of rat hippocampus: potential implication of voltage-gated calcium channels. Neurochem. Res.. 2017;42(2):347-359.
- [CrossRef] [Google Scholar]
- Plant lectins–more than just tools for glycoscientists: occurrence, structure, and possible functions of plant lectins. Acta Anat.. 1998;161(1–4):130-152.
- [Google Scholar]
- Role of plant based lectins in identifying rare Bombay blood group. Pharmacogn. J.. 2016;8(1)
- [Google Scholar]
- Urtica dioica agglutinin (UDA) as a potential candidate for inhibition of SARS-CoV-2 Omicron variants: in silico prediction and experimental validation. Phytomedicine. 2023;111:154648
- [CrossRef] [Google Scholar]
- Potential application of combined therapy with lectins as a therapeutic strategy for the treatment of bacterial infections. Antibiotics. 2021;10(5):520
- [Google Scholar]
- Dioclea violacea lectin increases the effect of neomycin against multidrug-resistant strains and promotes the purificatiob of the antibiotic in immobilized lectin column. Int. J. Macomol.. 2023;1(236):123941
- [Google Scholar]
- A novel plant lectin, NTL-125, interferes with SARS-CoV-2 interaction with hACE2. Virus Res.. 2022;315:198768
- [CrossRef] [Google Scholar]
- Lectins: cell-agglutinating and sugar-specific proteins. Science (New York, N.Y.). 1972;177:949-959.
- [CrossRef] [Google Scholar]
- History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14(11):53R-62R.
- [Google Scholar]
- Parkia platycephala lectin enhances the antibiotic activity against multi-resistant bacterial strains and inhibits the development of Haemonchus contortus. Microb. Pathog.. 2019;135:103629
- [CrossRef] [Google Scholar]
- DVL, lectin from Dioclea violacea seeds, has multiples mechanisms of action against Candida spp via carbohydrate recognition domain. Chem. Biol. Interact.. 2023;382:110639
- [CrossRef] [Google Scholar]
- The tissue architecture of oral squamous cell carcinoma visualized by staining patterns of wheat germ agglutinin and structural proteins using confocal microscopy. Cells.. 2021;10(9):2466
- [Google Scholar]
- Lectins in drug delivery: a study of the acute local irritancy of the lectins from Solanum tuberosum and Helix pomatia. Eur. J. Pharm. Sci.: Off. J. Eur. Feder. Pharm. Sci.. 1999;9(1):93-98.
- [CrossRef] [Google Scholar]
- The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj. J.. 2013;30:641-657.
- [Google Scholar]
- Immunomodulatory and anti-infective effects of Cratylia mollis lectin (Cramoll) in a model of wound infection induced by Staphylococcus aureus. Int. Immunopharmacol.. 2021;100:108094
- [CrossRef] [Google Scholar]
- Artocarpus integrifolia seed lectin enhances membrane damage, oxidative stress and biofilm inhibition activity of silver nanoparticles against Staphylococcus aureus. Colloids Surf. A: Physicochem. Eng. Asp.. 2021;624:126842
- [CrossRef] [Google Scholar]
- Antinutritional properties of plant lectins. Toxicon. 2004;44(4):385-403.
- [CrossRef] [Google Scholar]
- Reaction of lectin-specific antibody with human tissue: possible contributions to autoimmunity. J. Immunol. Res.. 2020;2020:1438957
- [CrossRef] [Google Scholar]
- Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microbes Infect.. 2021;10(1):1519-1529.
- [CrossRef] [Google Scholar]
- Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal.. 1997;15(12):1867-1876.
- [CrossRef] [Google Scholar]
- Urtica dioica agglutinin prevents rabies virus infection in a muscle explant model. Pharmaceutics. 2023;15(5)
- [CrossRef] [Google Scholar]
- Preparation and nanoencapsulation of lectin from Lepidium sativum on chitosan-tripolyphosphate nanoparticle and their cytotoxicity against hepatocellular carcinoma cells (HepG2) Biomed. Res. Int.. 2020;2020:7251346
- [CrossRef] [Google Scholar]







