Translate this page into:
Renal toxicity of methylprednisolone in male Wistar rats and the potential protective effect by boldine supplementation
⁎Corresponding authors at: P.O. Box 22452, Riyadh 11459, Saudi Arabia. dibrahim@ksu.edu.sa (Dalia Fouad), 438203882@student.ksu.edu.sa (Esraa Shuker),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Intravenous corticosteroids, methylprednisolone (MPL), which is one of the drugs that induce glomerular kidney diseases, when used for a long time at high dose by doctors to treat acute renal inflammation, promote the development of reactive oxygen species (ROS)-induced renal toxicity. This study investigated the role of boldine, a natural antioxidant with anti-apoptotic and anti-inflammatory properties, against MPL-induced renal toxicity in male Wistar rats.
Methods
120 rats were divided into eight equal groups as follows: G1 (control), G2, 3, and 4 (rats orally administered 5, 10, and 50 mg boldine/kg/day; respectively, for 28 days), G5 (rats intramuscularly injected with 100 mg MPL/kg only on the last three days), G6, 7, and 8 (rats administered boldine + MPL). After the last MPL injection, rats were sacrificed at intervals of 1, 24, and 48 h.
Results
There was a significant increase in body weight, blood glucose, and urea levels, as well as a decrease in albumin level in serum. Oxidative stress markers levels increased at all times, and gene expression of anti-oxidant enzymes increased at 24 h. According to immunohistochemistry, there was a considerable increase in P53 and TNF-α levels following MPL therapy. The cell death DNA assays revealed detectable necrosis and apoptosis, induced by MPL treatment.
Conclusions
Oral administration of boldine has a modulatory protective, anti-oxidant, anti-apoptotic, and anti-inflammatory effect against free radicals.
Keywords
Methylprednisolone
Renal toxicity
Oxidative stress
DNA damage
Boldine
Anti-oxidant properties
- Methylprednisolone
-
MPL
- Reactive oxygen species
-
ROS
- 11-beta-hydroxysteroid dehydrogenase type 1
-
11β-HSD1
- Lipid peroxidation
-
LPO
- Nitric oxide
-
NOx
- Reduced glutathione
-
GSH
- Glutathione reductase
-
GR
- Glutathione peroxidase
-
GSHPx
- Superoxide dismutase
-
SOD
- Lactate dehydrogenases
-
LDH
Abbreviations
1 Introduction
The kidneys are the excretory organs that filterate blood. So, they are common target organs for toxicity and injury after exposure of the body to medications or toxins. Abnormalities in urine properties and decreased excretory renal function are clinical signs of kidney disease (Romagnani et al., 2017). Intravenous corticosteroids (such as dexamethasone and methylprednisolone) are often used by physicians to treat acute renal inflammation (Jacob et al., 2015). Methylprednisolone (MPL) is a synthetic anti-inflammatory and immunosuppressive glucocorticoid having physiologic effects comparable to the naturally occurring ones. The effects of making it useful in human and animal medicine (Ocejo and Correa, 2021).
Glucocorticoids (GCs) influence renal development and function in both fetal and mature kidneys by regulating the cardiovascular system and influencing glomerular and tubular function. Treatment with excess GC can cause hypertension, increased cardiac output, renal blood flow and, in certain species, they increase renal vascular resistance (Smets et al., 2010). Prednisone, and non-steroidal anti-inflammatory medications cause intrinsic acute renal damage specific to the tubular and interstitial compartments of the kidney, as well as kidney toxicology (Radi, 2018) and they are one of the drug-induced glomerular pathologies. Glomerulopathy and/or proteinuria was discovered in dogs with Cushing syndrome (hyperadrenocorticism), a disease arising from excess production of adrenocorticotropic hormone, as well as in dogs and other species treated with prednisone (Smets et al., 2010). Direct alterations in glomerular permeability and effects related to increased blood pressure and glomerular filtration rate, increased blood glucose and insulin resistance, or obesity were also seen (Radi, 2018). Furthermore, renal response to large doses of glucocorticoids leads to an acute suppression of kidney function. Glucocorticoids also affect the concentrating and diluting capacity of the kidney.
Boldine (1, 10-dimethoxy-2, 9-dihydroxyaporphine) is a significant antioxidant alkaloid found in the leaves and bark of Peumusboldus Molina (P. boldus) (Monimiaceae), an important medicinal plant (Nabavi et al., 2017). This alkaloid is assumed to be responsible for most of the boldo extract's health-promoting and pharmacological benefits (O’Brien et al., 2006; Cassels et al., 2019). Boldine has cyto-protective, anti-atherogenic, anti-platelet, anti-tumor, anti-inflammatory, immunomodulatory, hepato-protective, and anti-pyretic properties (Heidari et al., 2019).
Boldine has been offered as a therapy for diabetes (Yang et al., 2018), and it is used to support bile production. It has a significant antioxidant activity that protects renal tissue and slows the progression of hyperglycemia and weight loss in streptozotocin-induced diabetic rats (Heidari et al., 2019). Boldine was used in several studies to prevent both cellular and renal alterations, as well as increases in glycemia, blood pressure, renal thiobarbituric acid (TBARS) reactive substances, and the urinary protein/creatinine ratio, which could be effective against tissue damage in diabetic rats (Hernández-Salinas et al., 2013). Gerhardt et al., (2014) showed that boldine could reduce cell viability and cell proliferation in human bladder carcinoma cells (T24). Furthermore, boldine inhibited cell growth and cell cycle.
This study has the objective to investigate the antioxidant, anti-apoptotic, and anti-inflammatory activity of boldine against changes induced by MPL renal toxicity in a male Wistar rat model.
2 Materials and methods
2.1 Experimental animals and handling
120 male Wistar rats weighing about 180–200 g were obtained from King Saud University’s animal house in Riyadh, Saudi Arabia and accommodated as described elsewhere (Fouad et al., 2019). All animals were handled per the recommendations of the King Saud University (KSU) Ethics committee in Riyadh, Saudi Arabia (KSU-SE-19-139), which received ethical approval on 13-02-2020.
Methylprednisolone Sodium Succinate 1000 mg was obtained from Pfizer Manufacturing Belgium NV Company. Commercial assay kits were obtained from Cayman and BioVision was used for measuring antioxidant defense enzyme and oxidative stress markers. Thermo Scientific provided the antibodies used for immunohistochemistry of TNF-α and P53 (Waltham, Massachusetts). The DNA extraction kit was obtained from Qiagen. All other chemicals and reagents were of high analytical grade.
2.2 Preparation of boldine
Boldine was supplied by Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and was freshly prepared. The dose of boldine used in this study was according to (Hernández-Salinas et al., 2013; Heidari et al., 2019).
2.3 Experimental design
The experimental animals were sorted into groups of 8, with 15 in each group. The rats were subsequently given the following treatments for four weeks in a row:
Group 1: Rats administered distilled H2O (Control group).
Group 2: Boldine (5 mg/kg) administered daily via oral tube for four weeks.
Group 3: Boldine (10 mg/kg) administered daily via oral tube for four weeks.
Group 4: Boldine (50 mg/kg) administered daily via oral tube for four weeks.
Group 5: Methylprednisolone Sodium Succinate (MPL) (100 mg/kg) was injected intramuscularly only on the last three days (Bardas et al., 2020).
Group 6: Boldine (5 mg/kg) + MPL administered daily via an oral tube for four weeks.
Group 7: Boldine (10 mg/kg) + MPL administered daily via an oral tube for four weeks.
Group 8: Boldine (50 mg/kg) + MPL administered daily via an oral tube for four weeks.
MPL (100 mg/kg) was injected intramuscularly once a day on the last three days of the experiment.
After the last administration, five rats from each group were sacrificed at 1, 24, and 48hrs intervals. The body weight of the rats was measured throughout the experimental period, especially on the day of dissection.
2.4 Sample preparation
A serum samples were separated from the blood for biochemical analysis. Serum was kept at −80 ℃, awaiting more analysis. The kidney was excised. The first portion was instantly conveyed to 10 % buffered formaldehyde for histological and immunohistochemistry examination. The second portion was immediately stored at −80 ℃ for DNA and RNA extraction, and for detecting antioxidant defense enzymes and oxidative stress markers.
2.5 Biochemical analysis
The blood glucose level, the concentration, and activities of kidney function (creatinine, urea, and albumin) in rat serum were determined using the ReflotronPlus Dry-Chemistry Analyzer (Roche, Germany). The results were expressed in mg/dl. All Reflotron strips were purchased from Roche (Germany).
2.6 Measurement of 11-beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme activities
11β-HSD1 enzyme activity was analyzed in kidney tissues homogenates using commercial sandwich enzyme immunoassay (ELIZA) kits (Cat. NO. RDR-HSD11b1-Ra, Reddotbiotech Company).
2.7 Histopathological examination
The kidney sample was preserved in formaldehyde, serial dehydration, clearing, and embedding. The tissue sections were stained using hematoxylin and eosin (H&E) (Yousef and Hussien, 2015).
2.8 Antioxidant defense enzyme and oxidative stress biomarkers
A portion of the kidney was homogenized to determine lipid peroxidation (LPO), and nitric oxide (NOx) using kits from (Cat. NO. K454-100, K262-200, BioVision Incorporated, 155 S. Milpitas Blvd, USA). Reduced glutathione (GSH), Glutathione reductase (GR), glutathione peroxidase (GSHPx), and superoxide dismutase (SOD) were measured using commercial assay kits from (Cat. NO. 703002, 703202, 703102, 706002, Cayman Chemical Company, AnnArbor, MI, USA).
2.9 Molecular analysis
Extraction of total RNA from kidney tissue was done using RNA Mini Kit (Cat. No.12183018A, Invetrogen, USA). Complementary DNA (cDNA) was synthesized from RNA by using High-Capacity cDNA Reverse Transcription Kits (Cat. NO. 4368814, Applied Biosystems by Thermo Fisher Scintific, USA). Real-time PCR analysis was done on triplicate samples. Integrated DNA Technologies, Inc., Illinois, USA, synthesized the PCR primers for SOD, GSHPx, GR, and 11b-HSD1 genes.
Primers for the genes of interest were designed as follows: SOD [(F) 5′-GAGCAGAAGGCAAGCGGTGAA-3′, (R) 5′-CCACATTGCCCAGGTCTC-3′]; GPx [(F) 5′-AACGTGGCCTCGCAATGA-3′, (R) 5′-GGGAAGGCCAGGATTCGTAA-3′]; GR [(F) 5′-TTCTGGAACTCGTCCACTAGG-3′, (R) 5′-CCATGTGGTTACTGCACTACTTCC-3′]; 11b-HSD1 [(F) 5′-CAATGGAAGCATTGTTGTCG-3′, (R) 5′-GAAGAACCCATCCAAAGCAA-3′]. The housekeeping gene was Glyceraldhyde-3-phosphate dehydrogenase (GAPDH).
2.10 Biomarker of cell death
2.10.1 Total DNA Extraction, and fragmentation
Total DNA was extracted from kidney tissue using DNeasy blood & tissue kit (Cat. No. 69504, Qiagen, USA) and its integrity was evaluated using agarose gel (1.5 %) electrophoresis.
2.10.2 Determination of lactate dehydrogenases (LDH)
Extracellular lactate dehydrogenase, as an index of necrosis, was measured in a culture medium using kit (Cat. No. 601170, Cayman Chemical Company, Ann Arbor, MI, USA).
2.11 Immunohistochemical for detection of tumor necrosis factor-alpha (TNF-α) and P53
To detect TNF-α and P53, paraffin kidney sections incubation for 12 h at 4 ℃ in a blocking solution with TNF-α antibody (Clone 7H8.2C1) (mouse monoclonal) and P53 antibody (Potocnjak and Domitrovic, 2016).
2.12 Statistical analysis
Analysis of data was done using one-way analysis of variance (ANOVA) with the SPSS software (ver.22) Chicago, IL, USA. The p-values in this study were two-sided, and ≤0.05 was considered significant. Further group comparisons were made using the post hoc LSD test. Continuous and categorical variables were presented as means ± Standard Deviation (SD) and percentages, respectively.
3 Results
3.1 Body weight and biochemical parameters
After 4 weeks of the experiment, the body weight, blood glucose, and serum urea levels in the MPL-treated groups increased significantly at 1, 24, and 48 h when compared to the control group. However, albumin levels decreased significantly at all-time intervals in the MPL-treated group compared to the control group (Tables 1, 2). Furthermore, the boldine (BOL) 5, 10, 50 mg/kg + MPL treatment group showed a significantly decreased body weight, blood glucose, and urea levels compared to the MPL groups, while albumin levels were increased at all-time intervals.
Groups parameter
Control
BOL 5 mg
BOL10 mg
BOL 50 mg
MPL100mg
BOL 5 mg + MPL 100 mg
BOL 10 mg + MPL 100 mg
BOL 50 mg + MPL 100 mg
Body weight (g) 1 h
177.2 ± 2.51 a
176.2 ± 2.059 a
177.4 ± 1.166 a
174.2 ± 3.54 a
197.6 ± 0.979b
190.6 ± 1.208c
189.6 ± 0.600c
188.2 ± 0.860c
Body weight (g24 (hrs
176.6 ± 2.712a
177.8 ± 2.457 a
179.4 ± 3.218 a
177.2 ± 3.979 a
208 ± 1.224b
189 ± 1.183c
186.8 ± 1.854c
185.4 ± 0.979c
Body weight (g) 48 hrs
167.8 ± 1.529 a
175.2 ± 1.392 a
180 ± 1.449 a
175 ± 2.408 a
188.6 ± 1.777b
184.6 ± 1.720c
182.4 ± 1.122c
181.6 ± 2.111c
Glucose (mmol/L) 1 h
4.720 ± 0.252 a
4.620 ± 0.205 a
4.740 ± 0.116 a
4.680 ± 0.193 a
6.760 ± 0.098b
6.260 ± 0.169c
6.160 ± 0.166c
6.020 ± 0.159c
Glucose (mmol/L) 24 hrs
4.860 ± 0.132 a
4.820 ± 0.135 a
4.940 ± 0.132 a
4.720 ± 0.232 a
7.740 ± 0.098b
5.560 ± 0.193c
5.440 ± 0.191c
5.380 ± 0.120c
Glucose (mmol/L) 48 hrs
4.680 ± 0.153 a
4.540 ± 0.132 a
4.700 ± 0.189 a
4.540 ± 0.213 a
5.660 ± 0.082b
5.460 ± 0.067c
5.280 ± 0.120c
5.180 ± 0.215c
Groups parameter
Control
BOL 5 mg
BOL 10 mg
BOL 50 mg
MPL 100 mg
BOL 5 mg + MPL 100 mg
BOL 10 mg + MPL 100 mg
BOL 50 mg + MPL 100 mg
Creatinine (U/L) 1 h
0.534 ± 0.003 a
0.527 ± 0.001 a
0.529 ± 0.002 a
0.536 ± 0.003 a
0.541 ± 0.003 a
0.537 ± 0.008 a
0.530 ± 0.001 a
0.534 ± 0.002 a
Creatinine (U/L) 24 hrs
0.535 ± 0.001 a
0.537 ± 0.006 a
0.540 ± 0.001 a
0.540 ± 0.002 a
0.545 ± 0.002 a
0.544 ± 0.003 a
0.538 ± 0.002 a
0.536 ± 0.002 a
Creatinine (U/L) 48 hrs
0.534 ± 0.002 a
0.531 ± 0.001 a
0.536 ± 0.002 a
0.537 ± 0.003 a
0.544 ± 0.002 a
0.541 ± 0.001 a
0.538 ± 0.002 a
0.540 ± 0.003 a
Urea)mg/dl)1h
45.58 ± 1.785 a
44.58 ± 1.313 a
46.38 ± 1.262 a
47.18 ± 1.132 a
67.28 ± 1.776b
63.28 ± 1.231c
62.28 ±.972c
61.68 ± 0.423c
Urea) mg/dl) 24 hrs
46.72 ± 1.172 a
45.32 ± 1.019 a
46.32 ± 0.692 a
45.52 ± 0.861 a
84.02 ± 1.812b
68.28 ± 0.992c
67.48 ± 1.042c
66.68 ± 1.187c
Urea)mg/dl) 48 hrs
45.06 ± 1.582 a
43.46 ± 1.080 a
44.26 ± 1.192 a
44.14 ± 1.664 a
57.28 ± 2.136b
52.68 ± 1.299c
52.48 ± 0.915c
51.48 ± 0.772c
Albumin (g/dl1 (h
4.160 ± 0.018 a
4.170 ± 0.045 a
4.128 ± 0.078 a
4.220 ± 0.025 a
2.800 ± 0.066b
3.100 ± 0.042c
3.134 ± 0.064c
3.615 ± 0.024c
Albumin) g/dl24 (hrs
4.178 ± 0.016 a
4.160 ± 0.018 a
4.110 ± 0.055 a
4.200 ± 0.044 a
2.230 ± 0.020b
3.700 ± 0.126c
3.754 ± 0.100c
3.764 ± 0.096c
Albumin (g/dl)48 hrs
4.020 ± 0.048 a
3.960 ± 0.040 a
3.920 ± 0.048 a
3.960 ± 0.097 a
2.496 ± 0.098b
3.240 ± 0.067c
3.385 ± 0.045c
3.516 ± 0.048c
3.2 11-Beta-Hydroxysteroid dehydrogenase type 1 (11β-HSD1) enzyme activities
There was a significant decrease in the 11β-HSD1 enzyme activities in kidney tissues homogenates in MPL-treated groups at 1, 24, and 48 h compared to the control group. However, in the BOL 5, 10, 50 mg/kg + MPL treated group, the activities of 11β-HSD1 were significantly increased at all-time intervals compared to the MPL group (Table 3).
Groups parameter
Control
BOL 5 mg
BOL 10 mg
BOL 50 mg
MPL 100 mg
BOL 5 mg + MPL 100 mg
BOL 10 mg + MPL 100 mg
BOL 50 mg + MPL 100 mg
11β-HSD1 ((nmol/g) 1 h
4.960 ± 0.134 a
4.851 ± 0.070 a
4.937 ± 0.115 a
4.981 ± 0.131 a
4.217 ± 0.09b
4.374 ± 0.154c
4.452 ± 0.130c
4.504 ± 0.121c
11β-HSD1 ((nmol/g) 24 hrs
4.976 ± 0.026 a
4.913 ± 0.049 a
4.979 ± 0.072 a
4.908 ± 0.057 a
3.504 ± 0.051b
4.721 ± 0.047c
4.843 ± 0.056c
4.863 ± 0.021c
11β-HSD1 ((nmol/g) 48 hrs
5.048 ± 0.147 a
4.957 ± 0.099 a
4.912 ± 0.16 a
5.029 ± 0.100 a
4.529 ± 0.062b
4.620 ± 0.186c
4.652 ± 0.108c
4.702 ± 0.107c
3.3 Oxidative stress markers and antioxidant defines enzymes in kidney tissue
There was a significant increase in LPO and NOx levels in the MPL treated group, while GSH levels were significantly decreased in the kidney tissues at 1, 24, and 48 h (Table 4). In addition, the MPL-treated group demonstrated a significant decrease in the SOD, GSHPx, and GR activity levels in kidney tissue at all-time intervals, when compared to the control group (Table 5). Boldine treatment (5, 10, 50 mg) + MPL caused a reduction in oxidative stress, and increased significantly in SOD, GSHPx, and GR levels compared to the MPL group, particularly at 24 h with 50 mg/kg boldine dose.
Groups parameter
Control
BOL 5 mg
BOL 10 mg
BOL 50 mg
MPL 100 mg
BOL 5 mg + MPL 100 mg
BOL 10 mg + MPL 100 m
BOL 50 mg + MPL 100 mg
MDA (nmol/g) 1 h
19.88 ± 0.048 a
19.3 ± 0.122 a
19.25 ± 0.161 a
19.60 ± 0.164 a
56.74 ± 0.193b
52.56 ± 0.229c
52.16 ± 0.191c
51.37 ± 0.406c
MDA (nmol/g) 24 hrs
20.58 ± 0.156 a
19.92 ± 0.120 a
20.36 ± 0.142 a
20.60 ± 0.066 a
58.92 ± 0.287b
43.54 ± 0.163c
43.04 ± 0.430c
42.52 ± 0.422c
MDA (nmol/g) 48 hrs
20.06 ± 0.116 a
19.4 ± 0.187 a
19.72 ± 0.080 a
20.20 ± 0.122 a
52.9 ± 0.367b
39.02 ± 0.182c
38.36 ± 0.221c
38.23 ± 0.206c
NOx (nmol/g) 1 h
1.192 ± 0.004 a
1.174 ± 0.004 a
1.150 ± 0.003 a
1.196 ± 0.006 a
1.942 ± 0.004b
1.750 ± 0.004c
1.74 ± 0.003c
1.706 ± 0.007c
NOx (nmol/g) 24 hrs
1.262 ± 0.004 a
1.254 ± 0.006 a
1.234 ± 0.004 a
1.246 ± 0.017 a
2.444 ± 0.004b
1.974 ± 0.006c
1.94 ± 0.020c
1.934 ± 0.013c
NOx (nmol/g) 48 hrs
1.174 ± 0.004 a
1.154 ± 0.009 a
1.158 ± 0.003 a
1.182 ± 0.003 a
2.222 ± 0.008b
1.850 ± 0.004c
1.840 ± 0.003c
1.812 ± 0.006c
GSH (nmol/g) 1 h
57.99 ± 0.444 a
58.4 ± 0.659 a
58.7 ± 0.788 a
58.89 ± 0.78 a
47.77 ± 0.670b
52 ± 1.095c
52.9 ± 0.671c
53.09 ± 0.768c
GSH (nmol/g) 24 hrs
60.70 ± 0.75 a
60.00 ± 0.72 a
60.25 ± 0.51 a
60.72 ± 0.75 a
44.26 ± 0.81b
55.8 ± 0.80c
56.2 ± 0.71c
56.70 ± 0.73c
GSH (nmol/g) 48 hrs
55.70 ± 0.693 a
55.5 ± 0.418 a
55.84 ± 1.016 a
56.52 ± 0.388 a
47.33 ± 0.895b
51.5 ± 0.400c
51 ± 0.836c
51.58 ± 0.788c
Groups parameter
Control
BOL 5 mg
BOL 10 mg
BOL 50 mg
MPL 100 mg
BOL 5 mg + MPL 100 mg
BOL 10 mg + MPL 100 mg
BOL 50 mg + MPL 100 mg
GSHPx (nmo/ml) 1 h
526.8 ± 1.62 a
518.4 ± 0.904 a
515.0 ± 0.625 a
521.4 ± 1.218 a
293.9 ± 0.813b
337.7 ± 0.470c
342.9 ± 0.697c
343.6 ± 0.918c
GSHPx (nmol/ml) 24hrs
525.0 ± 0.632 a
521.9 ± 0.562 a
526.6 ± 1.162 a
528.4 ± 1.720 a
270.6 ± 0.556b
344.5 ± 0.619c
346.5 ± 0.82c
348.9 ± 0.40c
GSHPx (nmol/ml) 48hrs
493.3 ± 0.496 a
486.1 ± 1.205 a
496 ± 1.140 a
500.8 ± 1.319 a
320.8 ± 0.550b
343.6 ± 0.974c
349.4 ± 1.692c
352.6 ± 2.063c
GR (nmol/g) 1 h
473.4 ± 1.568 a
466.8 ± 1.074 a
473.2 ± 0.736 a
476.8 ± 0.527 a
409.9 ± 1.143b
433.9 ± 0.548c
434.7 ± 0.572c
435.9 ± 1.488c
GR (nmol/g) 24 hrs
475.9 ± 1.14 a
471.9 ± 0.84 a
473.6 ± 1.31 a
477.6 ± 0.927 a
381.9 ± 0.833b
455.6 ± 1.46c
459.7 ± 0.485c
462.2 ± 0.743c
GR (nmol/g) 48 hrs
473.2 ± 0.978 a
476.2 ± 0.935 a
479.3 ± 1.479 a
478.9 ± 0.825 a
410.6 ± 0.871b
431.2 ± 1.223c
433.7 ± 1.285c
435.8 ± 1.505c
SOD (U/ml) 1 h
1.564 ± 0.006 a
1.576 ± 0.002 a
1.560 ± 0.013 a
1.580 ± 0.004 a
0.696 ± 0.002b
0.770 ± 0.008c
0.778 ± 0.014c
0.790 ± 0.005c
SOD (U/ml) 24 hrs
1.646 ± 0.008 a
1.650 ± 0.019 a
1.672 ± 0.013 a
1.680 ± 0.01 a
0.342 ± 0.005b
0.956 ± 0.012c
0.966 ± 0.002c
0.984 ± 0.005c
SOD (U/ml) 48 hrs
1.588 ± 0.004 a
1.586 ± 0.002 a
1.600 ± 0.003 a
1.612 ± 0.004 a
0.724 ± 0.009b
0.874 ± 0.014c
0.892 ± 0.004c
0.902 ± 0.005c
Depending on the biochemical parameters, oxidative stress markers and antioxidant enzymes activation were found in boldine (50 mg/kg) concentration at 24 has the most suitable time and dose to complete the current study using the other parameters.
3.4 Histopathological examination
Histological examination of kidney tissues in several experimental groups of rats in 24 h was observed by H&E staining according to Fig. 4. Control group rats (Fig. 1A) and rats administrated with boldine (50 mg/kg) (Fig. 1B) had the normal histological structure of the glomerulus, proximal and distal tubules, with no significant differences between them. Methylprednisolone (MPL) treated rats showed histopathological changes in kidney tissues at 24 h, including loss of normal histological structure, glomerular congestion, swelling of the glomerular and renal tubular lining epithelium, and cellular degeneration, all of which are associated with decreased luminal spaces (Fig. 1C). However, in boldine (50 mg/kg) treatment with MPL, the histological abnormalities in the kidney were decreased to a minor degree, with less renal damage and less granular and renal tubular epithelium degeneration (Fig. 1D).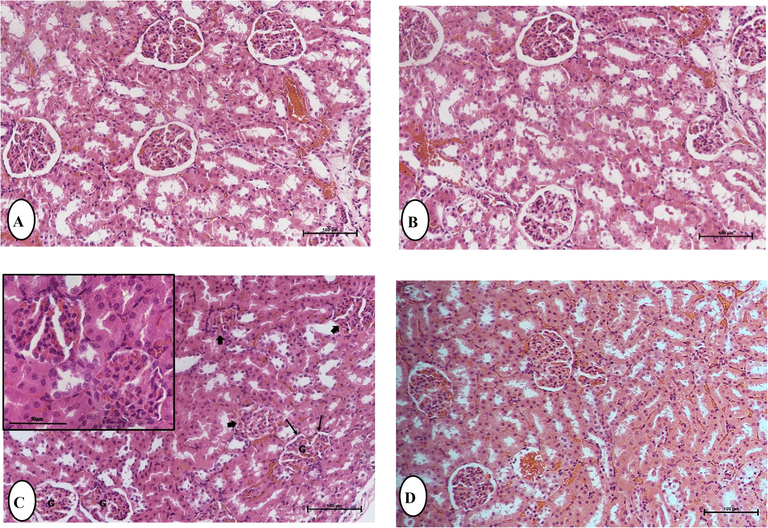
Histological examinations by hematoxylin and eosin staining demonstrating the effect of Boldine (BOL) on Methylprednisolone (MPL) induced kidney damage in rats. Light micrographs of the kidney of control (A) and treated group; (B) BOL 50 mg/Kg b.w./ day, showing the normal renal glomeruli, and renal tubules lined by tubular cells with vesicular nuclei. (C) MPL showing loss of normal histological structure, glomerular (G) congestion, swelling of the glomerular (), cellular degeneration, all of which are associated with decreased luminal spaces (), blood congestion, and increase in pyknotic and karyorrhectic debris in necrotic tubules. (D) BOL (50 mg/kg) + MPL, showing reduced renal damage and regain of typical architecture to a small extent with less renal tubular epithelium degeneration. (H and E), Original magnification is ×40, (scale bar 50 µm).
3.5 Molecular analysis
3.5.1 Real - time PCR
The control group and the group given boldine (50 mg/kg) had no notable effect on the levels of all the genes expressed throughout the 24 h after 4 weeks of the experiment. In contrast, the group treated with MPL demonstrated a significant decrease in the level of SOD, GPx, and 11β-HSD1 genes expression when compared to the control group. However, concomitant boldine (50 mg/kg) + MPL caused a significant increase in the level of SOD, GPx, and 11β-HSD1 genes compared to the MPL group (Fig. 2). Meanwhile, the GR gene was not expressed in the renal tissue.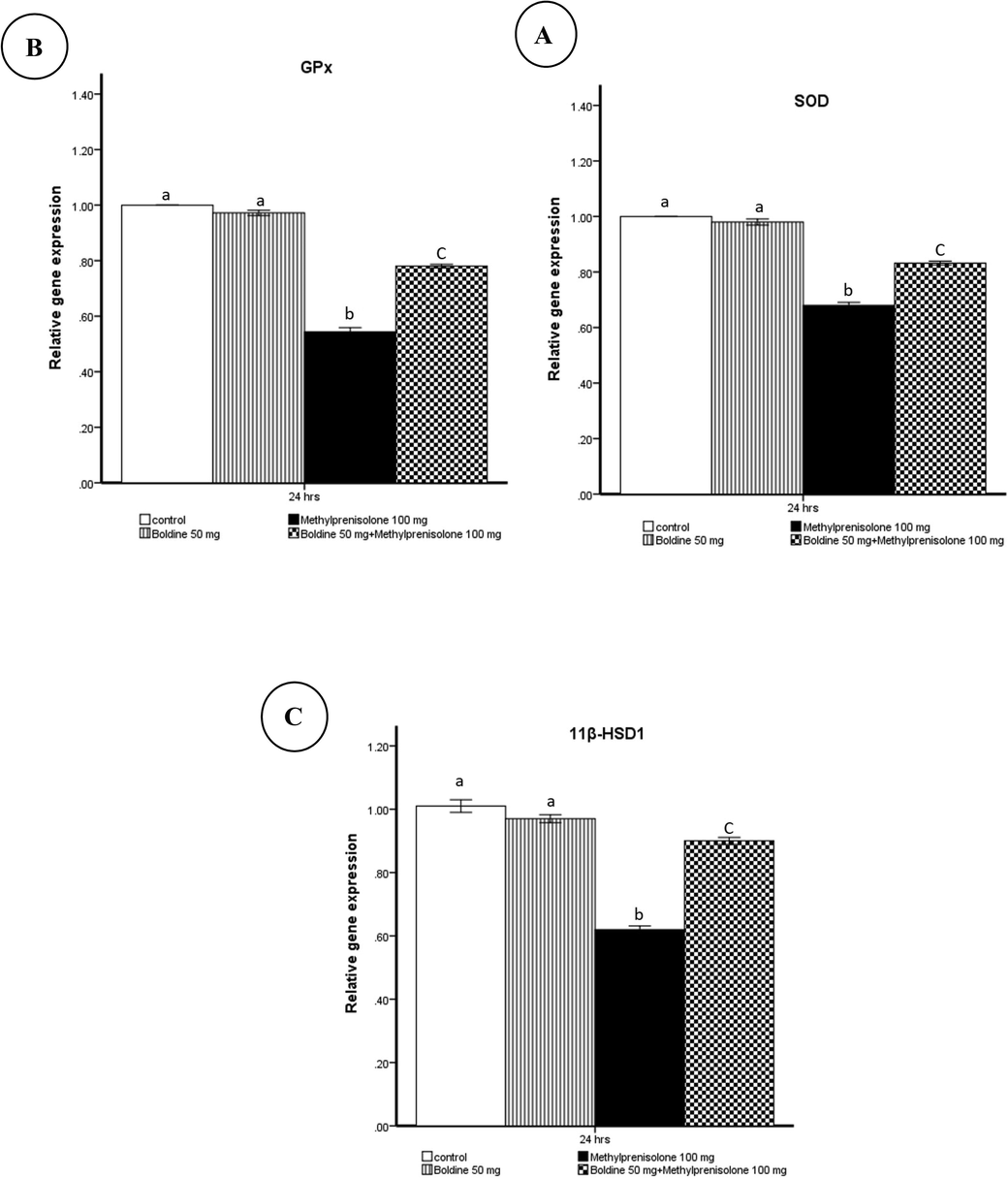
Effect of methylprednisolone on SOD(A), GPx(B), and 11β-HSD1(C) mRNA levels determined by the real-time PCR method were normalized to the quantity of GAPD mRNA.
3.5.2 Biomarkers of cell death:
3.5.2.1 DNA fragmentation
Apoptosis is indicated by DNA fragmentation. Agarose gel electrophoresis was used to investigate the qualitative measurement of the integrity of the renal genomic DNA (Fig. 3). DNA extracted from the control rats (lane 1) and BOL 50 mg/kg treated (lane 2) were of high -quality, while MPL treatment-induced DNA fragmentation at 24 h (lane 3). However, the groups treated with BOL 50 mg/kg + MPL (lane 4) demonstrated less DNA damage.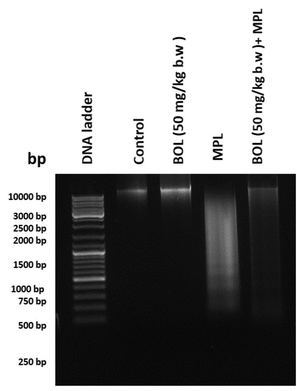
DNA fragmentation in control and experimental rats. Lane 1: control group; lane 2: group treated with BOL (50 mg/kg); lane 3: group treated with MPL (100 mg/kg); lane 4: group treated with BOL (50 mg/kg) + MPL (100 mg/kg).
3.5.2.2 Lactate dehydrogenase (LDH)
When compared to the control group, serum LDH levels in the MPL-treated groups were significantly higher at 24 h. Furthermore, concomitant boldine (50 mg/kg) administration with MPL significantly decreased LDH levels, compared to MPL groups. (Fig. 4).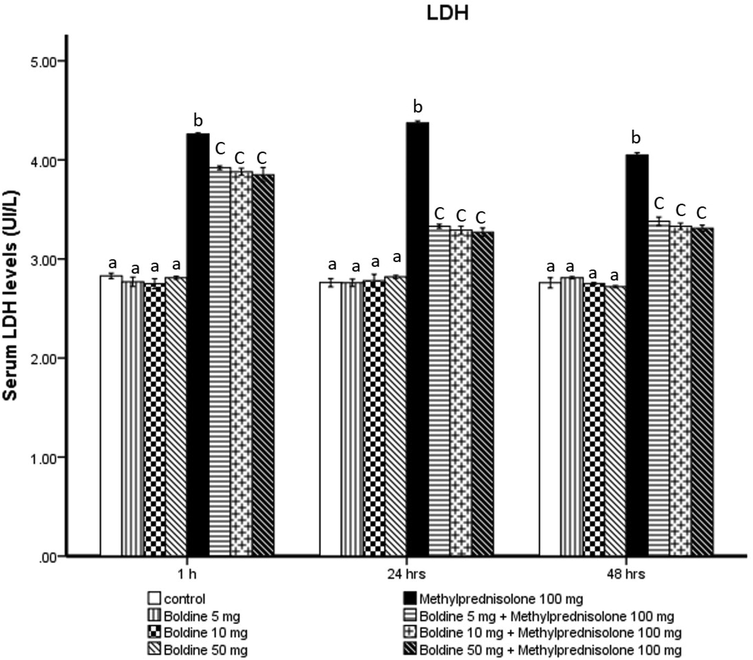
Effect of Boldine on methylprednisolone induces changes in the level of lactate dehydrogenase (LDH) (Ul/L).
3.6 Immunohistochemical observations of the expression of P53 (as apoptosis markers) and TNF-α (as inflammatory response markers)
The immunohistochemical analysis of P53 and TNF-α expression in the kidney in the control and boldine (50 mg/kg) supplemented groups revealed low P53 and TNF-α immunoreactivity (Fig. 5). In contrast, strong P53 and TNF-α immuno-reactivity was found in the renal tubular cells in rats treated with MPL, with many positive nuclei. Renal expression of P53 and TNF-α in rats treated with boldine (50 mg/kg) + MPL was evident as reduced immunoreactivity. Positive p53 protein expression was demonstrated by brown staining in the cytoplasm.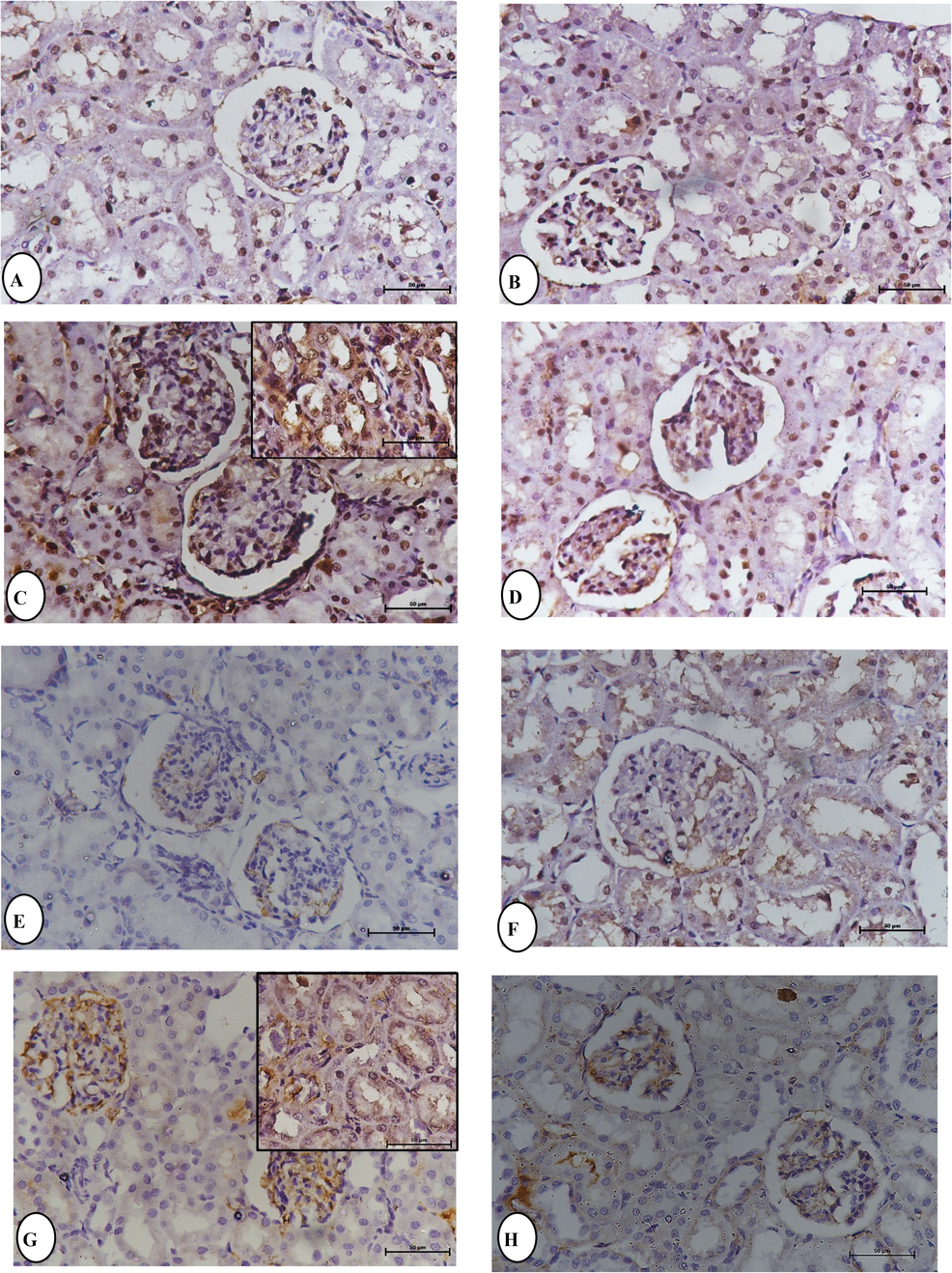
Immunohistochemical staining for P53 and TNF-α expression in the kidney section, the expression of P53 observations in: (A) Normal control rats. (B) Boldine BOL (50 mg/kg) rats. (C) Methylprednisolone MPL (100 mg/kg b.w). (D) BOL (50 mg/kg) + MPL (100 mg/kg b.w) rats at 24 h. The expression of TNF-α observation in: (E) Normal control rats. (F) Boldine BOL (50 mg/kg) rats. (G) Methylprednisolone MPL (100 mg/kg b.w). (H) BOL (50 mg/kg) + MPL (100 mg/kg b.w) rats at 24 h.
4 Discussion
In this study, treatment with MPL significantly increased blood glucose levels. High concentrations of blood glucose are the main features of diabetes. According to the American Diabetes Association, this abnormal increase in blood glucose concentration occurs during glucocorticoid use in patients with or without diabetic history, resulting in steroid-induced diabetes mellitus (American Diabetes Association, 2017). Corticosteroids can indirectly increase glucose production by increasing the substrate quantity for hepatic gluconeogenesis via their muscles and adipose tissue actions by impairing insulin sensitivity and limiting insulin's metabolic actions (Vegiopoulos and Herzig, 2007). Mollataghi et al., (2012) observed a reduction in blood glucose levels after boldine treatment in diabetic rats. Boldine may lower blood glucose levels by acting as an insulin sensitizer. Urea and creatinine are indicators of renal glomerular filtration rate and kidney disease (EL-Sawi et al., 2012). The current study showed a notable increase in urea serum level and a significant decrease in albumin serum level in the MPL-treated group compared to the control group, indicating kidney damage. These agree with previous findings by El-Sawy et al. (2018), who observed significant serum urea and creatinine level increase and a significant serum total protein and globulin decrease in male rats after dexamethasone (DEX) treatment. Gómez and Velarde (2018) discovered a significant prevention of the increase in the renal function (urinary protein/creatinine) ratio after boldine (50 mg/kg) supplementation in treating 5/6NX rats. As a result, boldine is thought to protect kidney function by preserving glomerular filtration and preventing kidney injury. In this study, histopathological findings in the renal tissues of MPL-treated rats supported the results of biochemical changes. Swelling of the glomerular and renal tubular lining epithelium associated with decreased luminal spaces was seen in the kidney. These results support previous findings by Salman and Hassooni, (2020) observed vacuolation of renal tissue and tubules shrinkage after DEX treatment. Chronic progressive glomerulonephritis, severe fibrosis, glomerulosclerosis, atrophy, and partial tubular system regeneration are all symptoms of GCs (Kamphuis et al., 2007). In addition, prenatal administration of GC and DEX cause a notable reduction in glomeruli number (Choi et al., 2013).
While the minor reduction in histological abnormalities in the kidney with less renal damage in the boldine + MPL-treated rat is a clear indicator of boldine’s ability to protect renal damage, the elevated renal damage observed in GCs treated rats was due to the increase in oxidative stress. GCs induce oxidative stress, generate free radicals, modify the renin–angiotensin system, and reduce the nephron number, all of which contribute to kidney injury (Moisiadis and Matthews, 2014). In this study, MPL treatment significantly affected the activities of a variety of oxidative stress markers. When compared to the control group, renal LPO and NOx levels were significantly higher in the MPL-treated group, while GSH levels were significantly lower. These results are in agreement with the previous findings by Pourmehdi et al. (2020) discovered an increase in LPO marker and a decrease in antioxidant enzymes in prednisolone-treated animals, indicating the generation of oxidative stress. Increased renal LPO suggested the potential for nephrotoxicity with this medication.
Several studies have shown that nutrients found in natural products can help prevent the progression of chronic illnesses caused by oxidative stress. Boldine is an alkaloid derived from the boldo tree that has been shown in numerous experimental models to be a powerful antioxidant, preventing or delaying the adverse effects associated with excessive production of ROS (O’Brien et al., 2006). Gómez and Velarde (2018) discovered a notable reduction in oxidative stress and a reduction in the renal TBARS level after boldine supplementation in the treatment of 5/6NX rats. In the presence of boldine, TBARS accumulation in renal tissue is reduced, but they are still eliminated in the urine. Without boldine, TBARS can accumulate in the cell membrane of renal cells rather than in urine or plasma, in diabetic patients.
Another study by Mondal et al. (2020) found a significantly increased levels of SOD and GSH after administering Boldine and nano-Boldine with Cisplatin and reduced levels of LPO, as a marker of oxidative stress. The formation of ROS and the depletion of GSH levels are linked to nephrotoxicity induction (Battin and Brumaghim, 2009). ROS may have aided the translocation of Bax from the mitochondria to the cytosol, causing apoptotic induction. Furthermore, an increase in ROS levels can damage DNA, resulting in cellular death (Rowe et al., 2008).
GCs have been reported to produce inflammation in several investigations. Dietary DEX caused kidney damage, resulting in mild to severe inflammation (Goodwin, 2019). Many intriguing biological activities of boldine have been investigated, but more research is required to fully understand its potential as a future therapeutic treatment. Boldine has been shown to help reduce the negative effects of synthetic glucocorticoids on normal cells and the body. This study aimed to demonstrate that boldine has renal protective effect against methylprednisolone-induced kidney damage. Through methylprednisolone, boldine was shown to be safe and effective in reducing toxicity, maintaining cellular homeostasis, and possessing antioxidant and anti-inflammatory properties in the kidney. We propose that boldine be included in daily diets under medical supervision. It can also be used as an adjuvant treatment in combination with synthetic glucocorticoids.
Acknowledgments
The authors would like to thank Deanship of scientific research in King Saud University is appreciated for funding and supporting this research through the initiative of DSR Graduate Students Research Support (GSR).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 2. Classification and diagnosis of diabetes. Diabetes care. 2017;40(Suppl. 1):S11-S24.
- [Google Scholar]
- Vitamin E and selenium reduce prednisolone side effects in rat hearts. Int. J. Vitam. Nutr. Res.. 2020;90(3–4):309-317.
- [Google Scholar]
- Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys.. 2009;55(1):1-23.
- [Google Scholar]
- Boldo, its secondary metabolites and their derivatives. Curr. Traditional Med.. 2019;5(1):31-65.
- [Google Scholar]
- Glucocorticoids attenuate septic acute kidney injury. Biochem. Biophys. Res. Commun.. 2013;435(4):678-684.
- [Google Scholar]
- EL-Sawi, N.M., Al-Seeni, M.N., Younes, S.H., Al-Jahdali, S.M., and Ali, S.S., 2012. Biochemical and histological studies on the effect of zearalenonemycotoxin and thymoquinone on male mice kidney. J. Am. Sci. 8 (10), 378–388.
- Role of silymarin in restoring the deleterious effects induced by dexamethasone in male rats. Alex. J. Vet. Sci.. 2018;59(2):125-135.
- [Google Scholar]
- Hepatoprotective activity of raspberry ketone is mediated via inhibition of the NF-kB/TNF- a /caspase axis and mitochondrial apoptosis in chemically induced acute liver injury. Toxicol. Res.. 2019;8:663-676.
- [Google Scholar]
- Gerhardt, D., Bertola, G., Dietrich, F., Figueiró, F., Zanotto -Filho, A., Fonseca, J.C.M., Morrone, F.B., Barrios, C.H., Battastini, A.M.O., and Salbego, C.G., 2014. Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β. In Urologic Oncology: Seminars and Original Investigations. 32(1), 36-e1. Elsevier.
- Boldine improves kidney damage in the goldblatt 2K1C model avoiding the increase in TGF-β. Int. J. Mol. Sci.. 2018;19(7):1864.
- [Google Scholar]
- Role of the glucocorticoid receptor in glomerular disease. Am. J. Physiol. Renal Physiol.. 2019;317(1):F133-F136.
- [Google Scholar]
- Boldine supplementation regulates mitochondrial function and oxidative stress in a rat model of hepatotoxicity. Pharm. Sci.. 2019;25(1):1-10.
- [Google Scholar]
- Boldine prevents renal alterations in diabetic rats. J. diabetes Res.. 2013;2013:593672
- [CrossRef] [Google Scholar]
- Intraoperative high-dose dexamethasone and severe AKI after cardiac surgery. J. Am. Soc. Nephrol.. 2015;26(12):2947-2951.
- [Google Scholar]
- Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr. Res.. 2007;61(1):72-76.
- [Google Scholar]
- Glucocorticoids and fetal programming part 2: mechanisms. Nat. Rev. Endocrinol.. 2014;10(7):403-411.
- [Google Scholar]
- Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia.. 2012;83(2):298-302.
- [Google Scholar]
- Improved drug carriage and protective potential against Cisplatin-induced toxicity using Boldine-loaded PLGA nanoparticles. J. Ayurveda Integrative Med.. 2020;11(1):24-36.
- [Google Scholar]
- Aporphine alkaloids and their antioxidant medical application: From antineoplastic agents to motor dysfunction diseases. Curr. Org. Chem.. 2017;21(4):342-347.
- [Google Scholar]
- Boldine and its antioxidant or health-promoting properties. Chem. Biol. Interact.. 2006;159(1):1-17.
- [Google Scholar]
- Methylprednisolone. StatPearls[Internet]. Island (FL): StatPearls Publishing; 2021. PMID 31335060.Retrieved 2020–11-10
- Carvacrol attenuates acute kidney injury induced by cisplatin through suppression of ERK and PI3K/Akt activation. Food Chem. Toxicol.. 2016;98:251-261.
- [Google Scholar]
- Betaine effects against asthma-induced oxidative stress in the liver and kidney of mice. Mol. Biol. Rep.. 2020;47(8):5729-5735.
- [Google Scholar]
- DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Rad. Biol. Med.. 2008;45(8):1167-1177.
- [Google Scholar]
- Morphological and Histological effect induced by each of Cefotaxime, Dexamethazon and mixture of them to the stomach, liver, kidney and lung in rats. Drug Invent. Today.. 2020;11(12):3313.
- [Google Scholar]
- Cushing’s syndrome, glucocorticoids and the kidney. Gen. Comp. Endocrinol.. 2010;169(1):1-10.
- [Google Scholar]
- Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol.. 2007;275(1–2):43-61.
- [Google Scholar]
- Anti-inflammatory effects of boldine and reticuline isolated from Litseacubeba through JAK2/STAT3 and NF-κB signaling pathways. Planta med.. 2018;84(01):20-25.
- [Google Scholar]
- Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem. Toxicol.. 2015;78:17-25.
- [Google Scholar]







