Translate this page into:
Residual fate of fenazaquin (10EC) in apple fruit and soil
⁎Corresponding authors. munazah_123@rediffmail.com (Munazah Yaqoob), mjavedansari@gmail.com (Mohammad Javed Ansari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
A field trial was carried out in Kashmir valley to determine the persistence of fenazaquin 10EC (Magister) in Red Delicious variety of apple at recommended (0.004%) and double the recommended (0.008%) application rates.
Methods
The spray was conducted one month prior to harvest. The plants treated with simple tap water were treated as control. Samples were collected at 0, 3, 7, 10, 15, 20 days and harvest. The procedure followed for extraction and cleanup was that of Luke et al. (1985) modified by Sharma (2007) and the final analysis was carried out on a Varian 450 (Walnut Creek, CA, USA) gas chromatograph (GLC) equipped with Thermionic Specific detector (TSD).
Results
After computation of data, the initial deposit was recorded as 3.18 ± 0.03 μg g − 1 and 6.98 ± 0.08 μg g − 1 at two concentrations, respectively. Fenazaquin (0.004%) dissipated to 96.91 per cent in 20 days after application and was not detectable beyond this period. Fenazaquin (0.008%) however, persisted upto 30 days recording 95.84 per cent dissipation at that time.
Conclusions
The progressive dissipation of fenazaquin (0.004%) and fenazaquin (0.008%) residues down to their tolerance limits suggested a waiting period of 18.55 and 30.49 days with a half-life period of 3.62 and 4.12 days, respectively. The terminal residue of fenazaquin at the lower rate was below maximum residue limit (MRL) set by European Union, however above MRL at the higher rate.
Keywords
Persistence
Fenazaquin
Apple
Residues
Half-life
Soil
1 Introduction
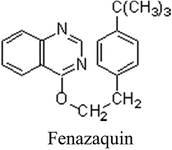
Apple grown in Kashmir holds national and international pride for its delicacy and superb aroma. The state has been declared as Agro-export zone for apple where 30 lakh people directly or indirectly are taking out their livelihood from the Industry. The export of the fruit holds a promising status and is very important for boosting economy of the state and promotion in that direction. The apple crop is subject to attack from a wide range of insects and mite pests which besides causing crop loss deteriorate the quality of fruit. Recent past, however, has witnessed an increase in the arthropod pest problems especially mite infestation due to changing climatic conditions. Mites not only cause crop loss but also adversely affect the quality as their infestation results in discoloration of leaves (Gupta, 2003). The chemical control of these acarine pests dominates the apple growing environment in Kashmir (Bhat et al., 2010). However, one of the major disadvantages of pesticide use is that residue might be present in this crop in amounts above maximum residue limits (MRLs) at the time of consumption which would pose health hazards to consumers. The problem is being viewed seriously by international organizations (US EPA, Codex Alimenterious Commission, WHO and FAO of the United Nations). Further, the development of resistance in arthropods in general and mites in particular to commonly used pesticides is one of the grave concerns of present day. Pesticide resistance development in mites infesting the apple crop in Kashmir too is reported (Sherwani et al., 2011). This has led to introduction and use of a myriad of pesticides especially when the orchardists of the valley are yet to conceive the idea of Integrated Pest Management. One of the commonly used acaricide for management of mites in apple in Kashmir is fenazaquin (IUPAC name: 4-tert-butylphenethyl quinazolin-4-yl ether).
Fenazaquin is a white to tan crystalline solid from the quinazoline category of pesticides, with an acceptable daily intake (ADI) of 0.005 mg/kg body weight/day (Dow Elanco, 1993). Under different trade names such as Magister, Matador, Totem, Demitam, and Magus, fenazaquin is primarily sold in two formulation types: 200 g/l aqueous suspension concentrate and 100 g/l emulsion concentrate. Fenazaquin is a non-systemic acaricidal compound with a wide spectrum of activity in controlling phytophagus mites infesting a number of crops, including fruits and vegetables (Solomon et al., 1993). It is one of the several acaricides and insecticides that are reported to act by inhibiting NADH:ubiquinone oxidoreductase (Complex I) (EC 1.6.99.3) in the range 1–10 nM. Fenazaquin has excellent contact activity against tetranychid and eriophid mites both in laboratory and field tests. This compound is non-phytotoxic and its activity is independent of temperature within the range 12.6–30C (Dreikorn et al., 1991; Shanker et al., 2001). It acts as an electron transport inhibitor, acting at complex I of the mitochondrial respiratory chain (Hollingworth et al. 1992). It is intended for controlling mites infesting a variety of crops namely apples, pears and citrus fruits. It has good knockdown activity on motile forms, as well as true ovicidal activity (Anonymous, 2017). Adequate analytical methods are available for the monitoring of fenazaquin residues in the environmental matrices. Since this acaricide had never been estimated from apples in Kashmir, therefore, the present study was carried out to find the residue dyanamics of fenazaquin on apple in Kashmir.
2 Material and methods
2.1 Field trial
The experiment was carried out on a 20 year old commercial orchard at Tel Bal area of Srinagar, Jammu & Kashmir, where apple variety “Red Delicious” was selected for testing fenazaquin 10 EC (Magister) for dissipation of residues at the recommended (0.004%) and double the recommended (0.008%) application rates. The trees were planted in contour system with plant-to-plant distance of 8.0 m. The trees were at a good bearing stage producing good quality fruit. The experiment was conducted in a single tree plot replicated five times in a randomized block layout. A total of fifteen trees including control (water spray) were selected and marked for the study. The trees were sprayed with fenazaquin one month prior to harvest and a complete coverage of plants was assured.
2.2 Meterological data
The weather conditions play an important role in the dissipation of pesticide residues. Meteorological data including temperature, rainfall and humidity during the period of field experiments were obtained from Meteorology Section of Division of Agronomy, SKUAST-Kashmir. The average maximum and minimum daily air temperatures (oC) recorded during sampling period ranged from 17.5 to 33.0 and 8.0 to 12.6, respectively. The minimum and maximum relative humidity in the range of 36–97 per cent and the rainfall below 2 mm were recorded during the experimental period.
2.3 Field sampling
Samples of apple from the sprayed trees were obtained randomly from each replication for the estimation of pesticides. Samples were collected at 0, 3, 7, 10, 15, 20 days and at harvest. Zero day samples consisted of fruits collected within one hour of spraying when the spray fluid had dried up. Harvest samples were taken 30 days after the application of treatment. The samples were collected in polythene bags (I kg capacity) with dry ice and taken to the laboratory within 1/2h from the time of collection. The samples were stored in the deep freezer (-20 °C) in order to avoid any degradation of residues between sampling and analysis.
2.4 Extraction and clean up
Apple fruits (1 kg) were chopped (with peel and pulp intact) on a cutting board and put in a blender and blended at 1000 rpm. The samples were processed for fenazaquin residues as described by Kadenczki et al. (1992). The representative 50g of finely homogenized sample was extracted with 200 ml acetone and hexane (1:1 V/V) and shaken for two hours at 80 cycles per minute in a horizontal shaker. The extracted samples were filtered through glass funnel using glass wool. The sample was transferred to the separating funnel and partitioned twice with 75 ml ethyl acetate after dilution with 10 per cent NaCl solution. The organic phase was passed through anhydrous Na2SO4 and concentrated in a rotary evaporator to reduce the volume up to 10 ml.
Glass column (60 cm long and 22 mm diameter) was packed compactly with activated charcoal and activated florisil (1:5 W/W). The column was pre-wetted with 10 ml hexane. The concentrated extract was loaded in the column and eluted with 125 ml hexane and ethyl acetate mixture (1:1 V/V). The eluate was concentrated on vacuum evaporator. The final volume was made up to 5 ml in n-hexane. The final extract was analysed on a Varian 450 (Walnut Creek, CA, USA) gas chromatograph (GLC) equipped with an Thermionic Specific detector (TSD Ni63), capillary column CP SIL 8 CB (25 m × 0.25 µm ID × 0.25 µm film thickness of 5% diphenyl and 95% dimethylpolysiloxane). The operating parameters of the instrument were as follows:
Injector temperature : 240 °C
Detector temperature : 250 °C
Oven temperature : 170 0C for 2 min
06° C/min upto 230° C and held for 5 min
10° C/min upto 260° C and held for 5 min
10° C/min upto 270° C and held for 5 min
Nitrogen was used as carrier gas with flow of 1 ml min − 1 through the column and detector make-up of 30 ml min − 1. Under these operating conditions, the retention time of fenazaquin was 9.43 min. Total run time was 31 min. Galaxy chromatography data system version 1.9.302.530 was used for the instrument control and data processing.
2.5 Preparation of standard
Analytical grade reference standard procured from Dr. Ehrenstorfer GmbH, Germany was used for standard curve preparation. A stock solution of 1000 μg g − 1 was prepared from which different concentrations ranging between 0.01 and 1.0 μg g − 1 were obtained for the preparation of standard curve. The data was subjected to regression analysis from which it is evident that the pesticide followed a linear relationship showing corresponding increase in respective pesticide concentrations with coefficient of determination (R2) of 0.9988.
2.6 Recovery
Before analyzing actual sample of fruits, the efficiency of the method was evaluated in recovery experiments by spiking untreated samples of fruits (collected from control plots) with different pesticides. A 50 g well homogenized sample of fenazaquin was spiked with known amount of standard pesticides at three concentrations each replicated thrice. The fortified samples were extracted and cleaned with the method followed for analyzing actual samples. The recoveries for the three levels fell within the acceptable tolerance of 70 to 120 per cent range (SANCO/12571/2014) indicating good performance of extraction, clean up and chromatographic parameters for residue determination in fruits (Table 1). The relative standard deviations (RSDs) were less than 20 per cent for all the levels. In the present study, fenazaquin was used against European red mite (Tetranychus ulmi koch) and analysed for residues. Crop yield and crop health were satisfactory as other pesticides were also used for the comprehensive protection of the apple crop.
Amount fortified(μg g − 1)
Amount recovered(μg g − 1)a
Average recovery(%)
Relative standarddeviation(%)
0.01
0.008
80.0 ± 1.283
1.60
0.10
0.086
87.0 ± 0.857
0.98
0.20
0.189
94.5 ± 1.796
1.90
2.7 Analysis of data
The data was subjected to statistical analysis as per Hoskin (1961):
The half-life value of insecticides as indices of the rates of residue dissipation was calculated as per Hoskins (1961):
b = slope of regression equation
Withholding period (Ttol) based on the prescribed maximum residue limits (MRLs) by European Union was worked out as indices of the safety to consumers.
2.8 Determination of residues of fenazaquin in soil under treated apple tree canopy
In this study, the soil samples (1 kg each) were collected in labeled wide mouth amber glass bottle using stainless steel auger (15 cm deep and 3–5 cm diameter) around the trees along the four directions that were sprayed with various pesticides for study of dissipation at various intervals. 5 samples were thoroughly mixed to ensure that the soil collected was truly representative of each treatment. Soil samples were subsequently taken to the laboratory where they were air dried at room temperature, powdered in a pestle and mortar, sieved through a 2 mm sieve and stored at −20 0C until further chemical processing. The sampling was carried out at 0, 3, 7, 10, 15, 20 days after treatment and at harvesting of the fruit.
2.9 Extraction and clean up of soil sample
The extraction and clean up method for analysis of fenazaquin from soil samples was carried out as per Kadenczki et al. (1992). The soil sample was extracted with 150 ml acetone and hexane (1:1V/V). The extract was partitioned with ethyl acetate after dilution with 10 per cent NaCl solution and concentrated in a rotary evaporator. A final analysis was carried out on GLC/NPD.
2.10 Recovery
GC method was used for analysis of fenazaquin residues in soil. Before analyzing the actual samples of soil, the efficiency of the method was evaluated in recovery experiments by spiking untreated samples (collected from control plots) with fenazaquin. A 50 g well ground soil sample of, fenazaquin was spiked with known amount of fenazaquin at three levels. For spiking level, three replicated samples were extracted with respective solvents and cleaned up on florisil column and analyzed by GC.
Where,
RA = Residue in aliquat (µg) estimated by analytical procedure
TS = Total solvent (ml) added per sample
SA = Size of aliquat (ml)
SW = Sample weight (g)
Half life values (T1/2) corresponding to rate of dissipation were calculated by the method of Hoskins (1961). where b = slope of regression equation
The waiting period (T tol) required to be elapsed for the pesticide deposits to reach the maximum residue limit required for the safe consumption of the fruit after pesticide application was also worked out by the method of Hoskins (1961).
3 Result and discussion
The data on persistence and dissipation of fenazaquin 10 EC applied at 0.004 and 0.008 per cent on Red Delicious apple is presented in Table 2. The acaricide left an initial deposit of 3.180 ± 0.020 and 6.980 ± 0.083 μg g−1 on the fruits at lower and higher concentrations, respectively, which degraded with time reaching 0.098 ± 0.001 on day 20 at the lower rate with 96.91 per cent dissipation where no residue could be detected beyond that period. The acaricide applied at higher rate however, dissipated slowly recording a residue level of 0.290 ± 0.036 μg g−1 at day 30 which is above MRL (0.1 μg g−1) set by the European Union and hence rendered apple fruit unsafe for consumption at that time. The residues dissipated with a half life value (T½) of 3.62 and 4.12 days. A waiting period (Ttol) of 18.55 and 30.49 days was worked out at two rates, respectively (Table 3). Sharma et al. (2006) observed an initial deposit of fenazaquin applied at 100 and 200 g a.i./ha on apple ranging 0.46–0.65 and 0.78–1.05 mg kg−1 with half-life of 1.9–5.3 and 3.6–5.2 days at two doses, respectively. Studies carried out by Duhan and Kumari (2011) on fenazaquin @ 125 and 250 g a.i./ha in field and in pot under field capacity moisture in laboratory and analyzed on GC-NPD revealed that it dissipates almost 90 per cent in 90 days with half-life period of 32.04 and 31.35 days, respectively, at field conditions and 30.10 and 28.94 days under laboratory conditions approximating to first order kinetics in both conditions having correlation coefficient ranging from −0.9848 to −0.9914. The present findings are in agreement with the fact that dissipation of fenazaquin follows Ist order kinetics (Anonymous, 2017). The variation observed between studies, however, may be attributed to different concentrations and formulations used and application on different variety of apple. *Mean of 5 replications. MRL = 0.1 (μg g−1). BDL = Below detectable limit. T1/2 = half-life, Ttol = waiting period, R2 = Regression coefficient.
Days after treatment(X)
Residues* (μg g − 1) ± SD(Y)
Dissipation(%)
0.004 (%)
0.008 (%)
0.004 (%)
0.008 (%)
0
3.180 ± 0.034
6.980 ± 0.083
–
–
3
2.092 ± 0.035
5.120 ± 0.252
34.21
26.65
7
1.015 ± 0.687
4.018 ± 0.054
68.08
42.43
10
0.787 ± 0.058
3.126 ± 0.325
75.25
55.21
15
0.100 ± 0.060
1.994 ± 0.056
96.85
71.43
20
0.098 ± 0.003
0.982 ± 0.016
96.91
85.93
30 (Harvest)
BDL
0.290 ± 0.036
BDL
95.84
Apple variety
Rate of application of fenazaquin 10 EC (%)
Regression equation (Y = a − bx)
R2
T1/2
Ttol
Red Delicious
0.004
3.540–0.080 X
0.09277
3.62
18.55
0.008
3.340–0.087X
0.9188
4.12
30.49
The studies carried out on the persistence and dissipation of fenazaquin (0.004% and 0.008%) in soil under treated apple tree canopy of Red Delicious variety revealed that the pesticides left no initial deposit at zero day after application and the residues continued to be undetectable up to the harvest. The present observations are, however in contradiction with the findings of various workers who have detected residues of pesticides in soil of many agricultural crops including apple (Redondo et al., 1997; Sharma et al., 2006; Vig et al., 2008; Chai, et al., 2009; Anwar, et al., 2012; Bhattacharyya et al., 2010; Guo et al., 2010; Mukhopadhyay, et al., 2011. Duhan and Kumari, 2011). Pesticides may reach the soil through direct application to the soil surface, incorporation in the top few inches of soil, or during application to crops (Akan et al., 2013). Fenazaquin, however during present study was applied on 20 year old healthy and foliage rich trees which hardly allowed pesticides to drift and fall on ground surface. Moreover, the soil basin under treated tree canopy during the present study was covered with grass cover and the soil samples for the purpose of pesticide analysis were collected only after removing the grass cover to expose the soil surface. Further, the method of application during present study was quite scientific which could possibly reduce the soil contamination with fenazaquin during application.
4 Conclusion
The fruit samples of Red Delicious variety of apple treated with fenazaquin 10 EC and soil samples from the basin under treated tree canopy were collected at 0, 3, 7, 10, 15, 20 and 30 days after pesticide application. Fenazaquin 10 EC applied at recommended concentration on Red Delicious apple one month prior to harvest left residues below MRL set by European Union at the time of harvest as compared to the higher concentration at which residues were detected above Maximum residue level. It indicates, that the fruit can be produced residue free and safe for consumption if judicious use of pesticides with respect to concentration and time is followed. However, waiting period based on the prescribed maximum residue limits needs be adopted strictly as index of safety to consumers. Pesticides applied properly and scientifically avoiding drift to environment can reduce the contamination of soil in apple orchards.
Acknowledgement
The authors thank Director Research SKUAST-K for financial assistance received during the study. This project was supported by Researchers Supporting Project number (RSP-2020/283) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Organophosphorus pesticide residues in vegetable and soil samples from Alau Dam and Gongulong agricultural areas, Borno State, Nigeria. Int. J. Environ. Monit. Anal.. 2013;1(2):58.
- [Google Scholar]
- Anonymous. 2017. Fenazaquin. http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation2017/FENAZAQUIN__297_.pdf
- Determination of pesticide residues in soil of Nawabshah, Sindh, Pakistan. Pakistan J. Zool.. 2012;44(1):87-93.
- [Google Scholar]
- Pesticides and brain cancer linked in orchard farmers of Kashmir. Indian J Med. Paediat. Oncol.. 2010;31(4):110-120.
- [Google Scholar]
- Effect of pH on the fate of fenazaquin in soil and water under laboratory-simulated condition. Toxicol. Environ. Chem.. 2010;92(6):1083-1092.
- [Google Scholar]
- Dissipation of acephate, chlorpyrifos, cypermethrin and their metabolites in a humid-tropical vegetable production system. Pest Manag. Sci.. 2009;65(2):189-196.
- [Google Scholar]
- Dow Elanco, 1993. Fenazaquin—A Profile. Department of Regulatory Toxicology and Environmental Affairs, Agriculture Products Research and Development, Oxfordshire, UK.
- Dreikorn BA, Thompson GD, Suhr RG, Worden TV, Davis NL (1991) The discovery and development of fenazaquin (EL-436),a new broad spectrum acaricide. In: Frehse H, Kesseler-Schmitz E, Conway S (eds) Proceedings of seventh international congress on pesticide chemistry, (1) 01-33.
- Degradation Studies of fenazaquin in soil under field and laboratory conditions. Bulletin of Environmental Contamination. Toxicology. 2011;87(2):180-183.
- [Google Scholar]
- Determination and safety evaluation of difenoconazole residues in apples and soils. Bull. Environ. Contaminat. Toxicol.. 2010;85(4):427-431.
- [Google Scholar]
- Mite pests of agricultural crops in India, their management and identification. In: Yadav P.R., Chauhan R., Putatunda B.N., Chillar B.S., eds. Mites, their identification and management. Hisar: CCS HAU; 2003. p. :48-61.
- [Google Scholar]
- R.M. Hollingworth K.I. Ahammadsahib G.G. Gadelhak J.L. Melaughlin Complex I of mitochondrial respiratory transport chain: a target for pesticide development by both man and nature In: Proceedings of American Chemical Society 1992 Abs:156.
- Mathematical treatment of rate of loss of pesticide residues. FAO Plant Prot. Bull.. 1961;9(9):163-168.
- [Google Scholar]
- Column ex traction of residues of several pesticides from fruits and vegetables; A simple multi residue analysis method. J. AOAC.. 1992;75(1):53-61.
- [Google Scholar]
- Dissipation study of difenoconazole in/on chili fruit and soil in India. Bull. Environ. Contamin. Toxicol.. 2011;87(1):54-57.
- [Google Scholar]
- Dissipation and distribution of atrazine, simazine, chlorpyrifos, and tetradifon residues in citrus orchard soil. Arch. Environ. Contaminat. Toxicol.. 1997;32(4):346-352.
- [Google Scholar]
- Persistence of fenazaquin in apple fruits and soil. Pesticide Res. J.. 2006;18(1):79-81.
- [Google Scholar]
- Determination of pesticide efficacy against field populations of European Red mite, Panonychus ulmi Koch. J. Insect Sci.. 2011;24(2):142-146.
- [Google Scholar]
- Fenazaquin, a selective acaricide for use in IPM in the UK. Crop Prot.. 1993;12:255-258.
- [Google Scholar]
- Dissipation and leaching of (14)C-monocrotophos in Soil Columns under subtropical climate. J. Environ. Sci. Health B 2008
- [Google Scholar]







