Translate this page into:
Resolution factor 15-epi-Lipoxin A4 modulates miRNA-499 induced differentiation of cardiosphere-derived stem cells through dual inhibition of Wnt/β-catenin and TGFβ/SMAD signalling axes
⁎Corresponding author at: Department of Cardiology, Shanghai General Hospital, No 100 of Haining road, Hongkou district, Shanghai 200080, China. ukhmfh@163.com (Guobing Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Cardiosphere derived stem cells (CDSCs) are signified as a valued source of stem cell therapeutics for treating a gamut of cardiovascular ailments. However, challenges in the cardiogenic differentiation process culminate in stem cell transplantation failure. Hence, it is imperative to understand the comprehensive mechanisms and the role of endogenous factors in cardiac differentiation.
Objectives
Objective of the current study is to check the effect of 15-epi-lipoxin A4 (15E-LXA4)—a stable analogue of lipoxin A4, a pro-resolving agonist—on miR-499 (a cardiac-specific microRNA)-induced differentiation of CDSCs.
Methods
CDSCs were transfected with lentiviral vectors bearing miR-499 and subjected to 15E-LXA4 treatment. Then, the treatment effects on surface markers (c-kit and CD105), cardiogenic gene markers (NKX2.5, GATA4, and cTnI), Wnt/β-catenin signaling (Wnt3a and phosphorylated/dephosphorylated β-catenin ratio) and TGFβ/SMAD signaling (TGFβ1 and SMAD3) were evaluated.
Results
15E-LXA4 treatment repressed miR-499 over-expression induced cardiac differentiation, manifested by enhanced expression of surface markers, Wnt/β-catenin and TGFβ/SMAD signaling parameters and reduced expression cardiogenic gene markers, plausibly through activation of estrogen receptor (ERα).
Conclusions
These findings give reason to understand the role of endogenous resolution factors like 15E-LXA4, as it displayed reciprocal effect on miR-499 induced cardiac differentiation in CDSCs.
Keywords
15-epi-lipoxin A4
miR-499
Wnt3a
TGFβ1
SMAD3
1 Introduction
Myocardial infarction (MI) is a major health problem throughout the world and epidemic proportions increased in South Asian countries. According to WHO estimates, in China, ischemic heart disease (IHD) is the disease causing a highest death toll, next to stroke; IHD is reported to have claimed above 1.9 million lives in 2016 (Gorabi et al., 2019). On the other side, lack of clinically resounding therapy which could reverse the myocardial functionalities post-MI or IHD, lead to ineffective treatment outcomes. In this milieu, stem cell therapy is burgeoning as a ray of hope in the treatment of various cardiac ailments. Of note, various preclinical and pragmatic clinical evidences accentuated that cardiosphere-derived stem cells (CDSCs) proffer promising outcomes in the treatment of MI/IHD (Cheng et al., 2014). CDSCs are resident cardiac cells with inherent cardiomyogenic, angiogenic, immunomodulatory, anti-fibrotic and anti-aging activities.
Interestingly, Malliaras et al. (2014), in the CADUCEUS trial, revealed that autologous intracoronary transplantation of CDSCs downscaled the scar mass, boosted myocardial viability and functionalities of the infarcted heart through its regenerative potential. In contrary, lack of noteworthy improvement in the homeostatic cardiac remodelling after CDSC therapy has questioned the efficacy of CDSCs in treating cardiac ailments (Kasai-Brunswick et al., 2017). However, induction of differentiation potential of CDSCs might be a honing strategy for formulating operative stem cell therapy based on CDSCs. In this line, Mundre et al. (2017) illustrated that co-treatment of 5-azacytidine with ascorbic acid induces differentiation of CDSCs to cardiomyocytes.
Characterization of epigenetic signatures of cardiac stem cells is becoming a pivotal tool in the discovery of targeted cardiotherapeutics in relation to various molecular circuitry components in the cardiogenesis and cardiac stem cell differentiation. In this context, treatment strategies based on microRNAs (miRNAs), a class of small non-coding RNAs, are gaining attention due to their disease modifying effects rather than conventional symptomatic remedies in the management of cardiovascular ailments. Ekhteraei-Tousi et al. (2015) illustrated that epigenetic regulation through miR-590 downmodulation, might improve TGFβ1-mediated differentiation of CDSCs. Another study reported that TrkC‐miR2, a novel TrkC gene-localized miRNA, exerts dual regulation of Wnt and TGFβ signaling cascades in the differentiation of CDSCs (Dokanehiifard and Soltani, 2018). Besides, various other miRNAs including miR-1, miR-23a/b, miR-193a, miR-133, miR-499 and miR-669a are known to partake in the physiological or pathological process of cardiac stem/progenitor cell differentiation (Gorabi et al., 2019).
Among them, miR-499—a notable miRNA in the cardiac tissue of human and also experimental animals including porcine, rodent and zebrafish—manifests a prominent slow‐to-fast shift in myofibers in the cardiac/skeletal tissues (Global Health Estimates, 2016). Zhang et al. (2012) found that miR-499 governs cardiac differentiation of murine mesenchymal stem cells via Wnt/β-catenin-mediated signaling mechanism. Another study by Wu et al. (2019) by using the C2C12 cell line, demonstrated that miR-499 provokes myoblast differentiation via TGFβR1-mediated mechanism. The role of resolution factors and endogenous lipid mediators in the amelioration of cardiovascular repercussions including myocardial infarction is known. However, the effect of 15-epi-lipoxin A4 (15E-LXA4)—a stable analogue of lipoxin A4, a pro-resolving agonist—on the differentiation of CDSCs has not been studied. In this evidence backdrop, the present study was designed to understand the effect of 15E-LXA4 on CDSC differentiation and the pertinent role of miR-499 and associated signalling mechanisms.
2 Materials and methods
2.1 Explant culture of CDSCs
CDSCs were generated from the cardiac tissue of four-week old Wistar rats as described previously (Mundre et al., 2017). Succinctly, following humane excision of heart tissues, the ventricles were fragmented into 1 mm3 size and subjected to 0.05% trypsin treatment for partial digestion. After confluency, the cardiac cells arising from the explants were extracted out by using 0.05% trypsin and seeded again in the culture plates with cardiosphere growth medium. Then, the non-adherent cardiospheres in the suspension were separated and cultured to form the CDSC monolayer, which is used for all the investigations after 3 passages. CDSCs were segregated into: i) control; ii) miR-499 transfected CDSCs; iii) miR-499 transfected CDSCs treated with 10 nm 15E-LXA4 (Cayman Chemicals, USA) for 24 h and then used for various evaluations.
2.2 Lentiviral transduction
CDSCs were plated in T25 flasks and transduced with miR-499 containing lentiviral particles (MOI:20) in the specified media. Post-transduction (after 1 day), viral particles in the media were separated out, and fresh growth media was added for recovery of cells for the same duration. Then, selection media was prepared and the CDSCs were allowed to grow for 7 days. Following selection, complete media was substituted.
2.3 Isolation of RNA and RT-PCR analysis
To extract total RNA from control, miR-499 transfected and miR-499 transfected + 15E-LXA4 treated cells, Rneasy (Qiagen, Valencia, CA), a total RNA isolation kit was used. Using GAPDH gene as the control, the target gene expression was normalized in each sample. PCR primers used for the analysis: GAPDH: F: CGGATTTGGTCGTATTGG and R: TCAAAGGTGGAGGAGTGG; NKX2.5: F: CCACCTGGCGCTGTGAGACC and R: GAAGCGCCGCTCCAGCTCAT; GATA4: F: GCCAACCCTGCGAGACACCC and R: TCAGCGTTACGGCGCCACAG; cTnI: F: TCTCTACCTCTGGAGATCAGCATGG and R: TGAAGTTTTCTGGAGGCGGAG.
2.4 Western blot analysis
The cells were homogenized in ice-cold RIPA using an electric homogenizer. The homogenate was centrifuged at 16,000×g at 4 °C for 20 min to obtain the supernatant containing the proteins. The protein was separated using SDS–polyacrylamide gel and transferred onto a polyvinylidenedifluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked using a blocking solution after washing followed by overnight incubation with specific primary antibodies: Wnt3a (Abcam, Cambridge, UK), ERα (Santa Cruz Biotechnology, USA), phospho-β-catenin (Ser33/37/Thr41) (Cell Signalling Technology, USA), Non-phospho-β-catenin (Ser33/37/Thr41) (Cell Signalling Technology, USA), TGF-β1 (Abcam, USA) and SMAD3 (Abcam, USA). The membranes were rinsed and consequently treated with secondary HRP (horseradish peroxidase)-conjugated antibodies (Santa Cruz Biotechnology, Inc., CA) and incubated. In the ultimate step, quantitative analysis of the reactive bands was done by using Tanon imaging system.
2.5 Statistical analysis
SPSS software (V13.0; SPSS, Inc., USA) was used for the statistical evaluation and the experimental data were indicated as mean ± SD. For the evaluations one-way ANOVA was applied using Tukey’s post-hoc test for comparisons among different treatments. Significant level was kept at P < 0.05.
3 Results
3.1 Effect of 15E-LXA4 on the expression of surface markers and ERα in miR-499 transfected CDSCs
c-kit and CD105 are the typical stem cell surface markers expressed by the CDSCs. These markers were significantly (P < 0.05) downregulated to 55% and 65% respectively in the miR-499 transfected CDSCs, when compared to that of the control cells (Fig. 1). In the similar line, protein expression of ERα, a receptor with robust co-existence with these surface markers, was significantly (P < 0.05) downregulated to 43% in the miR-499 transfected CDSCs. On the other edge, 15E-LXA4 treated miR-499 transfected CDSCs depicted increased expression of c-kit, CD105 and ERα, indicating a hindrance in CDSC differentiation process.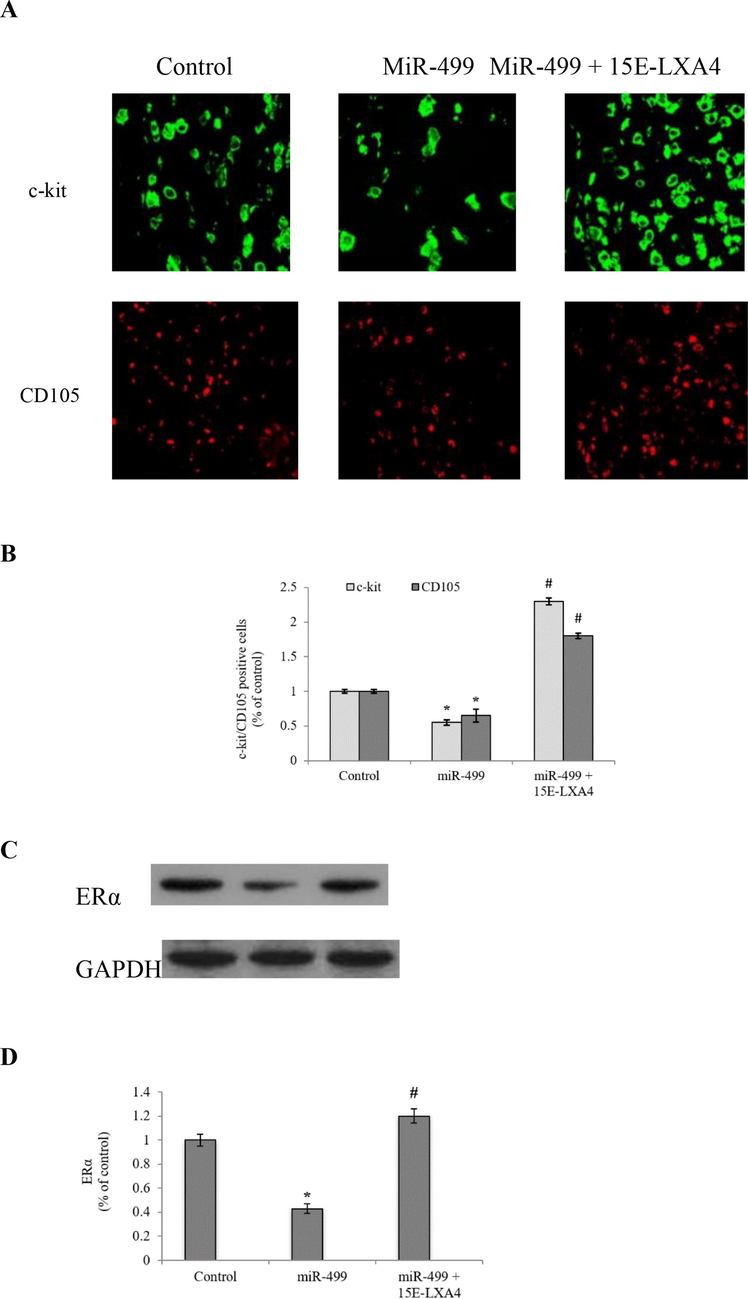
Effect of 15E-LXA4 on the expression of surface markers and ERα in miR-499 transfected CDSCs. A) Immunofluorescence analysis of CDSCs for c-kit and CD105 positive cells. B) Quantitative representation of c-kit and CD105 positive CDSCs. C) Indicative western blot images of ERα expression in all the CDSCs groups using β-actin control. D) Relative ERα expression level in each group. *P < 0.05 (miR-499 vs control); #P < 0.05 (miR-499 + 15E-LXA vs miR-499).
3.2 Effect of 15E-LXA4 on the expression of cardiogenic markers in miR-499 transfected CDSCs
NKX2.5 and GATA4 are the key transcription factors in cardiogenic differentiation. In addition to these factors, cTnI also serves as a pivotal marker of cardiomyocyte morphogenesis. In the current study, miR-499 transfected CDSCs revealed pointedly (P < 0.05) raised mRNA expression levels of NKX2.5, GATA4 and cTnI to about 4.5-, 5.1- and 4.6-folds, as compared to the control CDSCs (Fig. 2). In contrary, 15E-LXA4 treated miR-499 transfected CDSCs portrayed substantially (P < 0.05) lower mRNA expression levels of these markers. This underscores that miR-499 overexpression reinforced the cardiogenic differentiation, while 15E-LXA4 treatment showed reciprocal effect.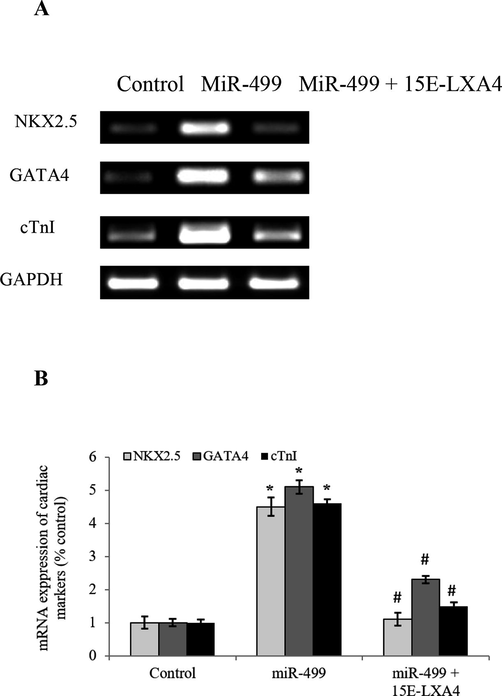
Effect of 15E-LXA4 on the expression of cardiogenic markers in miR-499 transfected CDSCs.A) Indicative RT-PCR images of NKX2.5, GATA4, and cTnI expressions in all the CDSCs groups using β-actin control. B) Relative NKX2.5, GATA4, and cTnI mRNA expression level in each group. *P < 0.05 (miR-499 vs control); #P < 0.05 (miR-499 + 15E-LXA vs miR-499).
3.3 Effect of 15E-LXA4 on the expression of Wnt signalling markers in miR-499 transfected CDSCs
Wnt signalling pathway is a fulcrum axis in the initiation of cardiogenic differentiation process in stem cells. In the current work, results revealed that the Wnt/β-catenin signalling axis was activated, as evidenced by a significant (P < 0.05) 3.7-fold increase in the Wnt3a expression in the LV-miR-499 transfected CDSCs (Fig. 3). Alongside, significantly (P < 0.05) diminished ratio of phosphorylated/dephosphorylated β-catenin reinstates the incremental differentiation activity in the LV-miR-499 transfected CDSCs. However, miR-499 overexpressed CDSCs exposed to 15E-LXA4 showed slowdown of the cardiac differentiation process, manifested by the inhibition of the Wnt/β-catenin pathway.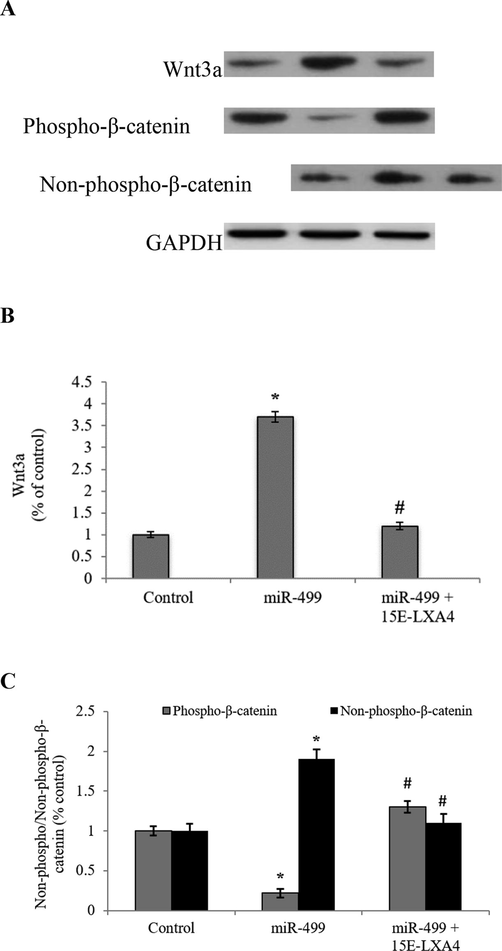
Effect of 15E-LXA4 on the expression of Wnt signalling markers in miR-499 transfected CDSCs. A) Indicative western blot images of Wnt3a and phosphorylated/dephosphorylated β-catenin expression in all the CDSCs groups using β-actin control. B) Relative Wnt3a expression level in each group. C) Relative phosphorylated/dephosphorylated β-catenin ratio in each group. *P < 0.05 (miR-499 vs control); #P < 0.05 (miR-499 + 15E-LXA vs miR-499).
3.4 Effect of 15E-LXA4 on the expression of TGFβ/SMAD signalling markers in miR-499 transfected CDSCs
The present study analyzed whether protein expressions of TGFβ1 and SMAD3 (a receptor-activated SMAD protein phosphorylated by TGFβ1) were modulated due to the incremental activity of miR-499 in the miR-499 overexpressed CDSCs. As anticipated, miR-499 significantly (P < 0.05) elicited the expression of TGFβ1 and SMAD3 to about 4.7-fold and 3.1-fold respectively, while 15E-LXA4 treatment mitigated the expression of these proteins (Fig. 4), accentuating reduction in the transition of CDSCs from progenitor to cardiomyogenic phase (see Fig. 5).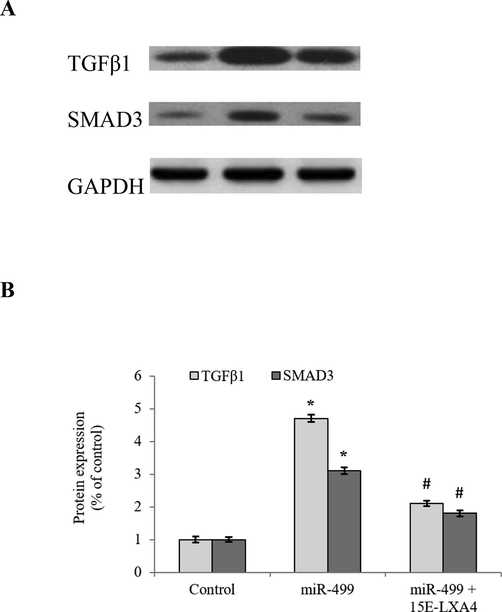
Effect of 15E-LXA4 on the expression of TGFβ/SMAD signaling markers in miR-499 transfected CDSCs. A) Indicative western blot images of TGFβ1 and SMAD3 expressions in all the CDSCs groups using β-actin control. B) Relative TGFβ1 and SMAD3 expression levels in each group. *P < 0.05 (miR-499 vs control); #P < 0.05 (miR-499 + 15E-LXA vs miR-499).
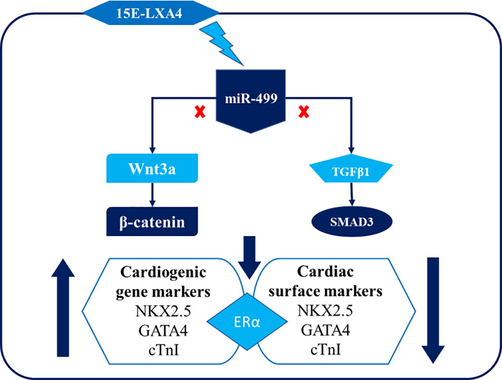
15E-LXA4 treatment blocks the effects of miR-499-mediated changes in TGFβ1/SMAD, Wnt/β-catenin signalling pathways, cardiogenic gene markers and cardiac surface markers in the CDSCs.
4 Discussion
An interesting study by Li et al. (2012) inferred that when compared with mesenchymal stem cells and mononuclear bone marrow cells, CDSCs posed supremacy in the context of paracrine profile, deterrence against ischemia and adverse remodelling, differentiation into cardiomyocytes, angiogenic potential and superior functional advantage in MI. Microembolization risk due to their larger size (∼150 µm) is regarded as a pitfall associated with the intracoronary administration of cardiospheres in cardiovascular ailments. However, modification of the cell culture conditions like optimization of the seeding density and time for harvest enables the production of safer CDSCs (Gallet et al., 2015). Although CDSCs are known to ‘clinically’ regenerate damaged myocardial tissue (Malliaras et al., 2014), the exact mechanism by which it accords benefits is still an enigma.
Ibrahim et al. (2014) identified that exosomes are the pivotal arbitrators of the CDSCs-orchestrated differentiation; notably, by using a miR-146a mimic, they proposed that microRNA-based therapeutics could, at least partially, replicate the benefits of the exosomal components of CDSCs. MiR-499, an intronicmiRNA of the sarcomeric β-myosin heavy chain (MyHC) gene family, Myh7β is a lucrative therapeutic target for accomplishing functionally differentiated cardiac cells in an array of cardiac complications. Recently, Elovic et al. (2019) demonstrated that miR-499 could function as a mainstay shut-down switch in the Bax-mediated cardiomyocyte apoptosis, thus permitting the cardiac differentiation of human embryonic stem cells, whilst eradicating undifferentiated stem cells. Hence, it makes sense to explore the role of miR-499 and its associated molecular signals in the differentiation of CDSCs.
Li et al. (2012) illustrated that CDSCs are harboured with abundant expression of CD105 (endoglin), limited expression of CD117 (c-kit) and CD90, and negligible expression of other markers (CD31, CD34, CD45 and CD133). C-kitpos cardiac stem cells exert the potential to differentiate into cardiac myocytes, endothelial and smooth muscle cells (Bao et al., 2017). Notably, Ellison et al. (2013) indicated that endogenous C-kitpos cardiac stem cells in the adult myocardium displayed robust regenerative/reparative potential in cardiac injury. CD105, a component of the TGFβ1 receptor complex, is one of the most predominant CDSC-specific surface markers. In this context, present study was attempted to understand the interplay between these CDSC surface markers and miR-499. The present study revealed that miR-499 over-expression downmodulated the expression of CD105 and c-kit in the CDSCs, indicating a plausible transition of CDSCs from progenitor to cardiomyogenic phase. However, treatment with 15E-LXA4 upmodulated the expressions of CD105 and c-kit in CDSCs. There is a possibility that hindrance of differentiation of CDSCs by 15E-LXA4 is mediated through its stimulatory effect on the estrogen receptor α (ERα) (Xiong et al., 2013). In fact, membranous ERα has a robust positive correlation with c-kit; also, ERα is reported to play a beneficial role in cardiac ischemic repair. However, ERα might have pleiotropic effects in the context of cardiac repair and CDSCs differentiation processes. In congruence with our notion, Brinckmann et al. (2009) reported that triggering ERα enhanced cardiomyocyte proliferation, but stalled the differentiation of undifferentiated myoblasts. Upon further probing, the present study revealed that ERα expression was mitigated in the CDSCs with miR-499 over-expression. On the other end, 15E-LXA4 treatment enhanced the ERα expression in the CDSCs over-expressing miR-499. From these observations, it became obvious that 15E-LXA4 allays CDSC differentiation, at least in part, through enhancement of ERα.
Cardiac troponin I (cTn-I), a myocardial contraction regulatory protein, is one of the pivotal markers to ascertain the differentiation of CDSCs in human and experimental models. In addition to cTn-I, GATA4 and NKX2.5 (cardiac transcription factors) are also considered as important cardiac-specific markers in the human embryonic stem cells and also in the rat mesenchymal stem cells (Wilson et al., 2010; Zhang et al., 2012). Besides, gene/protein expression of these markers are enhanced during miR-499-triggered cardiogenic differentiation via activation of Wnt/β-catenin signaliing pathway. In this study, further explored whether these cardiac-specific genes in the CDSCs with miR-499 transfection are altered by the treatment with 15E-LXA4. We observed that cTn-I, GATA4 and NKX2.5 expressions were elevated in the miR-499 overexpressed CDSCs, while 15E-LXA4 treatment mitigated the expression of these markers, indicating the repression of cardiomyogenic differentiation of CDSCs.
Wnt/β-catenin signalling pathway is a key nexus in the regulation of cardiac stem cell differentiation and morphogenesis. Of note, activation of Wnt signalling elicits cardiac differentiation, manifested by the increased expression of cardiac transcription factors (e.g., NKX2.5) and gap junction proteins (connexin-43) in human embryonic stem cells (Paige et al., 2010). Zhang et al. (2012) demonstrated that miR-499 overexpression using lentiviral vectors-mediated infection in murine bone marrow-derived mesenchymal stem cells resulted in stimulation of various genes pertinent to cardiogenic differentiation. In this study, increased protein expression of Wnt3a (a canonical Wnt ligand) as well as diminished ratio of phosphorylated/dephosphorylated (Ser33/37/Thr41) β-catenin in the miR-499 overexpressed CDSCs indicates the activation of Wnt/β-catenin axis. On the other end, 15E-LXA4 treated cells reciprocated these expression patterns in miR-499 overexpressed CDSCs, thereby indicating inhibitory action of 15E-LXA4 on Wnt/β-catenin axis.
TGFβ1 displays an established role in the induction of cardiogenic differentiation into myocardial cells. SMAD3 is a pivotal arbitrator of TGFβ/SMAD signalling axis (Dokanehiifard and Soltani, 2018). Upmodulation of TGFβ1 and SMAD3 in the miR-499 overexpressed CDSCs and downmodulation of TGFβ pathway in the 15E-LXA4 treated cells revealed that 15E-LXA4 is a negative regulator of the TGFβ signalling axis and cardiogenic differentiation. In congruence, Yang et al. (2019) illustrated that activation of LXA4 receptor and TGFβ1-mediated inhibition of AKT and SMAD results in inhibition of epithelial–mesenchymal transition, a differentiation process. Based on these outcomes, we could infer that 15E-LXA4, through its agonistic interaction with LXA4 receptor (Chiang et al., 2006), might have blocked TGFβ1-mediated differentiation of CDSCs.
On a clinical note, albeit 15E-LXA4 accords cardiac benefits in the post-myocardial infarction healing (Kain et al., 2017), 15E-LXA4 might display a repressive role in the cardiac differentiation process, at least in the initial phase of differentiation. However, the effects of 15E-LXA4 delayed (treatment after 7 days) or long-term treatment (up to 28 days) have not been investigated in this study. Furthermore, LXA4, being an endogenous component, overexpression of LXA4 receptor and its effect on the cardiac differentiation process have not been studied. Hence, further studies are warranted to ascertain the effects of 15E-LXA4 and role of LXA4 receptor activation in CDSCs, human embryonic stem cells and also in other cell lines across the differentiation phase to understand the latent effects and the pertinent comprehensive mechanisms.
5 Conclusions
Taken together, these results portray that miR-499 is a positive regulator of cardiogenic differentiation of CDSCs. Also, dual activation of Wnt/β-catenin and TGFβ/SMAD signalling axes is the functional mechanism behind miR-499-induced differentiation of CDSCs. On the other edge, 15E-LXA4, through the stimulation of LXA4 receptor negatively regulates the cardiogenic differentiation process through repression of miR-499 and associated Wnt/β-catenin and TGFβ/SMAD signalling pathways.
6 Source(s) of financial support
None
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- C-Kit Positive Cardiac Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells Synergistically Enhance Angiogenesis and Improve Cardiac Function After Myocardial Infarction in a Paracrine Manner. J. Card. Fail.. 2017;23:403-415.
- [Google Scholar]
- Estrogen receptor alpha supports cardiomyocytes indirectly through post-infarct cardiac c-kit+ cells. J. Mol. Cell Cardiol.. 2009;47:66-75.
- [Google Scholar]
- Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J. Am. Heart Assoc.. 2014;3:e001260
- [Google Scholar]
- The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev.. 2006;58:463-487.
- [Google Scholar]
- TrkC-miR2 regulates TGFβ signaling pathway through targeting of SMAD3 transcript. J. Cell Biochem.. 2018;120(2):2634-2641.
- [CrossRef] [Google Scholar]
- Inhibitory effect of hsa-miR-590-5p on cardiosphere-derived stem cells differentiation through downregulation of TGFB signaling. J. Cell Biochem.. 2015;116:179-191.
- [Google Scholar]
- Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell. 2013;154:827-842.
- [Google Scholar]
- MiR-499 Responsive Lethal Construct for Removal of Human Embryonic Stem Cells after Cardiac Differentiation. Sci. Rep.. 2019;9:14490.
- [Google Scholar]
- Intracoronary delivery of self-assembling heart-derived microtissues (cardiospheres) for prevention of adverse remodelling in a pig model of convalescent myocardial infarction. Circ. Cardiovasc. Interv.. 2015;8:002391
- [Google Scholar]
- Global Health Estimates, 2016. Deaths by Cause, Age, Sex, by Country and by Region, 2000- 2016. Geneva, Switzerland: World Health Organization 2018. Available from: URL:http://www.who.int/healthinfo/global_burden_disease/estimates/en/
- Regulation of cardiac stem cells by microRNAs: State-of-the-art. Biomed. Pharmacother.. 2019;120:109447
- [Google Scholar]
- Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep.. 2014;2:606-619.
- [Google Scholar]
- Resolution Agonist 15-epi-Lipoxin A4 Programs Early Activation of Resolving Phase in Post-Myocardial Infarction Healing. Sci. Rep.. 2017;7:9999.
- [Google Scholar]
- Cardiosphere-derived cells do not improve cardiac function in rats with cardiac failure. Stem Cell Res. Ther.. 2017;8:36.
- [Google Scholar]
- Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol.. 2012;59:942-953.
- [Google Scholar]
- Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlardySfunction) J. Am. Coll. Cardiol.. 2014;63:110-122.
- [Google Scholar]
- Synergistic role of 5-azacytidine and ascorbic acid in directing cardiosphere derived cells to cardiomyocytes in vitro by downregulatingWntsignaling pathway via phosphorylation of β-catenin. PLoS One. 2017;12:e0188805
- [Google Scholar]
- Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5:e11134
- [Google Scholar]
- Dynamic microRNA expression programs during cardiac differentiation of human embryonic stem cells: role for miR-499. Circ. Cardiovasc. Genet.. 2010;3:426-435.
- [Google Scholar]
- MiR-499 regulates myoblast proliferation and differentiation by targeting transforming growth factor β receptor 1. J. Cell Physiol.. 2019;234:2523-2536.
- [Google Scholar]
- Lipoxin A4 blocks embryo implantation by controlling estrogen receptor α activity. Reproduction. 2013;145:411-420.
- [Google Scholar]
- Lipoxin A4 ameliorates lipopolysaccharide-induced lung injury through stimulating epithelial proliferation, reducing epithelial cell apoptosis and inhibits epithelial-mesenchymal transition. Respir. Res.. 2019;20:192.
- [Google Scholar]
- MiR-499 induces cardiac differentiation of rat mesenchymal stem cells through wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun.. 2012;420:875-881.
- [Google Scholar]







